In this issue of Blood, Li et al1 provide compelling evidence for a protein kinase, serine/threonine kinase 10 (STK10), hitherto unknown in platelets, that promotes hemostasis and thrombosis, while also playing an important role in stroke and sepsis.
Platelets are critical players in hemostasis and thrombosis. The thrombotic process can amplify host inflammatory responses and trigger thromboinflammation, in part via platelet-neutrophil interaction. Platelet effector functions support the interconnected biological processes of hemostasis, thrombosis, and inflammation. Specifically, platelets are involved in adhesion, spreading, aggregation, secretion, and fibrin clot retraction, along with the formation of platelet-neutrophil complexes that support neutrophil extracellular traps (NETs). More importantly, these platelet functions are supported by critical signaling pathways.
Protein kinases are master regulators of signaling pathways, and serine/threonine (Ser/Thr) kinases phosphorylate their substrates on serine and/or threonine residues to regulate platelet function. Proteomic mass spectrometry in human and murine platelets have identified ∼110 serine/threonine kinases, including proteins with a kinase domain and those without a kinase domain and with only noncatalytic regulatory subunits.2 Despite the vast spectrum of platelet Ser/Thr kinases, our current knowledge of signaling pathways, protein-protein interaction and kinase-substrate relationships in platelets are largely derived from only a small number of (but highly investigated) kinases in the platelet kinome. A few examples include Ser/Thr kinases from the families of protein kinase A/B/C/G, calcium/calmodulin dependent kinase, mitogen activated protein kinase, cyclin dependent kinase, casein kinase, LIM kinase, and mammalian target of rapamycin. Using a quantitative phosphoproteomic approach, the authors previously demonstrated that platelet activation by collagen-related peptide (CRP) led to the robust phosphorylation of STK10 on Ser13 and Thr14.3 STK10 is a member of the Ste-20 family of Ser/Thr kinase and is well studied in lymphocytes, in which STK10 regulates lymphocyte migration by phosphorylating ezrin, radixin, and meosin (ERM) proteins.4 Platelets express 10 Ser/Thr kinases from the Ste-20 family.2 Given that STK10 was not among those reported in platelets, a functional role for platelet STK10 remained unknown.
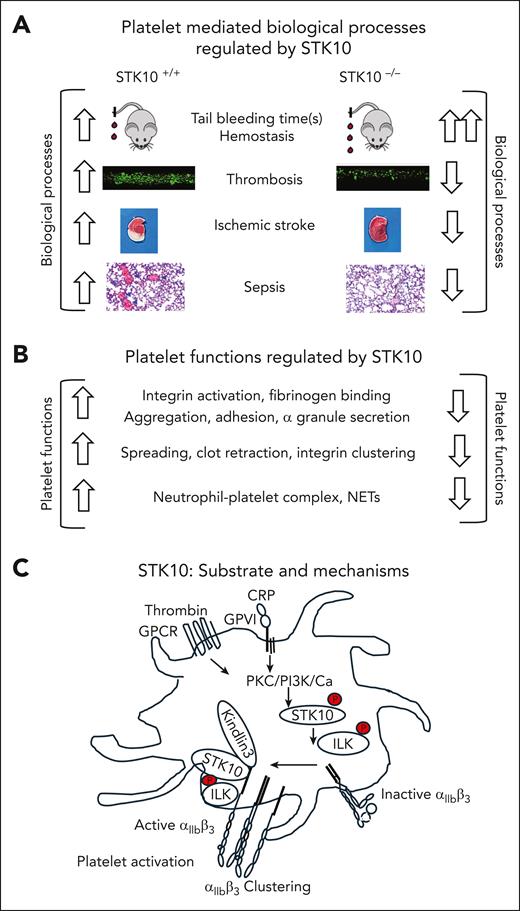
Li et al generated a new mice model with megakaryocyte-/platelet-specific deletion of STK10 and showed that STK10−/− mice had prolonged tail bleeding times (hemostasis), delayed occlusion time in a mesenteric ferric chloride injury (thrombosis), reduced infarct volume in a middle cerebral artery occlusion model (ischemic stroke) and increased survival in a cecal ligation and puncture (CLP) model (sepsis) (see figure panel A). STK10−/− platelets had reduced aggregation, α-granule secretion, integrin αIIbβ3 activation, and soluble fibrinogen binding in response to low doses of CRP and thrombin. Furthermore, decreased spreading on immobilized fibrinogen, reduced fibrin clot retraction, and associated Src and Syk signaling were observed in STK10−/− platelets (see figure panel B). The phosphoproteomic analysis of CRP-activated STK10−/− platelets identified dysregulation in the phosphorylation of focal adhesion proteins. Follow-up studies identified that STK10 directly phosphorylated Ser343 on integrin-linked protein kinase (ILK) and STK10−/− platelets displayed reduced phosphorylation of ILK, β3, AKT, P38, and extracellular signal-regulated kinase. Reduced platelet-neutrophil complex and NETs were noted in STK10−/− mice in a CLP model and platelets from human and mouse with sepsis showed increased Ser/Thr phosphorylation of STK10 and ILK. Mechanistically, these studies suggested that STK10 phosphorylated ILK, supported the binding of kindlin 3 to integrin β3, increased integrin αIIbβ3 clustering, and facilitated platelet hemostasis, thrombosis, and thromboinflammation (see figure panel C).
Key findings of Li et al on the role of STK10 in platelets. Role for STK10 in platelet-mediated biological processes (A) and platelet functions (B) along with signaling pathways that support STK phosphorylation, identification of the STK10 substrate, ILK, and potential mechanisms that support integrin activation (C). Studies were performed with platelet/megakaryocyte–specific deletion of STK10 mice (STK10−/−) and its flox/flox counterpart (STK10+/+). The representative figures shown in panel A are from the authors’ data in the accompanying article. Phosphorylated STK10 and ILK are shown with P in a red circle. ↑, increased; ↓, decreased. GPCR: G protein–coupled receptor; GPVI, glycoprotein VI receptor; PI3K, phosphatidylinositol 3-kinase.
Key findings of Li et al on the role of STK10 in platelets. Role for STK10 in platelet-mediated biological processes (A) and platelet functions (B) along with signaling pathways that support STK phosphorylation, identification of the STK10 substrate, ILK, and potential mechanisms that support integrin activation (C). Studies were performed with platelet/megakaryocyte–specific deletion of STK10 mice (STK10−/−) and its flox/flox counterpart (STK10+/+). The representative figures shown in panel A are from the authors’ data in the accompanying article. Phosphorylated STK10 and ILK are shown with P in a red circle. ↑, increased; ↓, decreased. GPCR: G protein–coupled receptor; GPVI, glycoprotein VI receptor; PI3K, phosphatidylinositol 3-kinase.
This comprehensive study by Li et al is timely and novel because it improves our understanding of understudied kinases in platelets. Specifically, this study illuminates signaling pathways and identifies the ILK Ser343 phosphorylation by STK10 in platelets and may reveal future therapeutic opportunities and novel drug targets. Overall, the finding that STK10 plays a central role in hemostasis, thrombosis, and thromboinflammation were a bit surprising given how little was known of the status of this kinase in platelets. These studies represent a good start and will spur additional questions. Does STK10 regulate migration in platelets? This is precedence for platelet haptotatic migration to limit bacterial invasion during infection and inflammation as a part of vascular surveillance.5 The phenotype of Ser485 Ala kindlin-3 mutant mice had unexpected similarities to STK10−/− mice, including prolonged bleeding, delayed thrombosis, reduced integrin activation, and diminished platelet functions.6 This raises the question whether STK10 or its substrate ILK phosphorylate kindlin 3 at Ser 485 in murine platelets promotes integrin activation. Considering that platelets are a determinant in cancer metastasis,7 it would be interesting to evaluate whether platelet STK10 contributes to tumor progression. For a rigorous characterization of this kinase, we need to better understand how STK10 is activated in platelets, discover new STK10 substrates, and identify STK10 copy number in human and murine platelets. Because prolonged tail bleeding time(s) was observed in STK10−/− mice, this study predicts a potential challenge related to bleeding in future preclinical models with pharmacological blockade of STK10. A more targeted pharmacological approach may involve disrupting STK10 binding to its substrate ILK, with the hope of selectively blocking integrin outside in signaling.
In conclusion, the study by Li et al reveals an essential role for STK10 in platelet function that underpins hemostasis, thrombosis, and thromboinflammation, thus revealing STK10 from the platelet kinome shadow.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal