Abstract
The zinc-finger transcription factor GATA-2 plays a critical role in maintaining the pool of early hematopoietic cells. To define its specific functions in the proliferation, survival, and differentiation of hematopoietic cells, we analyzed the hematopoietic potential of GATA-2−/− cells in in vitro culture systems for proliferation and maintenance of uncommitted progenitors or differentiation of specific lineages. From a two-step in vitro differentiation assay of embryonic stem cells and in vitro culture of yolk sac cells, we demonstrate that GATA-2 is required for the expansion of multipotential hematopoietic progenitors and the formation of mast cells, but dispensable for the terminal differentiation of erythroid cells and macrophages. The rare GATA-2−/− multipotential progenitors that survive proliferate poorly and generate small colonies with extensive cell death, implying that GATA-2 may play a role in both the proliferation and survival of early hematopoietic cells. To explore possible mechanisms resulting in the hematopoietic defects of GATA-2−/− cells, we interbred mutant mouse strains to assess the effects of p53 loss on the behavior of GATA-2−/− hematopoietic cells. Analysis of GATA-2−/−/p53−/− compound-mutant embryos shows that the absence of p53 partially restores the number of total GATA-2−/− hematopoietic cells, and therefore suggests a potential link between GATA-2 and p53 pathways.
PRODUCTION OF BLOOD cells through the life of the individual is dependent on the development and maintenance of pluripotent hematopoietic stem cells (HSCs), which are required to self-renew and yet provide sufficient numbers of multipotential progenitors and committed precursors. HSCs are thought to be quiescent and replicate only occasionally. The progenitor pool, on the other hand, is highly dynamic. In response to environmental signals, largely provided by hematopoietic cytokines, progenitor cells proliferate, die, or undergo further differentiation.1-3 Signals transmitted through cytokine receptors ultimately influence the function of nuclear transcription factors or associated factors. Accordingly, cells execute programs of gene expression and express lineage-restricted markers.4 5 Transcription factors critical to the formation or maintenance of HSCs and early hematopoietic progenitors are of particular interest in dissecting the mechanisms of lineage selection and maturation.
Three members of the GATA family of transcription factors, GATA-1, GATA-2, and GATA-3, have emerged as important transcription factors in hematopoietic cells.6,7 These proteins recognize highly similar (or identical) GATA motifs in target gene promoters or enhancers and function as transcriptional activators in conventional reporter assays. GATA-1 and GATA-3 have been studied primarily in relation to lineage-specific transcription. GATA-1, the founding member of this family, is expressed at a high level in erythroid cells, mast cells, megakaryocytes, and eosinophils, and at a low level in multipotential progenitors.8,9 Erythroid precursors lacking GATA-1 fail to differentiate beyond the proerythroblast stage either in vitro10 or in vivo.11 GATA-3, which is expressed in T lymphocytes,12,13 is essential for T-lymphoid cell development.14 The related GATA family member GATA-2 is expressed in broad distribution among hematopoietic cells, with particularly prominent expression in early progenitors, as well as megakaryocytes and mast cell lineages.15-18
To show the in vivo requirement for GATA-2 in hematopoiesis, we previously used gene-targeting in mouse embryonic stem (ES) cells and generated mouse embryos lacking GATA-2.19 GATA-2−/− mice die at approximately embryonic day 10 to 11 (E10-E11) with severe anemia. By chimeric analysis in which GATA-2−/− ES cells were introduced into wild-type blastocysts, we also observed a profound defect in the production of cells of all lineages during definitive (or adult) hematopoiesis in the absence of GATA-2. Furthermore, study of in vitro differentiation of GATA-2 null ES cells revealed significant impairment, particularly in responses to c-kit ligand [KL] or stem cell factor [SCF]. Taken together, these findings point to a critical role for GATA-2 in early hematopoietic cells, possibly influencing the proliferation or maintenance of progenitors.
In the study described here, we used in vitro differentiation of ES cells and in vitro culture of yolk sac hematopoietic cells to establish more precisely the position in the hematopoietic hierarchy at which GATA-2 is required. Our findings extend previous inferences regarding the role of GATA-2 in the proliferation and/or survival of multipotential progenitors, and also show that GATA-2 is required for generation of mast cells, but is dispensable for maturation of erythroid cells and macrophages. To examine whether p53, a key regulator of both cell-cycle arrest and apoptosis, is involved in the mechanisms leading to poor proliferation and cell death of GATA-2−/− hematopoietic cells, we generated GATA2−/−/p53−/− compound-mutant embryos and investigated their hematopoietic potential. We demonstrate that the absence of p53 partially counteracts the hematopoietic defects of GATA-2−/− cells with respect to the number of total hematopoietic cells, a finding that suggests a potential link between the functions of GATA-2 and p53.
MATERIALS AND METHODS
Cells and culture conditions.GATA-2−/− mutant ES cells were generated from CCE ES cells and wild-type and mutant ES cells were cultured as previously described.19
In vitro hematopoietic differentiation of ES cells.Undifferentiated ES cells were first grown on gelatin-coated plates in the absence of feeder fibroblasts for at least three passages. ES cells collected from 30% to 50% confluent plates were washed with ES medium without leukemia inhibitory factor (or ESgro) and then used in in vitro hematopoietic differentiation analysis.19 For two-step in vitro differentiation, ES cells (1,000 to 2,000 per 3.5-cm dish) were first plated into methylcellulose medium containing KL and interleukin-11 (IL-11) to form embryoid bodies (EBs). Plating efficiencies for EB formation of wild-type and two independent GATA-2−/− ES clones on primary plates were similar. Disaggregated cells (105/3.5-cm dish) from day 5 to day 15 EBs were replated into media containing various combinations of growth factors.20 The hematopoietic colonies grown on secondary plates were examined.
Hematopoietic Colonies Generated in the Two-Step In Vitro Differentiation of Wild-Type ES Cells
| Growth Factors . | Ery . | EryMeg . | Meg . | Mast . | G . | GM . | M . | Mix/Blast . | Total . |
|---|---|---|---|---|---|---|---|---|---|
| KL, IL-3, IL-11, and Epo | 25 | 11 | 4 | 26 | 28 | 37 | 70 | 44 | 247 |
| KL and Epo | 11 | 0 | 0 | 0 | 0 | 3 | 15 | 2 | 31 |
| Growth Factors . | Ery . | EryMeg . | Meg . | Mast . | G . | GM . | M . | Mix/Blast . | Total . |
|---|---|---|---|---|---|---|---|---|---|
| KL, IL-3, IL-11, and Epo | 25 | 11 | 4 | 26 | 28 | 37 | 70 | 44 | 247 |
| KL and Epo | 11 | 0 | 0 | 0 | 0 | 3 | 15 | 2 | 31 |
Abbreviations: Ery, erythroid cells; Meg, megakaryocytes; Mast, mast cells; G, granulocytes; M, macrophages; Mix/Blast, mixed or blast cells.
GATA-2−/− inbred embryos and GATA-2/p53 compound-mutant embryos.Germline transmitting chimeras produced by injecting GATA-2+/− cells into C57/B6 blastocysts were mated with 129/Sv female mice to generate 129/Sv inbred GATA-2+/− mice. 129/Sv inbred GATA-2−/− embryos were generated by crossing the GATA-2+/− inbred mice. For generating GATA-2/p53 compound-mutant mice, GATA-2+/− mice19 were crossed with p53+/− mice21 to generate GATA-2+/−/p53+/− compound-heterozygous mice. Embryos from intercrossing GATA-2+/−/p53+/− male and female mice were analyzed at E9-E10.
Yolk sac colony assay and in vitro liquid culture.Hematopoietic cells from E9-E10 yolk sacs were collected as previously described.19 One third of the total yolk sac cells were plated into medium containing KL, IL-3, IL-11, and erythopoietin (Epo). Hematopoietic colonies were examined and counted after day 6. For in vitro liquid culture, yolk sac cells were cultured in liquid medium containing KL, IL-3, IL-11, and Epo. Hematopoietic cells from colony assay and in vitro liquid culture were cytocentrifuged onto slides and stained with May-Grünwald/Giemsa or Toluidine blue for morphologic examination.
Reverse-transcriptase–polymerase chain reaction (RT-PCR) analyses.RNAs were isolated from hematopoietic colonies with RNAzole (TEL-Test Inc, Friendswood, TX). The RT-PCR and primers used for detecting specific mRNAs were as described previously.10
RESULTS
Establishment of a two-step in vitro hematopoietic differentiation assay for hematopoietic multipotential progenitors.To examine the effect of GATA-2 loss on hematopoietic multipotential progenitors more directly, we used a two-step in vitro ES cell differentiation assay. Specifically, we disaggregated wild-type EBs, grown in medium containing KL and IL-11, at selected times and replated the cells in the presence of different combinations of growth factors including KL, IL-3, IL-6, IL-11, granulocyte-macrophage colony-stimulating factor, and Epo. Our aim was to identify experimental conditions that favored the expansion and maintenance of hematopoietic progenitors, rather than the appearance of single-lineage committed colonies. In Table 1, the formation of hematopoietic colonies is compared between two media conditions (KL, IL-3, IL-11, and Epo v KL plus Epo) for secondary plates. The number of colonies containing cells of single lineage reflects the number of committed precursors in the original EBs, and the number of mixed or blast colonies provides a measurement of the number of multipotential hematopoietic progenitors in the EBs. We found that the minimum cytokine combination needed for the growth of hematopoietic progenitors includes KL, IL-3, and IL-11. Addition of Epo permits production of mature erythroid cells. In agreement with a previous finding,20 KL plus Epo favors the formation of erythroid cells at the expense of uncommitted progenitors.
Mixed or blast cell colonies are greatly reduced in number and size from GATA-2−/− ES cells.We next examined the behavior of GATA-2−/− ES cells under in vitro differentiation conditions favoring the development and maintenance of multipotential progenitors. The total number of hematopoietic colonies derived from GATA-2−/− cells disaggregated from day 9 EBs was reduced by approximately fourfold (Table 2). However, of particular note, the most profound deficit is displayed in mixed or blast cell colonies (mix/blast colonies) derived from uncommitted progenitors, which are disproportionately underrepresented upon replating of GATA-2−/− cells (1.6% of total colonies). Among colonies derived from committed precursors, non-red/G colonies (mostly mast cell colonies) were also markedly reduced in proportion (3.2% of total colonies). Nonetheless, the proportion of erythroid and macrophage colonies is not significantly altered as compared with wild-type cells.
Two-Step In Vitro Differentiation of Wild-Type and GATA-2−/− ES Cells (colonies/105 cells in secondary plates)
| Genotype . | Eryp . | Eryd . | Mac . | Non-Red/G . | Mix/Blast . | Total . |
|---|---|---|---|---|---|---|
| Wild-type | ||||||
| No. | 106 ± 31 | 62 ± 14 | 220 ± 11 | 78 ± 14 | 80 ± 11 | 546 ± 3 |
| % | 19.4 | 11.4 | 40.3 | 14.3 | 14.6 | 100 |
| GATA-2−/− | ||||||
| No. | 20 ± 3 | 15 ± 1 | 86 ± 6 | 4 ± 1 | 2 ± 1 | 127 ± 4 |
| % | 15.7 | 11.8 | 67.7 | 3.2 | 1.6 | 100 |
| Genotype . | Eryp . | Eryd . | Mac . | Non-Red/G . | Mix/Blast . | Total . |
|---|---|---|---|---|---|---|
| Wild-type | ||||||
| No. | 106 ± 31 | 62 ± 14 | 220 ± 11 | 78 ± 14 | 80 ± 11 | 546 ± 3 |
| % | 19.4 | 11.4 | 40.3 | 14.3 | 14.6 | 100 |
| GATA-2−/− | ||||||
| No. | 20 ± 3 | 15 ± 1 | 86 ± 6 | 4 ± 1 | 2 ± 1 | 127 ± 4 |
| % | 15.7 | 11.8 | 67.7 | 3.2 | 1.6 | 100 |
Results (mean ± SD) are from 2 experiments with 2 independent GATA-2−/− ES clones.
Abbreviations: Eryp, primitive erythroid colonies; Eryd, definitive erythroid colonies; Mac, macrophage colonies; Non-Red/G, non-red colonies with granulated cells (mostly mast cell colonies at this stage); Mix/Blast, mixed or blast cell colonies.
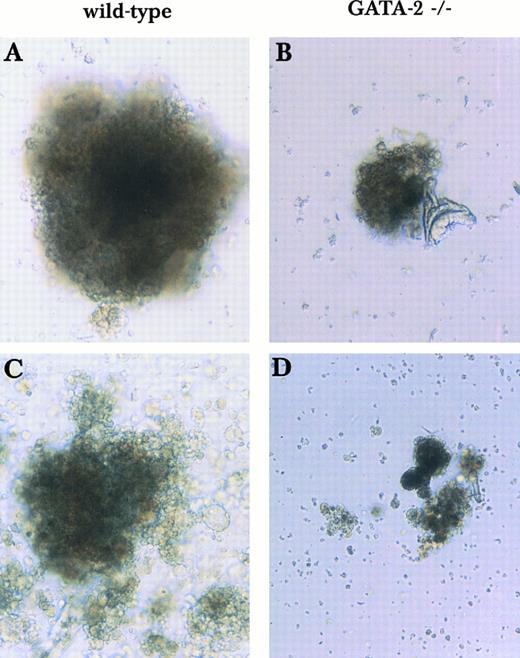
In addition to the disproportionate loss of mix/blast colonies, the few colonies observed are small and appear to contain dying cells (Figs 1 and 2). Similar results were observed upon replating of GATA-2−/− EBs at earlier times (days 5 and 7; data not shown). However, only macrophages and a few erythroid colonies appeared in secondary plates when cells from day 12 GATA-2−/− EBs were replated (data not shown). These findings from in vitro culture demonstrate that the defect in GATA-2−/− cells is manifested at early developmental stages in multipotential progenitors rather than in committed erythroid or myeloid precursors.
Mixed or blast-type hematopoietic colonies derived from wild-type and GATA-2−/− ES cells in the 2-step in vitro differentiation assay. Day 9 EBs were disaggregated and plated into secondary plates with medium containing KL, IL-3, IL-11, and Epo. Mixed and blast-type hematopoietic colonies of wild-type origin (A and C) or GATA-2−/− (B and D) origin were examined on day 9 (A and B) and day 13 (C and D). This finding was reproduced in 2 experiments using 2 independent GATA-2−/− clones.
Mixed or blast-type hematopoietic colonies derived from wild-type and GATA-2−/− ES cells in the 2-step in vitro differentiation assay. Day 9 EBs were disaggregated and plated into secondary plates with medium containing KL, IL-3, IL-11, and Epo. Mixed and blast-type hematopoietic colonies of wild-type origin (A and C) or GATA-2−/− (B and D) origin were examined on day 9 (A and B) and day 13 (C and D). This finding was reproduced in 2 experiments using 2 independent GATA-2−/− clones.
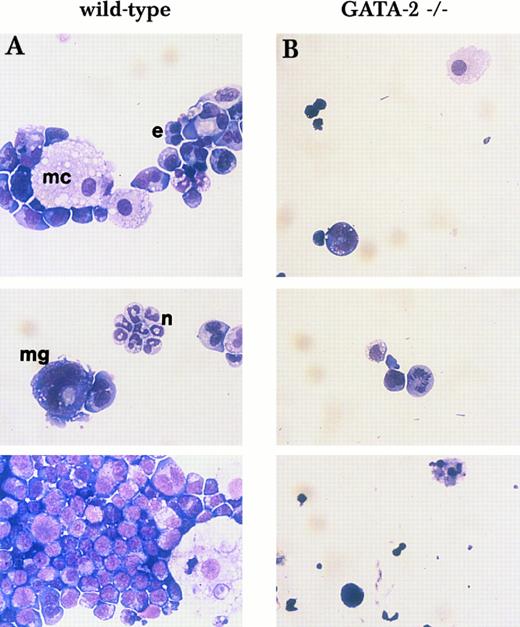
Morphology of mixed and blast-type colonies shown in Fig 1C and D. Cells from single mix/blast colonies shown in Fig 1C (A, wild-type) and D (B, GATA-2−/−) were examined by May-Giemsa staining. e, erythroid cell; mc, macrophage; n, neutrophil; mg, megakaryocyte.
Morphology of mixed and blast-type colonies shown in Fig 1C and D. Cells from single mix/blast colonies shown in Fig 1C (A, wild-type) and D (B, GATA-2−/−) were examined by May-Giemsa staining. e, erythroid cell; mc, macrophage; n, neutrophil; mg, megakaryocyte.
GATA-2 is dispensable for terminal differentiation of erythroid cells and macrophages.In contrast to the abnormal appearance of GATA-2−/− mix/blast colonies, erythroid and macrophage colonies of GATA-2−/− origin are similar in morphology to those of wild-type origin (Fig 3). To confirm the visual impression of normal erythroid maturation in the absence of GATA-2, RT-PCR analysis was used to estimate the expression of β-globin and GATA-1 in GATA-2−/− derived erythroid colonies. The results in Fig 4, demonstrate that RNA transcript levels for GATA-1 and globin (as compared with either actin or hypoxanthine phosphoribosyltransferase internal control) are normal in GATA-2−/− erythroid colonies. Consistent with this finding, the level of ζ and α-globin mRNAs per cell is also normal in GATA-2−/− yolk sac erythroid cells at E10 (data not shown). Thus, erythroid and macrophage precursors develop normally in the absence of GATA-2.
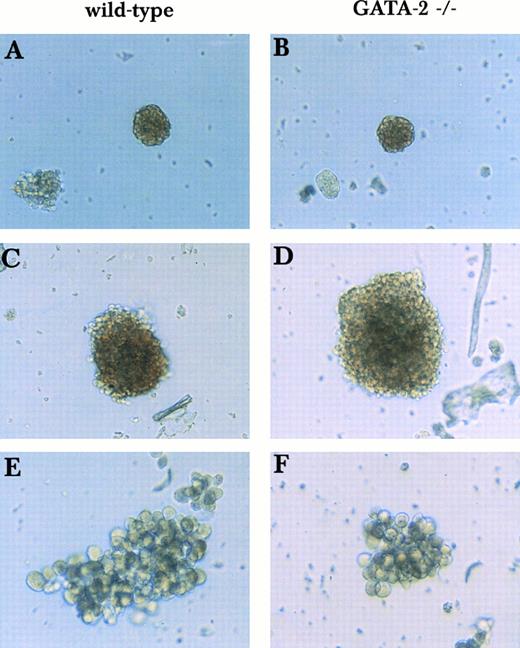
Primitive erythroid, definitive erythroid, and macrophage colonies derived from wild-type and GATA-2−/− ES cells in the 2-step in vitro differentiation. Day 9 EBS were disaggregated and plated into secondary plates with medium containing KL, IL-3, IL-11, and Epo. Colonies with cells of single lineages of wild-type origin (A, C, and E) and GATA-2−/− origin (B, D, and F ) were compared. Primitive erythroid colonies (A and B), definitive erythroid colonies (C and D), and macrophage colonies (E and F ) were examined on days 5, 6, and 13, respectively.
Primitive erythroid, definitive erythroid, and macrophage colonies derived from wild-type and GATA-2−/− ES cells in the 2-step in vitro differentiation. Day 9 EBS were disaggregated and plated into secondary plates with medium containing KL, IL-3, IL-11, and Epo. Colonies with cells of single lineages of wild-type origin (A, C, and E) and GATA-2−/− origin (B, D, and F ) were compared. Primitive erythroid colonies (A and B), definitive erythroid colonies (C and D), and macrophage colonies (E and F ) were examined on days 5, 6, and 13, respectively.
Comparison of β-globin and GATA-1 expression levels in wild-type versus GATA-2−/− definitive erythroid cells. Levels of β-globin and GATA-1 transcripts in definitive erythroid cells of wild-type colonies (1 and 2) and GATA-2−/− colonies (3 and 4) were compared by RT-PCR analysis with 2 different PCR cycles. Lanes 1 and 3, 24 PCR cycles for β-globin and 32 PCR cycles for GATA-1; lanes 2 and 4, 26 PCR cycles for β-globin and 34 PCR cycles for GATA-1. Actin and HPRT were used as internal controls for β-globin and GATA-1 expression, respectively.
Comparison of β-globin and GATA-1 expression levels in wild-type versus GATA-2−/− definitive erythroid cells. Levels of β-globin and GATA-1 transcripts in definitive erythroid cells of wild-type colonies (1 and 2) and GATA-2−/− colonies (3 and 4) were compared by RT-PCR analysis with 2 different PCR cycles. Lanes 1 and 3, 24 PCR cycles for β-globin and 32 PCR cycles for GATA-1; lanes 2 and 4, 26 PCR cycles for β-globin and 34 PCR cycles for GATA-1. Actin and HPRT were used as internal controls for β-globin and GATA-1 expression, respectively.
Hematopoietic cells from inbred GATA-2−/− yolk sac at E9.5-E10 contain few multipotential progenitors with poor expansion capacity and fail to generate mast cells upon in vitro culture.To evaluate whether the in vitro findings reflect the in vivo situation, we next examined the hematopoietic progenitor pool and mast cell formation of GATA-2−/− yolk sac hematopoietic cells in an inbred background. 129/Sv inbred GATA-2−/− embryos die at a similar stage as the 129/C57B6 outbred GATA-2−/− embryos studied previously.19 To examine the multipotential progenitor pool, cell suspensions were made from E9.5-E10 GATA-2−/− yolk sacs and plated in methylcellulose medium containing KL, IL-3, IL-11, and Epo. In agreement with our in vitro differentiation results already shown and colony assays performed previously on the 129/C57B6 outbred strain,19 the total number of colonies is reduced approximately 10-fold in GATA-2−/− relative to wild-type yolk sac, with the most profound deficit evident in mix/blast colonies (Table 3). Moreover, the few GATA-2−/− progenitors that are detected give rise to extremely small colonies that contain dying cells (Fig 5). Together, these results indicate that the loss of GATA-2 adversely affects both the number and the quality of multipotential hematopoietic progenitors in vivo.
Number of Colonies Generated From Wild-Type and GATA-2−/− Yolk Sacs
| Genotype . | Red . | Non-Red . | Mix/Blast . | Total . |
|---|---|---|---|---|
| Wild-type | ||||
| No. | 168 ± 110 | 1,104 ± 25 | 318 ± 102 | 1,590 ± 229 |
| % | 11 | 69 | 20 | 100 |
| GATA-2−/− | ||||
| No. | 9 ± 5 | 138 ± 13 | 8 ± 6 | 155 ± 24 |
| % | 6 | 89 | 5 | 100 |
| Genotype . | Red . | Non-Red . | Mix/Blast . | Total . |
|---|---|---|---|---|
| Wild-type | ||||
| No. | 168 ± 110 | 1,104 ± 25 | 318 ± 102 | 1,590 ± 229 |
| % | 11 | 69 | 20 | 100 |
| GATA-2−/− | ||||
| No. | 9 ± 5 | 138 ± 13 | 8 ± 6 | 155 ± 24 |
| % | 6 | 89 | 5 | 100 |
Results (mean ± SD) are from 2 experiments with 2 wild-type and 4 GATA-2−/− embryos.
Abbreviations: Red, red colonies; Non-Red, non-red colonies; Mix/Blast, mixed or blast cell colonies.
Mixed and blast-type hematopoietic colonies derived from wild-type and GATA-2−/− yolk sac cells at E9.5. Cells collected from the yolk sac at E9.5 were subjected to progenitor assay using medium containing KL, IL-11, IL-3, and Epo. Mixed and blast-type colonies from wild-type (A and C) and GATA-2−/− (B and D) cells were examined on day 9 in culture. This finding was reproduced in 4 GATA-2−/− embryos analyzed from 2 litters.
Mixed and blast-type hematopoietic colonies derived from wild-type and GATA-2−/− yolk sac cells at E9.5. Cells collected from the yolk sac at E9.5 were subjected to progenitor assay using medium containing KL, IL-11, IL-3, and Epo. Mixed and blast-type colonies from wild-type (A and C) and GATA-2−/− (B and D) cells were examined on day 9 in culture. This finding was reproduced in 4 GATA-2−/− embryos analyzed from 2 litters.
To examine mast cell formation, we performed long-term yolk sac culture in media containing KL and IL-3. After in vitro culture for longer than 3 weeks, wild-type yolk sac cells give rise to mainly macrophages and mast cells (Fig 6A). However, GATA-2−/− yolk sac cells generate only macrophages (Fig 6B). This observation is consistent with the results of the in vitro differentiation assay and indicates a critical role for GATA-2 in mast cell formation.
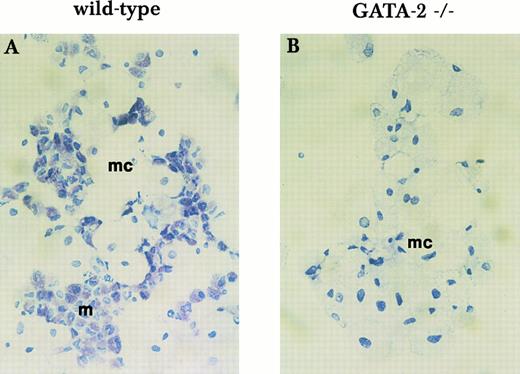
Mast cell formation in liquid culture of yolk sac cells. Yolk sac cells collected at E9.5 were subjected to long-term in vitro liquid culture with KL and IL-3 for mast cell formation. Mast cell formation from wild-type cells (A) and GATA-2−/− cells (B) was examined by Toluidine blue staining after 3 weeks in culture. mc, macrophage; m, mast cell. This finding was reproduced in 3 independent experiments.
Mast cell formation in liquid culture of yolk sac cells. Yolk sac cells collected at E9.5 were subjected to long-term in vitro liquid culture with KL and IL-3 for mast cell formation. Mast cell formation from wild-type cells (A) and GATA-2−/− cells (B) was examined by Toluidine blue staining after 3 weeks in culture. mc, macrophage; m, mast cell. This finding was reproduced in 3 independent experiments.
The hematopoietic defects of GATA-2−/− cells are partially reversed by the absence of p53.To investigate a potential role of p53 in mediating the phenotypes of GATA-2−/− hematopoietic cells, we generated and characterized GATA-2−/−/p53−/− compound-mutant embryos. Among 53 2-week-old offsprings from intercrossing of GATA-2+/−/p53+/− male and female mice, no GATA-2−/− mice (with or without p53 mutation) were found. Thus, GATA-2−/− mice are embryonic-lethal, independent of the presence or absence of p53 (Table 4). For embryos analyzed at E9-E9.5, all potential genotypes were observed at approximately the expected frequency (Table 4). At E9-E9.5, GATA-2−/−/p53+/+, GATA-2−/−/p53+/−, and GATA-2−/−/p53−/− embryos were still alive with detectable circulating blood (data not shown).
Genotype Analysis of E9-E9.5 Embryos and 2-Week-Old Mice Generated From GATA-2+/−/p53+/− Compound Heterozygous Mice
| G2 . | p53 . | No. . | Observed (%) . | Expected (%) . |
|---|---|---|---|---|
| E9-E9.5 embryos | ||||
| +/+ | +/+ | 2 | 5.3 | 6.25 |
| +/+ | +/− | 4 | 10.5 | 12.5 |
| +/+ | −/− | 1 | 2.6 | 6.25 |
| +/− | +/+ | 6 | 15.8 | 12.5 |
| +/− | +/− | 13 | 34.2 | 25 |
| +/− | −/− | 6 | 15.8 | 12.5 |
| −/− | +/+ | 1 | 2.6 | 6.25 |
| −/− | +/− | 3 | 7.9 | 12.5 |
| −/− | −/− | 2 | 5.3 | 6.25 |
| Total | 38 | 100 | 100 | |
| 2-Week-old mice | ||||
| +/+ | +/+ | 10 | 18.9 | 6.25 |
| +/+ | +/− | 9 | 17.0 | 12.5 |
| +/+ | −/− | 2 | 3.8 | 6.25 |
| +/− | +/+ | 12 | 22.6 | 12.5 |
| +/− | +/− | 17 | 32.1 | 25 |
| +/− | −/− | 3 | 5.6 | 12.5 |
| −/− | +/+ | 0 | 0 | 6.25 |
| −/− | +/− | 0 | 0 | 12.5 |
| −/− | −/− | 0 | 0 | 6.25 |
| Total | 53 | 100 | 100 |
| G2 . | p53 . | No. . | Observed (%) . | Expected (%) . |
|---|---|---|---|---|
| E9-E9.5 embryos | ||||
| +/+ | +/+ | 2 | 5.3 | 6.25 |
| +/+ | +/− | 4 | 10.5 | 12.5 |
| +/+ | −/− | 1 | 2.6 | 6.25 |
| +/− | +/+ | 6 | 15.8 | 12.5 |
| +/− | +/− | 13 | 34.2 | 25 |
| +/− | −/− | 6 | 15.8 | 12.5 |
| −/− | +/+ | 1 | 2.6 | 6.25 |
| −/− | +/− | 3 | 7.9 | 12.5 |
| −/− | −/− | 2 | 5.3 | 6.25 |
| Total | 38 | 100 | 100 | |
| 2-Week-old mice | ||||
| +/+ | +/+ | 10 | 18.9 | 6.25 |
| +/+ | +/− | 9 | 17.0 | 12.5 |
| +/+ | −/− | 2 | 3.8 | 6.25 |
| +/− | +/+ | 12 | 22.6 | 12.5 |
| +/− | +/− | 17 | 32.1 | 25 |
| +/− | −/− | 3 | 5.6 | 12.5 |
| −/− | +/+ | 0 | 0 | 6.25 |
| −/− | +/− | 0 | 0 | 12.5 |
| −/− | −/− | 0 | 0 | 6.25 |
| Total | 53 | 100 | 100 |
Since our results reveal profound hematopoietic deficits of GATA-2−/− cells in terms of multipotential progenitors and mast cell lineage, we performed in vitro hematopoietic culture of yolk sac cells from GATA-2−/−/p53−/− compound mutants to examine the effect of p53 loss on the hematopoietic defects of GATA-2−/− cells. In the liquid culture system with KL, IL-3, IL-11, and Epo, wild-type cells give rise to various types of hematopoietic cells, including definitive erythroid cells, macrophages, mast cells, megakaryocytes, and neutrophils (Fig 7). However, GATA-2−/−/p53+/+ cells generate only macrophages and erythroid cells (Fig 7) with a total cell number of approximately 5% of their wild-type counterpart (Table 5). In a GATA-2+/+ or GATA-2+/− background, the absence of p53 has no significant effect on cell number (Table 5) or cell composition (Fig 7) of hematopoietic populations in culture. However, loss of p53 leads to a significant enhancement (∼fourfold) in the number of total hematopoietic cells derived from GATA-2−/− yolk sac cells in liquid culture (Table 5). Furthermore, GATA-2−/−/p53−/− cells give rise to definitive erythroid cells, macrophages, megakaryocytes, and neutrophils, but no mast cells (Fig 7). Colony assays were also performed to further characterize the hematopoietic progenitors and precursors in GATA2−/−/p53−/− yolk sac cells. The absence of p53 in the GATA-2−/− background results in an increase of total colony number of approximately 2.5-fold (Table 6), an observation consistent with the increased cell number seen upon liquid culture (Table 5). Nonetheless, the GATA-2−/−/p53−/− yolk sac cells still fail to generate large mixed or blast-type hematopoietic colonies (data not shown), suggesting that p53 might be more critical for survival than for proliferation of GATA-2−/− hematopoietic cells.
Hematopoietic potential of yolk sac cells with various genotypes. Yolk sac cells collected at E9.5 were subjected to in vitro liquid culture with KL, IL-11, IL-3, and Epo. Hematopoietic cells grown after 10 days in culture were cytocentrifuged onto slides and stained with May-Grünwald/Giemsa. The arrow points to mast cells.
Hematopoietic potential of yolk sac cells with various genotypes. Yolk sac cells collected at E9.5 were subjected to in vitro liquid culture with KL, IL-11, IL-3, and Epo. Hematopoietic cells grown after 10 days in culture were cytocentrifuged onto slides and stained with May-Grünwald/Giemsa. The arrow points to mast cells.
Total Hematopoietic Cells in 10-Day In Vitro Liquid Culture of E9-E9.5 Yolk Sac Cells
| G2 . | p53 . | Cell No. (per yolk sac) . |
|---|---|---|
| Litter 1 (E9) | ||
| +/− | +/+ | 7.8 × 105 |
| +/− | +/− | 9.0 × 105 |
| +/− | −/− | 7.8 × 105 |
| −/− | +/+ | 1.2 × 105 |
| −/− | +/− | 8.4 × 104 |
| −/− | −/− | 4.6 × 105 |
| Litter 2 (E9.5) | ||
| +/+ | +/+ | 5.0 × 106 |
| +/+ | +/− | 4.7 × 106 |
| +/− | +/− | 4.1 × 106 |
| +/− | −/− | 4.5 × 106 |
| −/− | +/− | 2.1 × 105 |
| −/− | −/− | 8.0 × 105 |
| G2 . | p53 . | Cell No. (per yolk sac) . |
|---|---|---|
| Litter 1 (E9) | ||
| +/− | +/+ | 7.8 × 105 |
| +/− | +/− | 9.0 × 105 |
| +/− | −/− | 7.8 × 105 |
| −/− | +/+ | 1.2 × 105 |
| −/− | +/− | 8.4 × 104 |
| −/− | −/− | 4.6 × 105 |
| Litter 2 (E9.5) | ||
| +/+ | +/+ | 5.0 × 106 |
| +/+ | +/− | 4.7 × 106 |
| +/− | +/− | 4.1 × 106 |
| +/− | −/− | 4.5 × 106 |
| −/− | +/− | 2.1 × 105 |
| −/− | −/− | 8.0 × 105 |
Total Hematopoietic Colonies in 8-Day Methycellulose Culture of E9-E9.5 Yolk Sac Cells
| G2 . | p53 . | Total Colony No. Per Yolk Sac . |
|---|---|---|
| Litter 1 (E9) | ||
| −/− | +/− | 75 |
| −/− | −/− | 195 |
| Litter 2 (E9.5) | ||
| −/− | +/− | 62 |
| −/− | −/− | 158 |
| G2 . | p53 . | Total Colony No. Per Yolk Sac . |
|---|---|---|
| Litter 1 (E9) | ||
| −/− | +/− | 75 |
| −/− | −/− | 195 |
| Litter 2 (E9.5) | ||
| −/− | +/− | 62 |
| −/− | −/− | 158 |
DISCUSSION
In this study, we focused our attention on the progenitor pool and specifically distinguished the effects of GATA-2 loss on multipotential progenitors versus those on maturation of specific lineages. Multipotential hematopoietic progenitors obtained from GATA-2−/− yolk sac and GATA-2−/− in vitro differentiating EBs are greatly reduced in number relative to wild-type controls. Moreover, GATA-2−/− mixed or blast cell colonies derived from uncommitted progenitors were qualitatively different, as exemplified by their small size and the presence of dying cells. The impact of GATA-2 loss on hematopoietic progenitors is likely to be complex and includes disturbances of cellular proliferation and survival, as well as possible effects on the life span of progenitors or their capacity to maintain an uncommitted phenotype.
Mast cells are not present in long-term cultures of GATA-2−/− yolk sac cells. Two points suggest that GATA-2 may play a particularly important role in the expansion of mast cell precursors. First, the number of GATA-2−/− mast cell precursors is decreased to a far greater extent than the number of erythroid or macrophage precursors. Second, GATA-2 expression is largely restricted to proliferating mast cells.22 Nonetheless, GATA-2 may also function in the differentiation of mast cells, because no mature mast cells were obtained from GATA-2−/− cells in long-term yolk sac culture. These findings are consistent with the coexpression of GATA-1 and GATA-2 in mast cells and the formation of mast cells in the absence of GATA-1,23 and also suggest a specific function for GATA-2 in the production of mast cells.
Our experiments also allow us to conclude that loss of GATA-2 per se does not appear to affect maturation along erythroid or macrophage lineages. The observed decrease in the number of committed precursors for these lineages in GATA-2−/− EBs is best interpreted as an indirect consequence of deficits in the uncommitted, multipotential progenitors. After commitment to either lineage takes place, terminal differentiation of erythroid cells and macrophages can proceed in the absence of GATA-2. Comparison of specific mRNAs in wild-type and GATA-2−/− erythroid colonies is consistent with this view and shows that GATA-2 is not required for transcription of GATA-1 or globin genes in committed erythroid cells. Moreover, GATA-2 seems dispensable in lymphoid development and terminal differentiation of neutrophils, as indicated by the presence of occasional GATA-2−/− mature T cells in chimeras made with RAG-2–deficient blastocyts19 and the formation of some neutrophils upon in vitro culture of GATA-2−/− yolk sac cells (data not shown).
The status of HSCs in GATA-2−/− EBs and embryos is difficult to assess because of the low number of such cells even in wild-type embryos. The fact that mature blood cells are detectable both in vivo and in vitro with GATA-2−/− background clearly indicates that stem cells are formed, but it is still unclear whether GATA-2−/− stem cells are able to self-renew and survive normally. Alternative approaches such as conditional gene targeting might clarify the specific function of GATA-2 in HSCs.
The few GATA-2−/− multipotential hematopoietic progenitors in early embryos and EBs have low proliferative capacity, and cells derived from them are prone to die during in vitro culture. These findings strongly suggest that GATA-2 functions in the proliferation and survival of early hematopoietic cells. Among the factors controlling cell proliferation and survival, the tumor suppresser p53 acts as a negative regulator to prevent the expansion of specific cell populations by triggering cell-cycle arrest and apoptosis.24-27 p53 is frequently mutated in a variety of human cancers, including leukemia.28,29 A large proportion of mice lacking p53 are viable with high tumor susceptibility.21,30,31 However, some p53-null mouse embryos die in uteri with abnormal neural tube development.32 In hematopoiesis, apoptosis appears to play a role in controlling the number of early hematopoietic cells,33 and p53 has been shown to be involved in the induction of apoptosis in the hematopoietic cell line M1.34 In addition, the fact that p53-null hematopoietic cells can be immortalized by expressing raf or myc-raf oncogenes without additional genetic alternation implies a crucial role of p53 as a barrier for active proliferation of hematopoietic cells.35 The difference in hematopoietic potential between GATA-2−/−/p53+/+ and GATA-2−/−/p53−/− cells implies that wild-type p53 might play a role in triggering senescence and/or cell death of GATA-2−/− hematopoietic cells. Additionally, with an increase in the number of total hematopoietic cells, we were able to detect numerous macrophages, erythroid cells, megakaryocytes, and neutropils derived from GATA-2−/−/p53−/− cells, demonstrating again that GATA-2 is not strictly required for development of these cells. The absence of mast cells in the GATA-2−/−/p53−/− hematopoietic population further confirms a crucial role of GATA-2 in mast cell formation.
The involvement of GATA-2 in expanding early hematopoietic cells contrasts with the requirement for the related factor GATA-1 in terminal erythroid maturation.10,36 The reciprocal expression of these factors during erythroid development parallels the shift from proliferation to terminal maturation. Although the apparent roles of these factors differ, GATA-1 and GATA-2 are coexpressed during much of erythroid and hematopoietic development. The maturation of erythroid precursors to the proerythroblast stage, coupled with the failure to repress GATA-2 levels in the absence of GATA-1,10 23 suggests that GATA-2 can partially fulfill some of the transcriptional roles of GATA-1. Accordingly, it is possible that GATA-1 expressed at low levels in early hematopoietic cells may compensate partially for the absence of GATA-2, which results in few surviving multipotential progenitors at early developmental stages. As such, early hematopoietic cells might be even more severely impaired, or perhaps unable to form, if all GATA factors were absent. Creation of ES cells or embryos lacking both GATA-1 and GATA-2 should permit a direct test of these possibilities.
Address reprint requests to Stuart H. Orkin, MD, Division of Hematology/Oncology, Children's Hospital, 300 Longwood Ave, Boston, MA 02115.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal