Abstract
We previously reported the genetic abnormality in a Japanese family with type I congenital plasminogen deficiency caused by a Ser572 to Pro572 mutation. To characterize the molecular pathogenesis of the disease in this family, we expressed recombinant human wild-type and mutant (rS572P) plasminogens in COS-1 cells. Activation-resistant wild-type and mutant plasminogen stable transfectants in CHO-K1 cells also were established. Transient transfection and metabolic labeling experiments followed by immunoprecipitation analysis showed that the mutant plasminogen was secreted from COS-1 cells in reduced amounts, compared with the wild type. Endo H digestion of the wild-type and mutant plasminogen showed no shift in their migrations on sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis, indicating that both contain complex type oligosaccharide structures and could therefore be secreted. Furthermore, the secretion of activation-resistant mutant plasminogen was significantly reduced. Pulse-chase experiments and Northern blot analysis showed that the impaired secretion of the mutant plasminogen was the consequence of the accumulation of the mutant protein inside the cells but not of reduced plasminogen mRNA. Immunocytochemical staining of stable transfectants also revealed that CHO-K1 cells expressing the activation-resistant mutant plasminogen stained mainly in the perinuclear area, suggesting delayed processing of the mutant protein in the intracellular transport pathway. We conclude that the impaired secretion of mutant plasminogen, due to intracellular accumulation, is the molecular pathogenesis of type I congenital plasminogen deficiency caused by a Ser572 to Pro572 mutation.
PLASMINOGEN (PLG) is the proenzyme of the serine protease plasmin. It is a single-chain glycoprotein with 791 amino acid residues.1 Two molecular species of PLG, 93 kD and 90 kD, are present in human plasma. Approximately 40% of PLG is both N-glycosylated on Asn289 and O-glycosylated on Thr346. The remainder is only O-glycosylated on Thr346.2 Cleavage of the Arg561-Val562 bond by tissue PLG activator (tPA) or urokinase results in the formation of plasmin, which consists of a heavy chain (kringle domain) and a light chain (serine protease domain) held together by two disulfide bonds. The proteolytic activity of plasmin contributes to the digestion of insoluble fibrin clots and thrombi, leading to the recanalization of vessels and tissue repair.3
Genetic engineering techniques, including polymerase chain reaction (PCR), site-specific mutagenesis, and mammalian cell expression, have been used to investigate the structure-function relationship of PLG.4-10 However, the expression of recombinant PLG (rPLG) in mammalian cells is difficult due to the intracellular activation of rPLG by endogenous plasminogen activator in transfected cells and subsequent cell lysis.8
Congenital PLG deficiency is classified into two types, “true” deficiency, or type I, and dysplasminogenemia, or type II. In the former, the level of immunoreactive PLG is reduced in parallel with its functional activity, whereas in the latter the level of immunoreactive PLG is normal or only slightly reduced, although the functional activity is greatly reduced. An amino acid substitution in the PLG molecule was identified by amino acid analysis in a family with dysplasminogenemia.11 The amino acid substitution was Ala601 to Thr601, resulting in the impairment of proteolytic activity. The genetic change responsible for this substitution is a single G to A nucleotide transition in exon 15.12 The PCR technique developed in recent years has allowed us and others to detect small mutations, including point mutations, in the PLG gene.12-14 Type I congenital PLG deficiency was first reported in 1982. Since then, approximately 27 patients with thrombosis due to PLG deficiency have been reported.15
We previously reported, for the first time, the genetic abnormality in a Japanese family with type I congenital PLG deficiency caused by a Ser572 (TCC) to Pro572 (CCC) substitution.13 However, no reports concerning expression analysis in mammalian cells of mutant PLGs identified in patients with either type I or II congenital PLG deficiency have been published. To investigate further the molecular pathogenesis of our previously reported family with type I congenital PLG deficiency, we constructed recombinant plasmids containing either a wild-type or mutant full-length human PLG cDNA and expressed them transiently in green monkey kidney (COS-1) cells. Moreover, an activation-resistant rPLG (Arg561 → Ser561 ) was expressed in stable transfectants. Immunocytochemistry also was performed these stable transfectants to characterize in more detail the importance of the Ser572 residue in determining the inherent conformation of native human PLG.
MATERIALS AND METHODS
Construction of mammalian expression plasmids.A recombinant plasmid, pTH3/WT, was constructed to direct the synthesis of human PLG in mammalian cells. To generate pTH3/WT, a 2.7-kb HindIII/Sph I fragment containing the full-length cDNA of human PLG, prepared as reported previously,13 was ligated into the HindIII/Sph I site of pUC18 (Toyobo, Osaka, Japan). The resultant clone was labeled pTH1. The HindIII/BamHI insert of pTH1 (the BamHI site of pTH1 is within the polylinker of pUC18) was cloned into the HindIII/BamHI site of pBS/KS− (Stratagene, La Jolla, CA), and the resultant plasmid was designated pTH2. A similar cloning procedure was performed to construct the mammalian expression plasmid. The acquired sequence of the pBS/KS− polylinker sequence in pTH2 allowed the removal of the PLG insert after digestion with Xho I (5′ of the PLG initiating Met codon) and Not I (3′ to the PLG stop codon). The Xho I/Not I fragment was cloned into the Xho I/Not I site of the expression plasmid, pcDNAneo, which contains a neomycin resistance gene inserted in the parent plasmid pCDM8.16 The mammalian expression plasmid was designated pTH3/WT.
Site-directed mutagenesis of pTH3/WT was performed using pTH2. The pTH2 plasmid was used to transform the dut− ung− Escherichia coli strain, CJ236, and generate single-stranded uracil-containing DNA for site-directed mutagenesis.17 The mutagenic primer 5′-GTGGCCCACCCACATCCCTGGCCCTGGCAAG-3′ (the boldface letter indicates the mismatch), corresponding to nucleotide numbers 1876-1906 (numbering according to Petersen et al1 ), was used to replace the PLG Ser572 codon with a Pro572 codon. After verifying that the mutated coding sequence was correct by DNA sequence analysis, the mutant fragment was cloned into the Xho I/Not I site of pcDNAneo, by the same procedures used to construct pTH3/WT. The resultant plasmid was designated pTH3/S572P. Both the wild-type and the mutant single-stranded uracil-containing pTH2 were again mutagenized to produce activation-resistant PLG molecules, using the mutagenic primer 5′-GAAATGTCCTGGATCCGTTGTAGGGGGG-3′ (boldface letters indicate the mismatches, and the underline indicates the BamHI sequence), corresponding to nucleotide numbers 1845-1872 (numbering according to Petersen et al1 ). Colonies containing the desired codon substitution were screened using the new BamHI site in the mutagenic primer sequence and were confirmed by DNA sequence analysis. Two mammalian expression plasmids for the activation-resistant PLG molecules, containing wild-type or Pro572 mutant sequences, were constructed as described above, and the resultant clones were designated pTH3/R561S and pTH3/R561S + S572P, respectively.
Cell culture.COS-1 cells (green monkey kidney, SV40 transformed) (American Type Culture Collection [ATCC] No. CRL1650; Rockville, MD), CHO-K1 cells (Chinese hamster ovary) (ATCC No. CCL61), CV-1 cells (green monkey kidney) (ATCC No. CCL70), and HepG2 cells (human hepatoblastoma) (ATCC No. HB8065) were maintained in 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) (GIBCO-BRL, Gaithersburg, MD) supplemented with 10% fetal calf serum (Biocell Laboratories Inc, Rancho Domingues, CA) (10%-DMEM) and 2 mmol/L L-glutamine (GIBCO-BRL). Nonessential amino acids (500 μmol/L) (GIBCO-BRL) were added to the medium of the CHO-K1 cells.
DNA transfection and establishment of stable transfectants.Each plasmid DNA was isolated by the Birnboim and Doly procedure18 before mammalian cell transfection and purified in cesium chloride gradients generated by ultracentrifugation. DNA was introduced into cells using a calcium phosphate-mediated transfection procedure. For transient expression experiments, cells were subcultured at a density of 2.0 × 105 cells/60-mm dish for COS-1 cells and 7.5 × 105 cells/60-mm dish for HepG2 cells, 24 hours before transfection. Fifteen micrograms of DNA was applied onto each dish, and the cells were maintained in 10%-DMEM following transfection.
To establish stable transfectants, CHO-K1 and CV-1 cells were subcultured at a density of 1.0 × 105 cells/60-mm dish, split on the second day after transfection, and grown for 10 to 14 days in the presence of the antibiotic G418 (Sigma Chemicals, St Louis, MO), 0.8 mg/mL for CHO-K1 cells and 0.4 mg/mL for CV-1 cells. Independent colonies were picked into 12-well plates and grown to confluence. Transformed cells expressing PLG antigen were identified by spotting cell lysates on nitrocellulose (Bio-Rad Laboratories, Hercules, CA) and were confirmed by immunoblotting following sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 1,000-fold dilution of rabbit polyclonal anti-human PLG antibody (Behringwerke AG, Marburg, Germany). To prepare cell lysates, cells were washed with Dulbecco's phosphate-buffered saline (DPBS) (GIBCO-BRL), resuspended in 1 mL of disruption buffer (10 mmol/L Tris-HCl, pH 7.8, 150 mmol/L NaCl, 5 mmol/L EDTA, 10 mmol/L benzamidine, 1 mmol/L phenylmethylsulfonyl fluoride [PMSF], 1% Nonidet P [NP]-40), and lysed with three repetitive freeze-thaw cycles. Cellular debris was removed by centrifugation for 5 minutes at 15,000g. Following spotting on nitrocellulose or SDS-PAGE, samples were reacted with the polyclonal rabbit anti-human PLG antibody, followed by a goat anti-rabbit IgG horseradish peroxidase conjugate. Color was developed using an Immuno-Blot assay kit (Bio-Rad Laboratories). PLG antigen-positive clones were used for metabolic labeling experiments, as described below.
Metabolic labeling and immunoprecipitation analysis.For the transient expression experiments, COS-1 and HepG2 cells were rinsed twice with DPBS 40 hours posttransfection, incubated for 30 minutes in 2 mL of DMEM lacking methionine (GIBCO-BRL), and then supplemented with L-[35S] methionine (100 μCi/mL) (Amersham Corp, Arlington Heights, IL) for 4 hours. Cells were washed with DPBS and incubated with 3 mL of 10%-DMEM for an additional 16 hours. The culture media was collected, 10 mmol/L benzamidine and 1 mmol/L PMSF were added, and the media then was centrifuged at 1,300g for 5 minutes to remove cellular debris. Cells were lysed in 1 mL of disruption buffer and centrifuged as described above.
Aliquots (1.5 mL) of the metabolically labeled culture medium were immunoprecipitated by adding 1/10 vol of 10 × immunoprecipitation buffer (IPB; 1 × IPB = 10 mmol/L Tris-HCl pH 7.5, 150 mmol/L NaCl, 1 mmol/L EDTA, 1% NP-40) and incubated with 5 μg of goat polyclonal anti-human PLG (IgG fraction) (Cedarlane Lab, Ontario, Canada) for 16 hours at 4°C with continuous skaking. Immune complexes were precipitated with 30 μL of protein A-Sepharose beads (Sigma Chemicals), washed twice with 1× IPB, and then twice more with the same buffer lacking NP-40. The immunoprecipitation products were electrophoresed in a 10% SDS-PAGE gel, dried, and subjected to autoradiography.
Stable transfectants expressing rPLG were metabolically labeled with L-[35S] methionine and immunoprecipitated using a similar procedure, except that the incubation period in DMEM lacking methionine was extended to 2 hours. To investigate whether the rate of secretion of rR561S is higher than that of rWT, 15 μg of pTH3/WT or pTH3/R561S and 5 μg of pSV-β-galactosidase control vector (Promega, Madison, WI) plasmids were transiently cotransfected into COS-1 cells. Aliquots (1 mL) of metabolically labeled culture media were immunoprecipitated with both 5 μg of goat polyclonal anti-human PLG (IgG fraction) and 3 μg of rabbit polyclonal anti-human–β-galactosidase (IgG fraction) (Cappel, Durham, NC), and analyzed by electrophoresis and autoradiography, as described above.
For pulse-chase experiments, 15 μg of pTH3/WT or pTH3/S572P, and 5 μg of pSV–β-galactosidase control vector plasmids were cotransfected transiently into COS-1 cells. The transfected COS-1 cells were pulse-labeled with L-[35S] methionine for 30 minutes, and then chased for varying periods up to 24 hours with unlabeled methionine. Aliquots (700 μL) of metabolically labeled cell lysates were mixed with an equal volume of 1× IPB lacking EDTA and NP-40, and immunoprecipitated with 5 μg of goat polyclonal anti-human PLG (IgG fraction) and 3 μg of rabbit anti–β-galactosidase (IgG fraction) at 4°C for 1 hour, followed by incubation with 30 μL of protein A-Sepharose beads at 4°C for 1 hour. The quantity of immunoprecipitated β-galactosidase served as an internal control for both the transfection efficiency and for the recovery of the immunoprecipitated products. Aliquots (1 mL) of culture media were immunoprecipitated as described above. Radiolabeled rPLG and β-gal were quantitated by a laser densitometer (Pharmacia LKB XL, Uppsala, Sweden) to normalize for both the transfection efficiency and the immunoprecipitation efficiency.
SDS-PAGE analysis of 35S-labeled rPLG immunoprecipitated from the culture media of transfected COS-1 cells. (A) COS-1 cells were transiently transfected with pTH3/WT (WT) or pTH3/S572P (S572P) plasmids. Cells were metabolically labeled with L-[35S] methionine, and the culture media (1.5 mL) was immunoprecipitated with goat polyclonal anti-human PLG (IgG fraction). Immunoprecipitation products were analyzed by 10% SDS-PAGE and autoradiography under nonreducing conditions. The migration of protein standards is indicated. (B) Intracellular PLG purified from HepG2 cells (CE) and protein A-Sepharose beads containing adsorbed rPLG (CM) were treated with (+) or without (−) endo H as described in Materials and Methods. Each sample was boiled without a reducing agent, centrifuged, and the supernatant was subjected to 10% SDS-PAGE as described above.
SDS-PAGE analysis of 35S-labeled rPLG immunoprecipitated from the culture media of transfected COS-1 cells. (A) COS-1 cells were transiently transfected with pTH3/WT (WT) or pTH3/S572P (S572P) plasmids. Cells were metabolically labeled with L-[35S] methionine, and the culture media (1.5 mL) was immunoprecipitated with goat polyclonal anti-human PLG (IgG fraction). Immunoprecipitation products were analyzed by 10% SDS-PAGE and autoradiography under nonreducing conditions. The migration of protein standards is indicated. (B) Intracellular PLG purified from HepG2 cells (CE) and protein A-Sepharose beads containing adsorbed rPLG (CM) were treated with (+) or without (−) endo H as described in Materials and Methods. Each sample was boiled without a reducing agent, centrifuged, and the supernatant was subjected to 10% SDS-PAGE as described above.
Endo-β-N-acetylglucosaminidase H digestion of the recombinant plasminogens.The rPLG proteins were treated with endo-β-N-acetylglucosaminidase H (endo H) (Genzyme Corp, Boston, MA) as described by Johnston et al.19 In brief, protein A-Sepharose beads (Sigma Chemicals) containing adsorbed rPLG were incubated overnight at 37°C in 25 μL of 50 mU/L sodium acetate, pH 5.5, and 0.1% SDS, containing 50 mU/mL of endo H. Each sample was boiled in an equal volume of 2× SDS sample buffer (60 mmol/L Tris-HCl, pH 6.8, 5 mmol/L EDTA, 2% SDS, 10% glycerol, 0.5% bromphenol blue) without a reducing agent, and centrifuged at 15,000g. The supernatant was subjected to SDS-PAGE, followed by autoradiography. Intracellular PLG was purified from HepG2 cells using a lysine-Sepharose 4B affinity column (Pharmacia LKB) by the method of Deutsch and Mertz.20 Approximately 2 μg of intracellular PLG was digested with endo H to examine the enzymatic activity of the endo H.
Northern blot analysis.Total RNA was isolated from transfected COS-1 cells using a commercially available kit (Qiagen Inc, Chatsworth, CA). RNA samples were quantitated by absorbance at 260 nm, and 20 μg of RNA was loaded onto each lane of a 1% agarose/formaldehyde gel and electrophoresed. The gel was washed in 20× SSC (1× SSC = 150 mmol/L NaCl, 15 mmol/L sodium citrate, pH 7.0), and the RNA was transferred overnight at room temperature to a Hybond-N+ transfer membrane (Amersham Corp), followed by fixation to the membrane by baking at 80°C for 2 hours. Membranes then were prehybridized for 3 hours and hybridized at 43°C overnight in a solution containing 750 mmol/L NaCl, 20 mmol/L Tris-HCl (pH 7.5), 2.5 mmol/L EDTA, 1% SDS, 0.5× Denhardt's solution (1× Denhardt = 0.02% Ficoll, 0.02% polyvinylpyrrolidone, and 0.02% bovine serum albumin [BSA]), 50% deionized formamide, 10% dextran sulfate, and 50 μg/mL denatured salmon sperm DNA. The full-length PLG cDNA prepared as mentioned above was labeled with a Megaprime DNA labeling system (Amersham Corp) in the presence of deoxycytidine 5′-[α-32P]-triphosphate ([α-32P]dCTP) (Amersham Corp). Hybridized membranes were washed twice with 0.2× SSC/0.1% SDS at 65°C for 30 minutes and then exposed to Kodak X-Omat AR films (Eastman Kodak, Rochester, NY) at −80°C with an intensifying screen.
Immunocytological analysis of stable transfectants.Stable transfectants expressing rR561S or rR561S + S572P were plated on glass cover slips coated with poly-L-lysine (Sigma Chemicals). The cells were fixed with 4% paraformaldehyde in buffer A (DPBS containing 0.05% Tween 20) at room temperature for 15 minutes, washed with buffer A twice, permeabilized with 0.5% Triton X-100 in buffer A for 5 minutes, and washed again three times with buffer A. The cells then were blocked in buffer B (3% BSA and 5% normal goat serum in buffer A) for 20 minutes and reacted with a rabbit polyclonal anti-human PLG antibody (1:500 dilution in buffer B) for 1 hour at room temperature. After washing the cells three times with buffer A, the cover slips were incubated with biotinylated anti-rabbit IgG (Cappel; American Qualex, San Clemente, CA, 1:400 dilution in buffer B) for 1 hour, washed, and immunostained using an ABC kit (Vector Laboratories Inc, Burlingame, CA). Untransfected CHO-K1 cells also were treated as described above.
RESULTS
Expression and secretion of wild-type and mutant plasminogens in mammalian cells.Although both rPLG molecules, rWT and rS572P, were detected in the culture medium, a significant difference in the amount of the proteins secreted into the culture media was observed (Fig 1A). Their molecular sizes were identical to those of PLG produced by Hep G2 cells. Two close bands, of approximately 93 and 90 kD, were observed, suggesting the presence of fully and partially glycosylated molecules. In all cases, morphologic examination of the cells showed that little cell death had occurred 48 hours after the transfection (<5%, estimated by dye-exclusion using trypan blue). Although COS-1 cells have been successfully used for the transient expression of PLG, it is possible that, as in CHO and BHK cells, the recombinant PLG molecules in the culture medium might not have been secreted. Alternatively, they may have resulted from the activation of PLG to plasmin by endogenous PLG activators in the cells, leading to cell auto-degradation, and the subsequent release of intracellular proteins, including the rPLG. To exclude this possibility, we used the following two analyses: (1) endo H digestion of recombinant molecules, and (2) expression of the activation-resistant recombinant wild-type (rR561S) and mutant (rR561S + S572P) molecules.
SDS-PAGE analysis of stable transfectants secreting the activation-resistant rPLG. Stable transfectants secreting wild-type (R561S) and mutant (R561S + S572P) rPLGs were established in CHO-K1 cells and labeled with L-[35S] methionine at the time of 80% confluency. Immunoprecipitation products were electrophoresed under nonreducing conditions by 10% SDS-PAGE.
SDS-PAGE analysis of stable transfectants secreting the activation-resistant rPLG. Stable transfectants secreting wild-type (R561S) and mutant (R561S + S572P) rPLGs were established in CHO-K1 cells and labeled with L-[35S] methionine at the time of 80% confluency. Immunoprecipitation products were electrophoresed under nonreducing conditions by 10% SDS-PAGE.
SDS-PAGE analysis of 35S-labeled rPLG immunoprecipitated from the culture media of transfected COS-1 cells. COS-1 cells were transiently contransfected with pTH3/WT (WT) or pTH3/R561S (R561S) and pSV–β-galactosidase plasmids. The cells were metabolically labeled with L-[35S]-methionine, and the culture media (1.5 mL) was immunoprecipitated with goat polyclonal anti-human PLG (IgG fraction) and rabbit polyclonal anti–β-galactosidase (IgG fraction). The immunoprecipitation products were analyzed by 10% SDS-PAGE and autoradiography under nonreducing conditions. The migration of protein standards and β-galactosidase is indicated.
SDS-PAGE analysis of 35S-labeled rPLG immunoprecipitated from the culture media of transfected COS-1 cells. COS-1 cells were transiently contransfected with pTH3/WT (WT) or pTH3/R561S (R561S) and pSV–β-galactosidase plasmids. The cells were metabolically labeled with L-[35S]-methionine, and the culture media (1.5 mL) was immunoprecipitated with goat polyclonal anti-human PLG (IgG fraction) and rabbit polyclonal anti–β-galactosidase (IgG fraction). The immunoprecipitation products were analyzed by 10% SDS-PAGE and autoradiography under nonreducing conditions. The migration of protein standards and β-galactosidase is indicated.
Pulse-chase experiments of 35S-labeled rPLG. COS-1 cells were transiently cotransfected with either pTH3/WT (WT), pTH3/S572P (S572P), or pcDNAneo (mock) and pSV–β-galactosidase plasmids. After pulse-labeling with L-[35S] methionine for 30 minutes, the COS-1 cells then were chased for the times indicated (1 hour, 2 hours, 6 hours, 24 hours) in the presence of unlabeled methionine. In case of mock-transfected cells, culture medium and cell extract prepared 6 hours after pulse labeling were analyzed to examine the specificity of anti-PLG antibody. Both culture media (A, 1 mL) and cell lysates (B, 700 μL) were prepared, and immunoprecipitation samples were analyzed under nonreducing conditions as described above. β-Galactosidase present in the cell lysates was used as an internal control for both transfection and immunoprecipitation efficiency.
Pulse-chase experiments of 35S-labeled rPLG. COS-1 cells were transiently cotransfected with either pTH3/WT (WT), pTH3/S572P (S572P), or pcDNAneo (mock) and pSV–β-galactosidase plasmids. After pulse-labeling with L-[35S] methionine for 30 minutes, the COS-1 cells then were chased for the times indicated (1 hour, 2 hours, 6 hours, 24 hours) in the presence of unlabeled methionine. In case of mock-transfected cells, culture medium and cell extract prepared 6 hours after pulse labeling were analyzed to examine the specificity of anti-PLG antibody. Both culture media (A, 1 mL) and cell lysates (B, 700 μL) were prepared, and immunoprecipitation samples were analyzed under nonreducing conditions as described above. β-Galactosidase present in the cell lysates was used as an internal control for both transfection and immunoprecipitation efficiency.
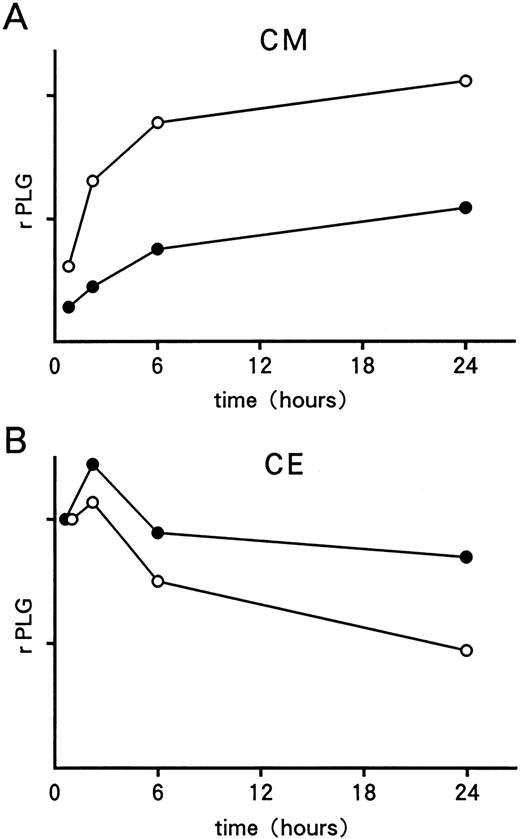
Pulse-chase analyses of 35S-labeled rPLG. Radiolabeled rWT (○) and rS572P (•) in culture medium (A) and cell extract (B) were quantitated by a laser densitometer. The quantity of β-gal immunoprecipitated at each time point was used to normalize both the transfection efficiency and the immunoprecipitation efficiency. Within each pulse-chase experiment, the number of counts is expressed in arbitrary units.
Pulse-chase analyses of 35S-labeled rPLG. Radiolabeled rWT (○) and rS572P (•) in culture medium (A) and cell extract (B) were quantitated by a laser densitometer. The quantity of β-gal immunoprecipitated at each time point was used to normalize both the transfection efficiency and the immunoprecipitation efficiency. Within each pulse-chase experiment, the number of counts is expressed in arbitrary units.
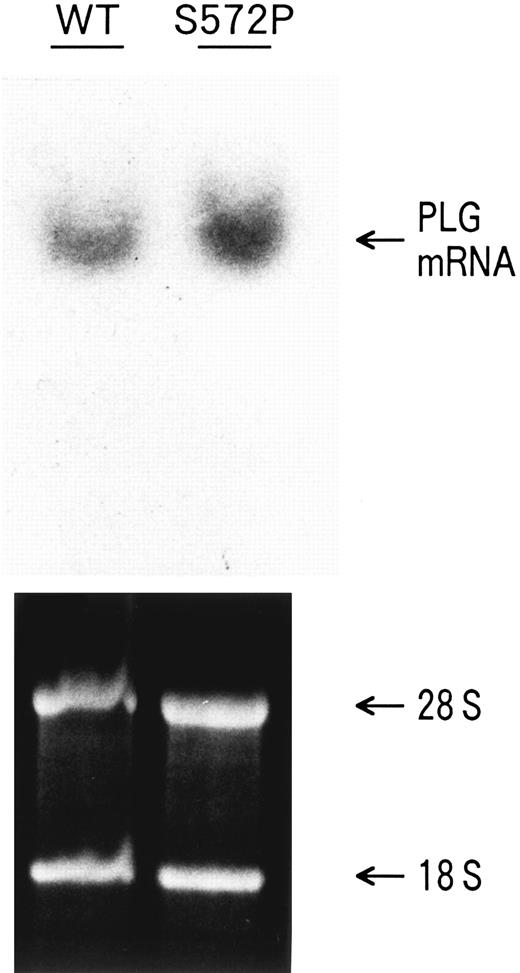
Northern blot analysis of transfected COS-1 cells. COS-1 cells were transiently transfected with pTH3/WT (WT) or pTH3/S572P (S572P) plasmid. Equal amounts of total RNA isolated from the transfected COS-1 cells were loaded in each lane, as shown by ethidium bromide staining (lower panel). The PLG mRNA was hybridized with a 32P-labeled full-length PLG cDNA probe (upper panel).
Northern blot analysis of transfected COS-1 cells. COS-1 cells were transiently transfected with pTH3/WT (WT) or pTH3/S572P (S572P) plasmid. Equal amounts of total RNA isolated from the transfected COS-1 cells were loaded in each lane, as shown by ethidium bromide staining (lower panel). The PLG mRNA was hybridized with a 32P-labeled full-length PLG cDNA probe (upper panel).
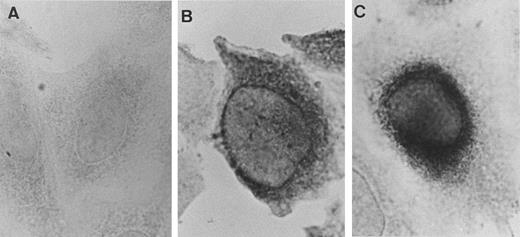
Immunolocalization of rPLG in stable transfectants. Untransfected CHO-K1 cells (A) and stably transfected CHO-K1 cells expressing rR561S (B) or rR561S + S572P (C) were fixed, permeabilized, and reacted with rabbit polyclonal anti-human PLG antibody. The cells then were incubated with biotinylated anti-rabbit IgG and immunostained using an ABC kit.
Immunolocalization of rPLG in stable transfectants. Untransfected CHO-K1 cells (A) and stably transfected CHO-K1 cells expressing rR561S (B) or rR561S + S572P (C) were fixed, permeabilized, and reacted with rabbit polyclonal anti-human PLG antibody. The cells then were incubated with biotinylated anti-rabbit IgG and immunostained using an ABC kit.
Because the glycoproteins residing in the endoplasmic reticulum contain high mannose carbohydrates on Asn residues, molecules in the culture medium derived from cell lysis should be cleaved by endo H treatment and produce a shift in migration toward a smaller apparent molecular weight on SDS-PAGE analysis (Fig 1B). For both rWT and rS572P, no shifts in migration occurred after enzymatic treatment, thereby indicating that these recombinant molecules contain complex-type oligosaccharide structures, and could be secreted from the transfected cells. When intracellular PLG purified from HepG2 cells was treated with endo H, the 93-kD band disappeared, indicating that the endo H we used was enzymatically active (Fig 1B). In addition, activation-resistant plasmids (pTH3/R561S, pTH3/R561S + S572P) were constructed and expressed stably in CHO-K1 cells. SDS-PAGE analysis showed that rR561S + S572P was detected only in reduced amounts in the culture medium, compared to rR561S, consistent with the result obtained in the transient expression experiments (Fig 2). When the cotransfection of pTH3/WT or pTH3/R561S and pSV–β-galactosidase plasmids into COS-1 cells was performed, no difference in the rate of secretion between rWT and rR561S was observed (Fig 3). Furthermore, a band of β-galactosidase was detected in the culture medium of COS-1 cells transfected with pTH3/WT, although no β-galactosidase band was observed in the culture medium of cells transfected with pTH3/R561S (Fig 3). Taken together, these results definitely show that rS572P, containing the same mutation as the patients' mutant PLG, is secreted only in reduced amounts from transfected COS-1 cells.
It should be noted that, although we did not measure the enzymatic activities of rWT and rS572P, rWT and rS572P appear to be functionally active in terms of plasmin activity. This is because of indirect evidence that, in both cases, independent colonies picked for establishing stable transfectants in CHO-K1 and CV-1 cells underwent cell lysis over the 5th to 10th days after splitting the transfectants. This resulted in a failure to establish stable transfectants in both type of cells (data not shown). However, as predicted, stable transfectants producing rR561S and rR561S + S572P were established successfully in CHO-K1 cells.
Pulse-chase and Northern blot analyses of wild-type and mutant plasminogens.To examine further whether the Ser572 to Pro572 substitution actually impairs the secretion of mutant molecules, a pulse-chase labeling study was performed. Recombinant WT molecules were detected in the culture medium from cells transfected with the pTH3/WT plasmid, and the intensity of the bands increased gradually over the chase time, up to 24 hours after pulse labeling (Figs 4A and 5A). However, only small amounts of rS572P were secreted into the culture medium during the 24-hour chase period (Figs 4A and 5A). At each time point, both the transfection efficiency and the immunoprecipitation efficiency of rPLG were monitored by the amount of immunoprecipitated β-galactosidase. Analysis of the cell lysates showed that rS572P accumulated inside cells transfected with the pTH3/S572P plasmid, even 24 hours after pulse-labeling (Figs 4B and 5B). On the other hand, intracellular rWT gradually decreased over the chase period (Figs 4B and 5B). Only the bands for β-galactosidase were detected in culture medium and cell extract, when the mock-transfected cells were immunoprecipitated with both anti-PLG antibody and anti–β-galactosidase antibody (Fig 4). In addition, when the cell lysates were immunoprecipitated without anti–β-galactosidase antibody, the 116-kD band was no longer observed, indicating that the bands were specific for β-galactosidase (data not shown). The results of the pulse-chase experiments, and those shown in Fig 1B, indicate that most of the rS572P molecules remained within the COS-1 cells, compared with the rWT molecules.
Northern blot analysis also was performed to investigate whether the reduced secretion of rS572P was caused by a corresponding reduction in mRNA. rS572P mRNA was not transcribed at a reduced level, compared with rWT mRNA (Fig 6). Therefore, the reduced secretion of rS572P by COS-1 cells is not caused by a reduction in rS572P mRNA.
Immunolocalization of rPLG in stable transfectants.To localize the distribution of rPLG in stable transfectants, immunocytochemistry was performed. Untransfected CHO-K1 cells were negative for PLG antigens (Fig 7A). When stable transfectants expressing rR561S were immunostained, PLG antigens were detected and distributed throughout the whole cytoplasm (Fig 7B). Stable transfectants expressing rR561S + S572P stained mainly in the perinuclear area (Fig 7C). These results suggest that the most of rR561S + S572P molecules accumulate inside the cells and have delayed intracellular transport.
DISCUSSION
The objective of this study was to elucidate the molecular pathogenesis of type I congenital PLG deficiency caused by a Ser572 to Pro572 substitution in a family we had previously identified.13 The impaired secretion of mutant proteins has been shown by genetic engineering techniques to be the cause of disease in most, if not all, patients with congenital deficiencies of plasma proteins.21-24 Therefore, we used a mammalian expression strategy, and metabolic labeling followed by immunoprecipitation analysis. We first examined whether the recombinant wild-type (rWT) and mutant (Ser572 → Pro572 ) (rS572P) PLGs could be detected in the culture medium of COS-1 cells, because transient expression of human PLG has been successfully used previously in this type of cell.4 We showed that rS572P was secreted only in reduced amounts from the cells, compared with rWT, and confirmed that the rPLG detected in the culture medium would not be the consequence of cell lysis. We then verified, using pulse-chase labeling, Northern blot analyses, immunocytochemical study, that impaired secretion of rS572P is the molecular mechanism of type I congenital PLG deficiency in this family.
The experimental expression of native human PLG in mammalian cells has been achieved in COS-1, HeLa, 293, L929, C-127, and BHKts13 cell lines.4,6,8 However, only low levels of secretion of intact-sized rPLG have been previously reported. In addition, CHO-K1, CHO/DVKX, BHK570, and CV-1 cell lines, with which stable transfectants secreting plasma proteins have been established, have been transiently or stably transfected with an expression plasmid containing the full-length PLG cDNA, resulting in the intracellular activation of rPLG, and the auto-degradation of the cells.8 The molecular mechanisms of this phenomenon have been investigated, and it has been postulated that the intracellular activation of rPLG by endogenous PLG activators, such as u-PA, leads to the generation of plasmin activity.8 Thus, cells possessing endogenous PLG activator activity would be inappropriate for our experiments. Gonzalez-Gronow et al4 and Browne et al6 have successfully expressed rPLG with authentic structure and glycosylation in COSm6 and HeLa cells, respectively. Therefore, we chose COS-1 cells. In addition, COS-1 cells contain the SV40 T antigen, and the expression vector we selected has an SV40 origin sequence, resulting in an efficient expression of rPLG.
Transient cotransfection of pTH3/WT or pTH3/S572P and pSV-β-galactosidase plasmids into COS-1 cells followed by pulse-chase experiments showed faint bands of β-galactosidase in the culture media from both rWT and rS572P (Fig 4A). As β-galactosidase is located in the cytoplasm of cells, the small amounts of β-galactosidase detected in the culture media might represent protein released from dead cells. Furthermore, when activation-resistant (pTH3/R561S) and pSV–β-galactosidase plasmids were transiently cotransfected into COS-1 cells, metabolically labeled, and immunoprecipitated with anti–β-galactosidase antibody, no β-galactosidase band was detected (Fig 3). Therefore, it is conceivable that the COS-1 cells we used possess endogenous PLG activators in small amounts, by which both rWT and rS572P were activated, and consequently, a small number of transfected COS-1 cells might have been autodegraded by activated rPLG (<5%, estimated by dye-exclusion using trypan blue).
SDS-PAGE analysis of rWT and rS572P in culture media followed by autoradiography detected two 93- and 90-kD species (Fig 1A). This result is in contrast to that of Gonzalez-Gronow et al,4 in which only a single 93-kD species was secreted when COSm6 cells were transfected. Recently, Horrevoets et al10 have shown that two species of rPLGs were detected in the culture medium of CV-1 cells, a parent cell line of COS-1 cells, transfected with recombinant vaccinia virus. The cause of this discrepancy is unknown at present. However, differences in the type of cells transfected, in the expression vectors used, or in the transfection procedure used might influence the glycosylation of the recombinant proteins. The treatment of cells with synthetic agents, such as dimethylsulfoxide (DMSO), is known to alter cellular glycosyltransferase activity in differentiated human promyelocytic leukemia (HL-60) or murine erythroleukemia (MEL) cells,25 and DMSO was used when Gonzalez-Gronow et al transfected their expression plasmid into COSm6 cells.
The higher intracellular accumulation of rS572P (Fig 4B) and the perinuclear localization of rR561S + S572P (Fig 7C) show the retarded transport of mutant PLG inside the cells. It is thought that the degradation of improperly folded proteins occurs in the rough endoplasmic reticulum or the Golgi apparatus.26 Furthermore, it is known that improperly folded proteins are either rescued to a natural conformation, or proteolysed if the proteins are denatured beyond repair, by molecular chaperones.27 28 Therefore, the Ser572 to Pro572 substitution of PLG may disrupt the inherent conformation of the proteins. Consequently, the mutant molecules may not be able to escape from their molecular chaperones.
The issue of whether the mutant PLG containing the Ser572 to Pro572 substitution is actually secreted from the patients' hepatocytes is unclear, because we have not attempted to detect the molecules in the patients' plasma. According to our expression data, small amounts of the mutant PLG should be circulating in patients' vessels. If this is the case, the mutant PLG secreted from hepatocytes may be as functionally active as normal PLG, because stable transfectants could not be established in CHO-K1 and CV-1 cells, although no functional study of rS572P was performed. Alternatively, the mutant PLG may be cleared faster from the circulation and removed more selectively than normal PLG.4 Both functional analysis and binding experiments with human endothelial cells29 using purified rWT and rS572P are needed to be performed.
ACKNOWLEDGMENT
We thank Dr M. Forsgren of the University of Lund, Sweden, for providing the plasmid, pPLGKG. The secretarial assistance of S. Ohnaka is also acknowledged.
Supported in part by a grant to H.A. from the Uehara Memorial Foundation.
Address reprint requests to Hiroyuki Azuma, MD, First Department of Internal Medicine, School of Medicine, The University of Tokushima, Kuramoto-cho 3, Tokushima 770, Japan.

![Fig. 1. SDS-PAGE analysis of 35S-labeled rPLG immunoprecipitated from the culture media of transfected COS-1 cells. (A) COS-1 cells were transiently transfected with pTH3/WT (WT) or pTH3/S572P (S572P) plasmids. Cells were metabolically labeled with L-[35S] methionine, and the culture media (1.5 mL) was immunoprecipitated with goat polyclonal anti-human PLG (IgG fraction). Immunoprecipitation products were analyzed by 10% SDS-PAGE and autoradiography under nonreducing conditions. The migration of protein standards is indicated. (B) Intracellular PLG purified from HepG2 cells (CE) and protein A-Sepharose beads containing adsorbed rPLG (CM) were treated with (+) or without (−) endo H as described in Materials and Methods. Each sample was boiled without a reducing agent, centrifuged, and the supernatant was subjected to 10% SDS-PAGE as described above.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/1/10.1182_blood.v89.1.183/2/m_bl_0016f1.jpeg?Expires=1769301746&Signature=gZo3nEuufGBsbDJ2PrM9PO8KsGlkFSHx7WRdgbTJYqCKYn5AbmeL5PgpgVPH0nCny8VF~jCuYPOdzcpN-YHheKTKLXxmSHpi8H2J0Qnxh9pSq2arQboHhMidy2g4~I22h4iEsyBxtPbO8JVGJWZIn0GkfmE~uH-YEbMDxmnAkWYEzqNa0l8uLj2oKJJZ1nRSO4CsyWSkDqzE8yHHklaHzuKCEUdHwTIn-aJc9-FGqXg1J8PP-svJ4RAzmHaLKAQ4bNgHA9mkAcDzUPGACYUc2qkTFgHao9PSRVuBB8PAG~NrP9sdffxQvzUNjUVpe4XJc6xq5TD-jJsEmPHT2g8qvA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. SDS-PAGE analysis of stable transfectants secreting the activation-resistant rPLG. Stable transfectants secreting wild-type (R561S) and mutant (R561S + S572P) rPLGs were established in CHO-K1 cells and labeled with L-[35S] methionine at the time of 80% confluency. Immunoprecipitation products were electrophoresed under nonreducing conditions by 10% SDS-PAGE.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/1/10.1182_blood.v89.1.183/2/m_bl_0016f2.jpeg?Expires=1769301746&Signature=DqIyR2H7NtWFDxQxMggx30Kri8zNO7x9-nIjoBA3-KD5wUsv0CUb~MclUz-bOFTkGBUGbH5L2sV9gM-xaAUE5zB~foIb~Oen5rogyMLT1QNk8Xb6BYuajRJA4XpM3jgFMgCI-9vFThUx~VZk4dct1vdMiOpeAbOvOST6CH8r46dImDI9XaEU1gU982jev-TVqyC7ujPA5FfOOsv8nU1BQmFY9OcdPv0ycobwmiFnvwuNzkKKj4e~F3pMG8z0q-javwOnGKgfN8Za5zfeQjkcGn9~x2bcPBpFR7QHIc78FqhrJHY-F3CrsfHwOKhRDnD2VeiDkxy-ShlRreexUAJJIQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. SDS-PAGE analysis of 35S-labeled rPLG immunoprecipitated from the culture media of transfected COS-1 cells. COS-1 cells were transiently contransfected with pTH3/WT (WT) or pTH3/R561S (R561S) and pSV–β-galactosidase plasmids. The cells were metabolically labeled with L-[35S]-methionine, and the culture media (1.5 mL) was immunoprecipitated with goat polyclonal anti-human PLG (IgG fraction) and rabbit polyclonal anti–β-galactosidase (IgG fraction). The immunoprecipitation products were analyzed by 10% SDS-PAGE and autoradiography under nonreducing conditions. The migration of protein standards and β-galactosidase is indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/1/10.1182_blood.v89.1.183/2/m_bl_0016f3.jpeg?Expires=1769301746&Signature=KSFRTWW3CV2gHfctQ4W7Qk7gPY5C2vISQfFZ179kCNdBLcP~W85QoBhQYsH2bzwCZ43n5HFZgTEssddnl6zOHkhrO5B5rrCbXp1G2vW97wBP8Y1lE6XG0-kETNOnmU3BiZvdkQLC8LI6uB73lX~yhwrckRZ8rw0uUfdidQKuG6G-~~zORiOmr2pI6VVRJ021FhgfuAoG2o5d3PZiQ-dFkWbaExiR5MgdtXYOMXHumWlxy8WT2Huu1GP4aRJdtX7xb0vJkXnLtXVtGSBuWqA0T7GcCRKoBHr7nsuGcn0JA-RRbv52AKo-OygGaAQmKyAKfq9siEQJ0rjudRuaEO7Jbg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Pulse-chase experiments of 35S-labeled rPLG. COS-1 cells were transiently cotransfected with either pTH3/WT (WT), pTH3/S572P (S572P), or pcDNAneo (mock) and pSV–β-galactosidase plasmids. After pulse-labeling with L-[35S] methionine for 30 minutes, the COS-1 cells then were chased for the times indicated (1 hour, 2 hours, 6 hours, 24 hours) in the presence of unlabeled methionine. In case of mock-transfected cells, culture medium and cell extract prepared 6 hours after pulse labeling were analyzed to examine the specificity of anti-PLG antibody. Both culture media (A, 1 mL) and cell lysates (B, 700 μL) were prepared, and immunoprecipitation samples were analyzed under nonreducing conditions as described above. β-Galactosidase present in the cell lysates was used as an internal control for both transfection and immunoprecipitation efficiency.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/1/10.1182_blood.v89.1.183/2/m_bl_0016f4.jpeg?Expires=1769301746&Signature=lJh5DXGSwF-bNppL6qzzhPyRgu7S1jUvMflvAxijw0h7DasVwqOh3q1N0Lk68J0CehNAhcN55EkMFUnjo8ObL3rEY0zCRY~hqkHpXYHrmjj9IdPdxLXfIC98KTz-mVH7JIAG0hixg9W2yqjsSnUi3LYZJ8i0U8xlDf4Ti-YG8hYMH8J4icqRXreyYWgE2FeIFfaENQueSsO4P54tXENFXhE65UnGWvuNlfxaOkvP9wdWW6SLDXiBCQR9lMySDazBz3NhEqlfE4yiYYjoCmCrrYPAa5mzJlJdcM9wjV6-FjnJutFi~0DOWRHmcI85KldMh9BQPHiL6~sMR7P0LlvIeg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal