Abstract
CD8+ T-cell clones were generated from peripheral blood mononuclear cells (PBMC) of three human immunodeficiency virus (HIV)-seronegative individuals and six HIV-seropositive individuals and assessed for their cytokine secretion profile, cytolytic potential, and chemokine production. While the great majority of CD8+ T-cell clones generated from HIV-seronegative individuals produced interferon (IFN)-γ, but not interleukin-4 (IL-4), that is a type 1 cytotoxic (Tc1) profile, high numbers of CD8+ T-cell clones generated from HIV-seropositive individuals produced IL-4 in addition to IFN-γ or IL-4 alone, thus showing a type 0 cytotoxic (Tc0)- or a type 2 cytotoxic (Tc2) profile, respectively. Tc0/Tc2 cells displayed lower cytolytic activity than Tc1 cells, including a reduced ability to lyse autologous targets pulsed with HIV or HIV peptides. By contrast, the production of chemokines RANTES and macrophage inflammatory protein-1α was comparable in Tc1, Tc0, and Tc2 clones irrespective of whether they were derived from HIV-seronegative or HIV-seropositive individuals. When CD8+ T-cell clones were generated from PBMC cultures of HIV-seronegative individuals conditioned with IL-4 plus an anti–IL-12 antibody (Ab), a shift towards the Tc0/Tc2-like profile was observed. Conversely, the addition to PBMC cultures of IL-12 plus an anti – IL-4 Ab shifted the differentiation of CD8+ T cells from HIV-infected individuals towards the Tc1-like profile, whereas IL-12 or anti–IL-4 Ab alone had a lower Tc1-promoting effect. Irradiated PBMC from HIV-infected individuals, used as feeder cells, shifted the differentiation of CD8+ T cells from a healthy HIV-seronegative individual towards the Tc0/Tc2-like profile. On the other hand, a shift towards the Tc1-like profile was noted in CD8+ T-cell clones generated from the skin specimens of two HIV-seropositive patients with Kaposi's sarcoma, successfully treated with IFN-α, in comparison to CD8+ clones generated from the same skin areas before treatment. The IFN-α–induced Tc1 shift could be prevented by the incubation of skin-infiltrating CD8+ T cells with IL-4 before cloning. Taken together, these data indicate that both defective production of IL-12 and abnormal IL-4 production in bulk PBMC populations of HIV-infected individuals may contribute to the development of high numbers of CD8+ T-cell clones showing a Tc0/Tc2-like phenotype and reduced cytolytic potential against HIV itself. They also suggest that the cytokine profile of CD8+ T-cell clones can be modulated by cytokines (or anticytokine Ab) both in vitro and in vivo.
CD8+ T CELLS PLAY A fundamental role in controlling human immunodeficiency virus (HIV) infection, inasmuch as they have been shown to act as cytotoxic T lymphocytes (CTL) against HIV-infected cells.1,2 Anti-HIV cytolytic activity has been detected in every phase of HIV infection and may also be present during the development of full-blown disease.3 However, precursor frequencies of CTLs are progressively lost during the course of HIV infection and the loss of CTL activity parallels the decrease in CD4+ T cells.4 CD8+ cells from asymptomatic HIV-seropositive individuals can also suppress HIV replication without killing the infected cells.5,6 Such a suppressor activity is not HLA-restricted, does not require cell-to-cell contact, occurs at the level of viral transcription,7 and seems to be mediated by some chemokines produced by CD8+ T cells, such as RANTES, macrophage inflammatory protein-1α (MIP-1α), MIP-1β, and interleukin-16 (IL-16).8,9 Progression of HIV infection to full-blown disease is associated with the loss of CD8 T-cell–mediated HIV-suppressive function.10 While activated CD8+ T cells usually play a protective role against HIV infection, the possibility that other CD8+ T-cell subsets can rather favor HIV replication has also been suggested.11 For example, at least in vitro, CD8+ T cells expressing the CD30 ligand are capable of enhancing viral replication in HIV-infected CD4+ T cells, and possibly their death, via the CD30L/CD30 interaction.12
Recently, it has been shown that not only CD4+, but also CD8+, T cells can be categorized into different subsets according to their profile of cytokine production.13,14 The great majority of CD8+ T cells produce interferon (IFN)-γ, but no IL-4 (type 1 cytotoxic or Tc1), whereas a minority of CD8+ T cells produce both IFN-γ and IL-4 (Tc0) or IL-4, but no IFN-γ (Tc2).14 We and others have shown that unusual high proportions of CD8+ Tc2 clones can be generated from both skin biopsies and peripheral blood mononuclear cells (PBMC) of HIV-infected individuals in advanced phase of infection.15,16 These cells show unusual phenotypic and functional features, such as CD30 and CD40L expression and B-cell helper activity for IgE synthesis.14-16
In this study, other functional properties of CD8+ Tc2 cell clones generated from PBMC of HIV-infected individuals, as well as the mechanisms responsible for the enhanced prevalence of these cells in HIV-infected individuals, have been investigated. The results show that CD8+ Tc2 clones from both seronegative or seropositive subjects have a lower cytolytic activity than Tc1 clones, but comparable production of both RANTES and MIP-1α. Moreover, we provide evidence that both decreased production of IL-12 by macrophages and enhanced IL-4 synthesis by T cells probably contributes to the shifting of CD8+ T cells into the Tc2 profile in HIV-infected subjects. Finally, the development of CD8+ T cells into Tc2 could be prevented by the in vivo treatment of HIV-infected individuals with IFN-α and restored by in vitro incubation of cells with IL-4.
MATERIALS AND METHODS
Reagents.Phytohemagglutinin (PHA) was purchased from GIBCO Laboratories (Grand Island, NY). Phorbol 12-myristate 13-acetate (PMA) was purchased from Sigma Chemical Co. (St Louis, MO). Recombinant interleukin-2 (rIL-2) was a kind gift of Eurocetus (Milano, Italy) and rIL-4 was purchased from Roche (Basel, Switzerland). rIL-12 and neutralizing anti–IL-12 (C8.6.2.1) monoclonal antibody (MoAb) were kind gifts of Dr G. Trinchieri (Wistar Institute, Philadelphia, PA). Anti-CD20, anti-CD16, anti-CD4, anti-CD8 MoAbs were purchased from Becton Dickinson (Mountain View, CA) and anti-CD30 from Immunotech (Marseille, France). Anti-CD3 antibodies were purchased from Ortho (OKT3, Ortho Pharmaceuticals, Raritan, NJ) and from Ancell (UCHT1, Ancell Corp, Bayport, MN). Neutralizing anti–IL-4 MoAb was purchased from R & D (R&D Systems, Minneapolis, MN). Anti–IFN-γ and anti–IL-4 Ab for intracytoplasmatic staining were a generous gift of C. Heusser (Ciba-Geigy, Basel, Switzerland). Eight gp120 HIV peptides (aa 88-98, 105-117, 312-319, 319-327, 413-424, 425-436, 437-448, 449-460), selected for appropriate size (<13 aa), were purchased from Neosystem-Labs (Strasbourg, France). The HIV-IIIB strain was kindly provided by Dr P. Balboni (University of Ferrara, Italy).
Subjects.PBMC were obtained from six HIV-infected patients and three HIV-seronegative healthy volunteers. All six HIV-infected patients were classified in the group IV, according to the Centers for Disease Control (CDC) criteria.17 Four of them belonged to subgroup C-1 and two to subgroup D (Kaposi Sarcoma [KS]). Biopsy specimens were taken for diagnostic purposes from the two patients with KS from the same skin areas before and after a cycle (12 weeks, 9 × 106 IU/week) of IFN-α (Wellferon; Wellcome, London, UK) treatment. All patients and controls gave informed consent.
Cell cloning of peripheral blood CD8+ T cells.Purified CD8+CD4− cell suspensions were obtained from PBMC of HIV-seropositive or HIV-seronegative individuals by a step of plastic adherence followed by a negative selection using anti-CD4 plus anti-CD16 and anti-CD20 MoAbs and magnetic beads coated with antimouse IgG (Dynabeads, Dynal, Oslo, Norway). By this technique, cell suspensions recovered are usually > 95% CD8+ T cells (and <1% CD4+, CD16+ and CD20+ cells). CD8+ T cells from HIV-seropositive individuals were preactivated in 24-well plates coated with insolubilized anti-CD3 MoAb (1 μg/mL; Ancell Corp, Bayport, MN) in the absence or in the presence of IL-12 (200 IU/mL), anti–IL-4 Ab (1 μg/mL), or both for 5 days in RPMI 1640 medium, supplemented with 2 mmol/L L-glutamine, 20 mmol/L 2-mercaptoethanol, 3% human serum, 10% fetal calf serum (FCS; Hyclone Laboratories, Inc, Logan, UT) (complete medium). CD8+ T cells from HIV-seronegative subjects were preactivated under the same experimental conditions in the absence or in the presence of IL-4 (4 ng/mL) plus anti–IL-12 MoAb (1 μg/mL). T-cell blasts were cultured further for 10 days in the presence of rIL-2 (20 U/mL) and then cloned under limiting dilution conditions (0.3 cells/well), in the presence of irradiated (6,000 rad) allogeneic (in some cases of autologous) PBMC as feeder cells (105 cells/well), PHA (1% vol/vol) rIL-2 (50 U/mL), as previously described.12,15 In some experiments, purified CD8+ T cells were directly cloned in limiting numbers in the presence of allogeneic feeder cells plus PHA and rIL-2. Growing microcultures were then supplemented, at weekly intervals, with rIL-2 (20 U/mL) and 105 irradiated feeder cells. Clonal efficiency was calculated according to Taswell.18
Processing of biopsies and generation of T-cell clones.To favor the expansion of activated T cells present in the tissue, skin biopsy specimens were cultured in complete medium and rIL-2 (50 U/mL). rIL-2 (50 U/mL) was then added three times a week and cultures were followed for an additional 6 days. Tissue specimens were then disrupted by repeated pipetting and viable T blasts were counted and cloned in limiting dilution conditions as previously described.12 14
Cell phenotyping.Cell phenotyping of purified CD8+ T cells or of CD8+ clones from HIV-seropositive patients and controls was performed on a Cytoron Absolute Cytofluorimeter (Ortho Pharmaceuticals; Raritan, NJ) by using fluoresceinated anti-CD4 and phycoerythrinated anti-CD8 MoAb.
Assays for detection of cytokines and chemokine production.The ability of T-cell clones to produce cytokines was evaluated after stimulation of 5 × 105/mL viable T-cell blasts for 36 hours with PMA (10 ng/mL) plus anti-CD3 MoAb (50 ng/mL).12,14 To avoid production of cytokines by contaminating feeder cells, the stimulation was performed at least 15 days after the last refeeding with irradiated allogeneic cells. For the quantitation of RANTES, MIP-1α, and IFN-γ in supernatants, the Quantikine Immunoassays (R & D Systems) were used, according to the manufacturer's instructions.12,14 Quantitation of IL-4 and IL-5 was performed by in homemade capture enzyme-linked immunosorbent assay (ELISA), as described.12 14 Values of the cytokine content 5 standard deviation (SD) over those of control supernatant (derived from irradiated feeder cells alone) were considered positive.
Cytolytic assays.Nonspecific cytolytic activity of T-cell clones was assessed as reported elsewhere.14 Briefly, T-cell blasts of each clone were extensively washed, counted, resuspended in complete medium, and assayed for redirected cytotoxicity against murine 51Cr-labelled P815 mastocytoma cells in the presence of anti-CD3 Ab (100 ng/mL) with different E/T ratios (4:1, 2:1, 1:1, 1:2).
Antigen-specific cytolytic activity was assessed on short term CD8+ polyclonal T-cell lines generated from HIV-infected patients with prevalent Tc1 or Tc2 profile. To this end, CD8+ T cells, purified as described earlier, were stimulated with insolubilized anti-CD3 Ab plus IL-2 and, after 4 days, were assessed for CD30 expression by flow cytometry. CD8+CD30+ T-cell blasts were separated by using anti-CD30 Ab plus antimouse Ig-coated magnetic beads (Dynal) from CD30− T-cell blasts and cultured for 10 days in the presence of IL-2 (20 U/mL). Flow cytometric determination of intracellular IFN-γ and IL-4 production was performed on both populations according to the method described by Assenmacher et al.19 Three types of 51Cr-labeled autologous target cells were used for the cytolytic assay: (1) purified CD4+ T blasts preactivated with PHA (48 hours) and pulsed (2 hours) with a mixture of eight gp120 peptides (160 μg/mL/106 cells), (2) Epstein-Barr virus (EBV)-induced lymphoblastoid cell line pulsed with the same peptides, and (3) purified activated CD4+ T-cell blasts cocultured (24 hours) in vitro with the supernatant of H9 cells infected with HIV IIIB strain (CCID50 6.8 × 106/mL; 37 μL/106 cells). Cells not pulsed with peptides and T-cell blasts cocultured with the supernatant of the noninfected H9 cell line were used as control targets, respectively. Effector cells were cultured in triplicates with targets at three E:T ratios (2.5; 10; 40). After 4 hours incubation (or 15 hours for IIIB-infected cells) at 37°C, 0.1 mL supernatant was removed for measurement of 51Cr release and specific cytolytic activity was calculated as described.15
Statistical analysis.For statistical analysis, t-test and χ2 test were used.
RESULTS
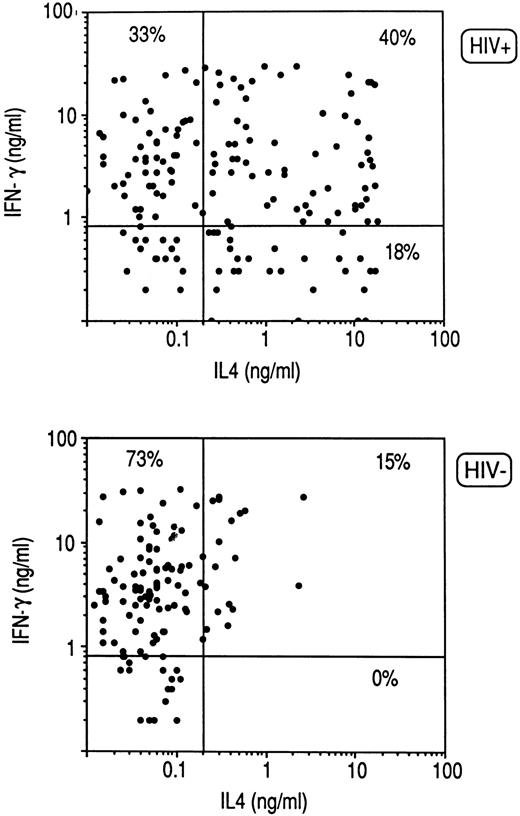
Cytokine profile of CD8+ clones from HIV-infected individuals.Purified CD8+ T cells derived from PBMC of three HIV-seronegative healthy donors and six HIV-infected patients were activated with insolubilized anti-CD3 Ab. CD8+ T-cell blasts were then cloned in limiting numbers with autologous irradiated PBMC as feeder cells, PHA, and IL-2. A total of 118 CD8+ T-cell clones were obtained from HIV-seronegative and 158 from HIV-seropositive donors, the clonal efficiency being much lower in the latter (31% ± 7% v 13% ± 6%). The cytokine profile of the two groups of clones in response to stimulation with PMA and anti-CD3 MoAb was then analyzed. As shown in Fig 1, the great majority of CD8+ T-cell clones generated from HIV-seronegative patients produced IFN-γ, but not IL-4 (Tc1), whereas high proportions of CD8+ T-cell clones generated from HIV-infected patients produced IL-4 in addition to IFN-γ (Tc0) or even IL-4 alone (Tc2), the differences being highly significant (P < .0001) for the three subsets of clones (Fig 1).
Cytokine profile of CD8+ T-cell clones generated from PBMC of three HIV-seronegative and six HIV-seropositive subjects. CD8+ cell suspensions were preactivated with insolubilized anti-CD3 (UCTH1) Ab for 5 days and then cloned under limiting dilution conditions with irradiated autologous feeder cells, PHA and IL-2, as described in Materials and Methods. T-cell blasts from each clone (106/mL) were stimulated for 36 hours with PMA and anti-CD3 (Ortho) Ab. IL-4 and IFN-γ produced in the supernatants were quantitated by appropriate immunoassays, as described in Materials and Methods. Lines represent mean values (± 5 SD) found in supernatants of stimulated cultures containing irradiated feeder cells alone.
Cytokine profile of CD8+ T-cell clones generated from PBMC of three HIV-seronegative and six HIV-seropositive subjects. CD8+ cell suspensions were preactivated with insolubilized anti-CD3 (UCTH1) Ab for 5 days and then cloned under limiting dilution conditions with irradiated autologous feeder cells, PHA and IL-2, as described in Materials and Methods. T-cell blasts from each clone (106/mL) were stimulated for 36 hours with PMA and anti-CD3 (Ortho) Ab. IL-4 and IFN-γ produced in the supernatants were quantitated by appropriate immunoassays, as described in Materials and Methods. Lines represent mean values (± 5 SD) found in supernatants of stimulated cultures containing irradiated feeder cells alone.
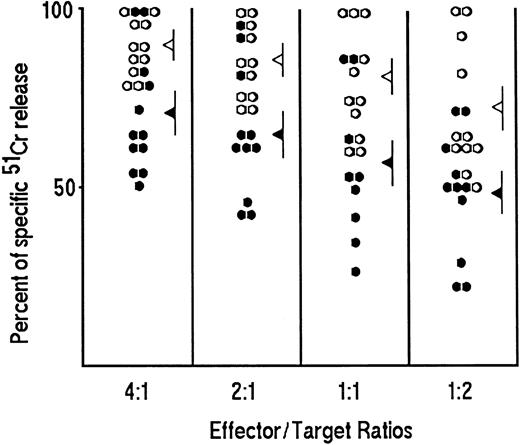
Cytolytic potential.To establish whether the Tc0/Tc2 profile associated with changes in the cytolytic potential of CD8+ T cells, redirected cytolytic activity was evaluated in randomly selected 33 clones with Tc2 profile (12 from HIV-seronegative and 21 from HIV-seropositive patients) in comparison with 57 clones showing a Tc1 profile (26 from HIV-seronegative and 31 from HIV-seropositive patients). The mean values (± standard error [SE]) of specific cytolytic activity of Tc2 clones (52 ± 5) were significantly lower (P < .01) than those of Tc1 clones (78 ± 6) when 1:1 effector/target ratio was used (data not shown). In 22 randomly selected clones from HIV-seronegative subjects, the mean values of the cytolytic activity of Tc1 and Tc2 clones were significantly different (P < .01) at each of the four effector/target ratios used (Fig 2), whereas no difference in the cytolytic potential between Tc2 clones derived from HIV-seronegative or HIV-seropositive patients was noted. Likewise, the cytolytic potential of Tc1 clones derived from HIV-seronegative or HIV-seropositive donors was comparable (data not shown).
Cytolytic activity of Tc1 and Tc2 clones generated from PBMC of HIV-seronegative subjects. T-cell blasts of 22 representative CD8 T-cell clones (11 Tc1, ○ and 11 Tc2, •) were incubated at four different effector/target ratios (4:1; 2:1; 1:1; 1:2) in triplicate with 5 × 103 51Cr-labeled P815 mastocytoma cells for 4 hours at 37°C in the presence of anti-CD3 Ab (100 ng/mL). The 51Cr release for each clone was calculated as described in Materials and Methods. The mean values (±SE) of specific cytolytic activity at each E/T ratio of Tc1 (◃ ) and Tc2 (◂ ) clones are also reported.
Cytolytic activity of Tc1 and Tc2 clones generated from PBMC of HIV-seronegative subjects. T-cell blasts of 22 representative CD8 T-cell clones (11 Tc1, ○ and 11 Tc2, •) were incubated at four different effector/target ratios (4:1; 2:1; 1:1; 1:2) in triplicate with 5 × 103 51Cr-labeled P815 mastocytoma cells for 4 hours at 37°C in the presence of anti-CD3 Ab (100 ng/mL). The 51Cr release for each clone was calculated as described in Materials and Methods. The mean values (±SE) of specific cytolytic activity at each E/T ratio of Tc1 (◃ ) and Tc2 (◂ ) clones are also reported.
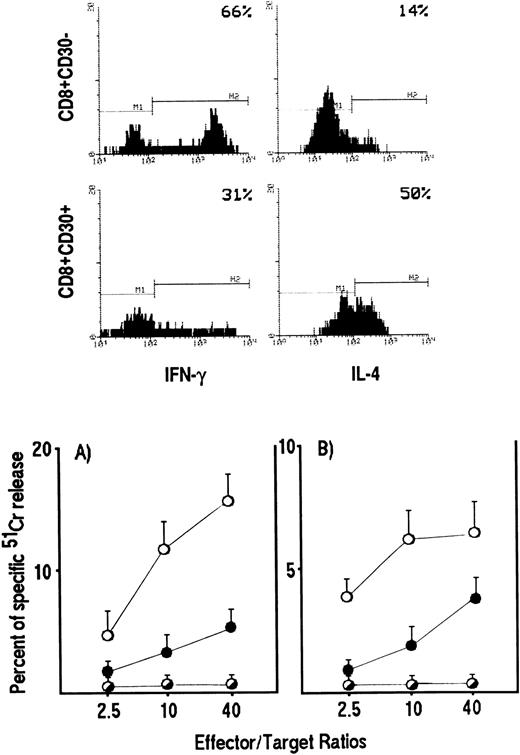
We then asked whether the reduction in cytolytic activity by Tc0/Tc2 clones also reflected a reduced ability of these cells to lyse HIV-infected targets. To answer this question, polyclonal CD8+ T-cell lines were generated from two HIV-infected patients by stimulation of purified CD8+ T cells with anti-CD3 Ab and IL-2. Based on our previous demonstration that CD8+ T cells, which develop into Tc0/Tc2 cells express CD30, whereas Tc1 precursors do not,20 CD8+CD30+ were separated from CD8+CD30− and assessed for both their cytokine profile by intracellular staining and their ability to lyse autologous EBV-transformed B-cell lines pulsed with HIV peptides or autologous CD4+ T-cell blasts pulsed with either HIV peptides or the HIV IIIB strain. As expected, T-cell lines generated from CD8+CD30+ cell suspensions contained higher numbers of IL-4– and lower numbers of IFN-γ–producing cells than T-cell lines generated from CD8+CD30− cell suspensions (Fig 3, upper panels). More importantly, the progenies of CD8+CD30+ T cells showed a markedly reduced ability to lyse both autologous EBV-transformed B cells and CD4+ T-cell blasts pulsed with HIV peptides (Fig 3 lower panels). A similar difference in the cytolytic activity of Tc2- and Tc1-oriented cell lines was observed when autologous CD4+ T-cell blasts infected in vitro with HIV-IIIB strain were used as targets (data not shown).
HIV-specific cytotoxicity by CD8+CD30+ and CD8+CD30− short-term T-cell lines generated from PBMC of HIV-infected patients. CD8+ T cells, stimulated with insoluble anti-CD3 Ab, were separated into CD30+ and CD30− cells and expanded as described in Materials and Methods. IL-4 and IFN-γ production by CD8+CD30+ and CD8+CD30− T cells was detected by cytokine intracellular staining as described in Materials and Methods (upper panels). CD8+CD30+ (•) and CD8+CD30− (○) effector cells were incubated for 4 hours at different E:T ratios with 5 × 10451Cr-labeled autologous PHA-induced CD4+ T-cell blasts (lower panel) (A) or EBV-transformed B-cell lines (lower panel) (B) pulsed with a mixture of eight gp120 peptides (○,•) or with medium alone (◑). The 51Cr release for each line was calculated as described in Materials and Methods. The mean values (±SE) of triplicate cultures from a representative experiment are reported.
HIV-specific cytotoxicity by CD8+CD30+ and CD8+CD30− short-term T-cell lines generated from PBMC of HIV-infected patients. CD8+ T cells, stimulated with insoluble anti-CD3 Ab, were separated into CD30+ and CD30− cells and expanded as described in Materials and Methods. IL-4 and IFN-γ production by CD8+CD30+ and CD8+CD30− T cells was detected by cytokine intracellular staining as described in Materials and Methods (upper panels). CD8+CD30+ (•) and CD8+CD30− (○) effector cells were incubated for 4 hours at different E:T ratios with 5 × 10451Cr-labeled autologous PHA-induced CD4+ T-cell blasts (lower panel) (A) or EBV-transformed B-cell lines (lower panel) (B) pulsed with a mixture of eight gp120 peptides (○,•) or with medium alone (◑). The 51Cr release for each line was calculated as described in Materials and Methods. The mean values (±SE) of triplicate cultures from a representative experiment are reported.
Production of chemokines.In view of the possible role of chemokines produced by CD8+ T cells in suppressing HIV replication,8 9 production of both RANTES and MIP-1α by Tc1 and Tc2 clones generated from either HIV-seropositive or HIV-seronegative patients was assessed. As shown in Fig 4, no significant difference in the amount of RANTES produced either by Tc1 or by Tc2 clones generated from HIV-seronegative or HIV-seropositive patients, was found. Accordingly, no difference in the production of MIP-1α between Tc1 (66.6 ± 7.2 ng/mL) and Tc2 (51.9 ± 9.2 ng/mL) clones was observed.
RANTES production by Tc1 (○) and Tc2 (•) clones derived from PBMC of HIV-seronegative and HIV-seropositive patients. Supernatants of 90 randomly selected CD8+ T-cell clones (26 Tc1 and 12 Tc2 generated from PBMC of HIV-seronegative subjects and 31 Tc1 and 21 Tc2 from PBMC of HIV-seropositive individuals), obtained as described in the legend to Fig 1, were assessed for the content of RANTES, as described in Materials and Methods.
RANTES production by Tc1 (○) and Tc2 (•) clones derived from PBMC of HIV-seronegative and HIV-seropositive patients. Supernatants of 90 randomly selected CD8+ T-cell clones (26 Tc1 and 12 Tc2 generated from PBMC of HIV-seronegative subjects and 31 Tc1 and 21 Tc2 from PBMC of HIV-seropositive individuals), obtained as described in the legend to Fig 1, were assessed for the content of RANTES, as described in Materials and Methods.
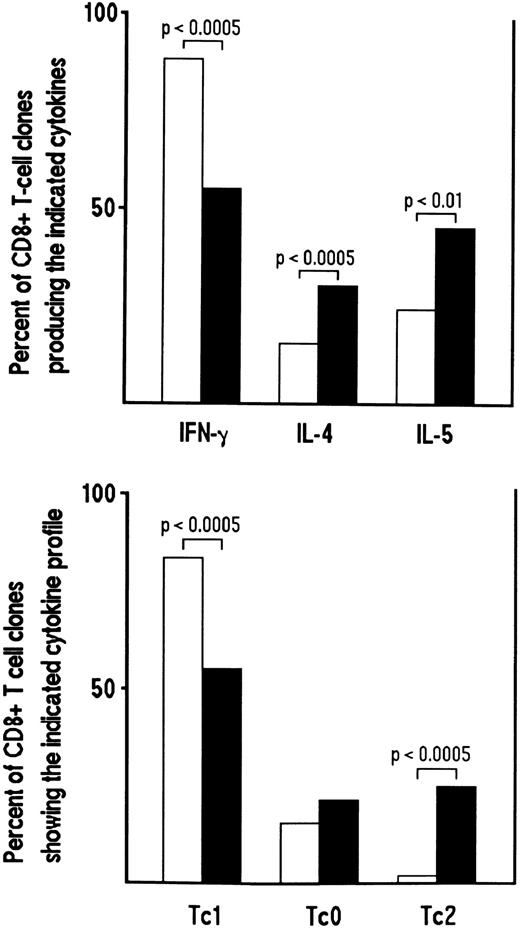
Influence of cytokines or anticytokine antibodies on the development of CD8+ Tc0 and Tc2 clones.We then asked whether the cytokine profile of CD8+ T-cell clones generated from HIV-seronegative or HIV-seropositive patients could be modulated by the addition in bulk culture before cloning of cytokine and/or anticytokine antibodies. To this end, CD8+ T-cell clones were generated from three HIV-seronegative donors in the absence or presence of recombinant IL-4 and anti–IL-12 MoAb. Conversely, CD8+ T-cell clones were generated from HIV-infected patients in the absence or in the presence of IL-12, anti–IL-4 MoAb or both. A total of 139 CD8+ T-cell clones were obtained from cultures conditioned with IL-4 plus anti–IL-12, the clonal efficiency being roughly similar to the 118 CD8+ T-cell clones obtained from unconditioned cultures (23% to 35% v 20% to 37%). Following stimulation with PMA plus anti-CD3 MoAb, the proportions of IFN-γ–producing CD8+ T-cell clones derived from cultures conditioned with IL-4 and anti–IL-12 MoAb were significantly lower than those of parallel clones derived from unconditioned cultures (P < .0005) (Fig 5). Conversely, the proportions of clones producing IL-4 and IL-5 derived from IL-4/anti–IL-12 MoAb-conditioned cultures were significantly higher in comparison to those derived from unconditioned cultures (P < .0005 and P < .01, respectively). The majority of T cells, however, still expressed a Tc1 pattern (Fig 5).
Effect of the addition of IL-4 plus anti-IL–12 MoAb on the development of Tc2 clones derived from CD8+ T cells of HIV-seronegative patients. Purified CD8+ cells (106/mL) from PBMC of three HIV-seronegative patients were preactivated with insolubilized anti-CD3 MoAb in the absence (□) or in the presence of IL-4 (4 ng/mL) plus anti-IL–12 MoAb (1 μg/mL) (▪). After 5 days, T-cell blasts were cloned in limiting numbers, as described in Materials and Methods. Supernatants of CD8+ T-cell clones (118 generated from unconditioned and 139 from IL-4 plus anti-IL–12 MoAb-conditioned cultures), obtained as described in the legend to Fig 1, were assayed for cytokine content, as described in Materials and Methods. The percentages of the two groups of clones producing IFN-γ, IL-4, and IL-5 (upper panel) or showing the Tc1/Tc0/Tc2 profile of cytokine production (lower panel), are reported.
Effect of the addition of IL-4 plus anti-IL–12 MoAb on the development of Tc2 clones derived from CD8+ T cells of HIV-seronegative patients. Purified CD8+ cells (106/mL) from PBMC of three HIV-seronegative patients were preactivated with insolubilized anti-CD3 MoAb in the absence (□) or in the presence of IL-4 (4 ng/mL) plus anti-IL–12 MoAb (1 μg/mL) (▪). After 5 days, T-cell blasts were cloned in limiting numbers, as described in Materials and Methods. Supernatants of CD8+ T-cell clones (118 generated from unconditioned and 139 from IL-4 plus anti-IL–12 MoAb-conditioned cultures), obtained as described in the legend to Fig 1, were assayed for cytokine content, as described in Materials and Methods. The percentages of the two groups of clones producing IFN-γ, IL-4, and IL-5 (upper panel) or showing the Tc1/Tc0/Tc2 profile of cytokine production (lower panel), are reported.
The numbers of CD8+ T-cell clones comparable to those obtained from unconditioned cultures (n = 22) of three HIV-infected patients were generated from cultures conditioned with rIL-12 (n = 15), anti–IL-4 MoAb (n = 18), or both (n = 29), the clonal efficiency being also similar (17%, 10%, and 7% v 11%). However, only CD8+ T-cell clones generated in the presence of both IL-12 and anti–IL-4 Ab showed a significant difference in their profile of cytokine production in comparison to those generated from unconditioned cultures. As shown in Fig 6 (upper panel), a significant increase in the proportion of IFN-γ–producing clones (P < .005) and a significant decrease in the proportions of IL-4– and IL-5–producing clones (P < .005) was observed. In contrast, the addition in bulk culture before cloning of anti–IL-4 Ab alone induced only a reduction in the proportion of IL-5–producing clones (P < .02), whereas the addition of IL-12 alone significantly enhanced the proportion of IFN-γ–producing clones (P < .005), but had no effects on the proportions of both IL-4– and IL-5–producing clones (Fig 6, upper panel). Moreover, analysis of the amounts of cytokines produced showed that T-cell clones generated in the presence of IL-12 plus anti–IL-4 Ab produced significantly higher amounts of IFN-γ (P < .005) and, conversely, significantly lower amounts of both IL-4 and IL-5 (P < .02) than unconditioned clones (Fig 6, lower panel). CD8+ T-cell clones generated in the presence of IL-12 or anti–IL-4 alone produced significantly higher amounts of IFN-γ in comparison to unconditioned clones (P < .01 and P < .005, respectively), whereas there was little or no difference in the amounts of both IL-4 and IL-5 produced (anti–IL-4 Ab on IL-5 production; P < .02) (Fig 6, lower panel).
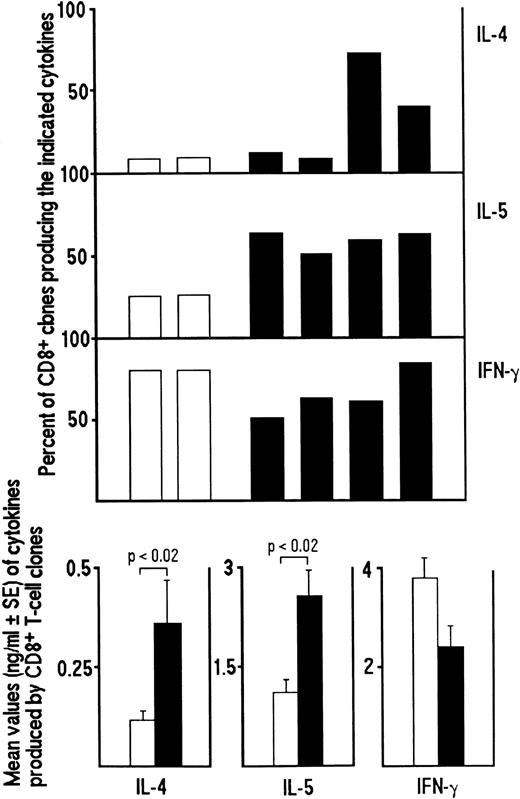
Inhibitory effect of the addition of IL-12, anti–IL-4 MoAb or both on the development of Tc0 and Tc2 clones generated from CD8+ T cells of HIV-infected patients. Purified CD8+ T cells from PBMC of four HIV-seropositive patients were preactivated with insolubilized anti-CD3 MoAb in the absence (□) or in the presence of IL-12 (200 IU/mL) (▨), anti–IL-4 MoAb (1 μg/mL) (▨) or both (▪). After 5 days, T-cell blasts from each culture were cloned in limiting numbers, as described in Materials and Methods. Supernatants of CD8+ T-cell clones, obtained as described in the legend to Fig 1, were assayed for cytokine content, as described in Materials and Methods. The percentages of the four groups of clones producing IFN-γ, IL-4 and IL-5 (upper panel) and the mean values (±SE) of cytokines produced by each group of clones (lower panel) are shown.
Inhibitory effect of the addition of IL-12, anti–IL-4 MoAb or both on the development of Tc0 and Tc2 clones generated from CD8+ T cells of HIV-infected patients. Purified CD8+ T cells from PBMC of four HIV-seropositive patients were preactivated with insolubilized anti-CD3 MoAb in the absence (□) or in the presence of IL-12 (200 IU/mL) (▨), anti–IL-4 MoAb (1 μg/mL) (▨) or both (▪). After 5 days, T-cell blasts from each culture were cloned in limiting numbers, as described in Materials and Methods. Supernatants of CD8+ T-cell clones, obtained as described in the legend to Fig 1, were assayed for cytokine content, as described in Materials and Methods. The percentages of the four groups of clones producing IFN-γ, IL-4 and IL-5 (upper panel) and the mean values (±SE) of cytokines produced by each group of clones (lower panel) are shown.
To establish whether the preferential development of CD8+ T cells from HIV-seropositive patients into Tc0 and Tc2 clones was due to an intrinsic property of CD8+ T cells or to a conditioning effect exerted by feeder cells, purified CD8+ T cells from one healthy individual were cloned by using irradiated allogeneic PBMC derived from six different donors (two HIV-seronegative- and four HIV-seropositive patients) as feeder cells. The T-cell clones generated under these different experimental conditions were then compared for their profile of cytokine production. With feeder cells from HIV-infected patients, clonal efficiencies were usually lower (range, 7% to 17%, mean, 14% ± 5%) than those obtained with feeder cells from the two HIV-seronegative subjects (17% and 35%, respectively). More importantly, the proportions of IL-4– and IL-5–producing CD8+ T-cell clones generated with feeder cells from HIV-seropositive donors were significantly higher (P < .05 and P < .01, respectively), whereas the proportions of IFN-γ–producing T-cell clones were lower (P = not significant), than those obtained with feeder cells from HIV-seronegative donors (Fig 6). Furthermore, analysis of the amounts of cytokines showed that T-cell clones obtained with feeder cells from HIV-seropositive donors produced significantly higher amounts of IL-4 and IL-5 (P < .02) than those of clones obtained with feeder cells from HIV-seronegative subjects (Fig 7). The shifting was not due to HIV infection of these clones, inasmuch as none of them showed detectable p24 Ag production in response to stimulation with PMA plus anti-CD3 Ab, which consistently induced high p24 Ag production by HIV-infected CD4+ T-cell clones (data not shown). Taken together, these data suggest that an imbalance between IL-4 and IL-12 production in bulk culture may be responsible for the preferential development of CD8+ cells into Tc0 and Tc2 clones in HIV-seropositive patients. Moreover, they also indicate that at least in vitro the cytokine profile of CD8+ T-cell clones from HIV-infected patients can be influenced by the addition of exogenous IL-12 and/or neutralization of endogenously produced IL-4.
Effect of allogeneic feeder cells from HIV-seronegative and HIV-seropositive patients on the development of Tc2 clones. Purified CD8+ T cells from one HIV-seronegative patient were directly cloned in limiting numbers in the presence of irradiated allogeneic feeder cells from two HIV-seronegative (□) and four HIV-seropositive patients (▪) plus PHA and IL-2. Clonal supernatants, obtained as described in the legend to Fig 1, were assayed for cytokine content, as described in Materials and Methods. The percentages of cytokine-producing clones generated in the presence of different allogeneic feeder cells (upper panel), and the mean values (±SE) of the cytokine produced by the two group of clones (clones derived in the presence of feeder cells from HIV-seropositive and HIV-seronegative patients) (lower panel) are reported.
Effect of allogeneic feeder cells from HIV-seronegative and HIV-seropositive patients on the development of Tc2 clones. Purified CD8+ T cells from one HIV-seronegative patient were directly cloned in limiting numbers in the presence of irradiated allogeneic feeder cells from two HIV-seronegative (□) and four HIV-seropositive patients (▪) plus PHA and IL-2. Clonal supernatants, obtained as described in the legend to Fig 1, were assayed for cytokine content, as described in Materials and Methods. The percentages of cytokine-producing clones generated in the presence of different allogeneic feeder cells (upper panel), and the mean values (±SE) of the cytokine produced by the two group of clones (clones derived in the presence of feeder cells from HIV-seropositive and HIV-seronegative patients) (lower panel) are reported.
Effect of in vivo treatment with IFN-α on the development of CD8+ Tc2 clones.We finally asked whether the cytokine profile of CD8+ T-cell clones generated from HIV-seropositive patients can be modified by cytokines administered in vivo. To answer this question, we took the opportunity to study two HIV-seropositive patients who were treated with IFN-α because they were suffering from KS. IFN-α has, indeed, been found to be effective in the treatment of KS21 and able to promote the development of Th1 cells both in humans in vitro22 and in the experimental animal models in vivo.23 To this end, specimens were taken from the same skin areas of the two KS patients before and after a cycle of IFN-α treatment and incubated in IL-2–supplemented medium to expand the skin-infiltrating T cells. Cytofluorimetric analysis performed after 5 days of culture showed that the majority of growing cells from both skin specimens taken either before (76% and 83%) or after (81% and 77%) IFN-α treatment were CD8+ T cells. T-cell blasts from all cultures were then cloned under limiting conditions in the presence of standard pooled irradiated PBMC as feeder cells, PHA, and IL-2. A total number of 248 CD8+ T-cell clones were generated from skin specimens taken before and 183 CD8+ T-cell clones from skin specimens taken after IFN-α treatment and assessed for production of both IFN-γ and IL-4 in response to stimulation with PMA plus anti-CD3 MoAb. The results of these experiments are summarized in Table 1. In both cases, the amounts of IFN-γ produced by CD8+ T-cell clones generated after IFN-α treatment were significantly higher (P < .005 and P < .0001, respectively) than those produced by CD8+ T-cell clones generated from the same skin areas before treatment. Conversely, the mean values of IL-4 produced by CD8+ clones derived after IFN-α treatment were lower (one case significant P < .01) in comparison to those of clones generated before therapy.
Effect of In Vivo Treatment With IFN-α on the Ability to Produce IL-4 and IFN-γ by CD8+ T-Cell Clones Generated From Skin Biopsies of HIV-Infected Patients With KS
| Patient No. . | No. of . | Mean Values of Cytokine Production* . | |
|---|---|---|---|
| (treatment) . | Clones . | IL-4 (ng/mL) . | IFN-γ (ng/mL) . |
| Patient no. 1 | |||
| Before | 146 | 1.66 ± 0.4† | 4.07 ± 0.2ρ |
| After | 119 | 1.00 ± 0.1† | 8.89 ± 1.5ρ |
| Patient no. 2 | |||
| Before | 102 | 0.51 ± 0.1‡ | 4.94 ± 0.41-155 |
| After | 64 | 0.17 ± 0.1‡ | 7.42 ± 0.21-155 |
| Patient No. . | No. of . | Mean Values of Cytokine Production* . | |
|---|---|---|---|
| (treatment) . | Clones . | IL-4 (ng/mL) . | IFN-γ (ng/mL) . |
| Patient no. 1 | |||
| Before | 146 | 1.66 ± 0.4† | 4.07 ± 0.2ρ |
| After | 119 | 1.00 ± 0.1† | 8.89 ± 1.5ρ |
| Patient no. 2 | |||
| Before | 102 | 0.51 ± 0.1‡ | 4.94 ± 0.41-155 |
| After | 64 | 0.17 ± 0.1‡ | 7.42 ± 0.21-155 |
Abbreviation: NS, not significant.
Clonal T cells were extensively washed, counted, and incubated at 106/mL in the presence of PMA (20 ng/mL) plus anti-CD3 Ab (100 ng/mL) for 36 hours. The content of IL-4 and IFN-γ in T-cell clone supernatants was evaluated by ELISA kits, as described in Materials and Methods.
P, NS.
P < .01.
ρ P < .005.
P < .0001.
In one of the two cases, the skin specimen obtained after IFN-α treatment was split into two parts; one was incubated in medium plus IL-2 alone and the other in medium plus IL-2 and IL-4. A total of 119 and 126 CD8+ clones were obtained from the two bulk cultures, respectively. As shown in Fig 8, the proportions of IFN-γ–producing CD8+ T-cell clones generated from the biopsy fragment incubated with IL-2 plus IL-4 were significantly lower than those derived from the skin fragment incubated in the presence of IL-2 alone (P < .0005). Furthermore, in the first group of clones, the proportions of those producing IL-4 and IL-5 were significantly higher than in the second group (P < .0005 and P < .0005, respectively). When clones derived under these two experimental conditions were categorized according to their cytokine pattern as Tc1, Tc0 and Tc2, a significant decrease in the number of Tc1 and a significant increase in the number of Tc2 clones (P < .0005 and P < .0005, respectively) was seen among those derived from the skin specimen cultured in IL-2 plus IL-4 (data not shown). Taken together, these data suggest that the potential of CD8+ T cells present in the skin of HIV-seropositive patients to develop into Tc0/Tc2 clones can be prevented by in vivo administration of IFN-α and can be restored by treatment of the cells with IL-4 in vitro.
Effect of in vitro treatment with IL-4 on the development of Tc2 cells from the skin of a KS patient treated with IFN-α. The skin specimens obtained after treatment with IFN-α were incubated in IL-2 alone (□) or IL-2 plus IL-4 (▪). After 5 days, T-cell blasts were cloned in limiting numbers with irradiated autologous feeder cells, PHA, and IL-2. The supernatants of CD8+ T-cell clones, obtained as described in the legend to Fig 1, were assayed for cytokine content, as described in Materials and Methods. The percentages of the two groups of clones producing IFN-γ, IL-4, and IL-5 are reported.
Effect of in vitro treatment with IL-4 on the development of Tc2 cells from the skin of a KS patient treated with IFN-α. The skin specimens obtained after treatment with IFN-α were incubated in IL-2 alone (□) or IL-2 plus IL-4 (▪). After 5 days, T-cell blasts were cloned in limiting numbers with irradiated autologous feeder cells, PHA, and IL-2. The supernatants of CD8+ T-cell clones, obtained as described in the legend to Fig 1, were assayed for cytokine content, as described in Materials and Methods. The percentages of the two groups of clones producing IFN-γ, IL-4, and IL-5 are reported.
DISCUSSION
The demonstration that long-term CD4+ T-cell clones segregate into subsets producing distinct types of cytokines, named Th1, Th2, and Th0,12 has recently provided the basis for similar studies with CD8+ T cells.13 Although the great majority of CD8+ T cells produce IFN-γ, but no IL-4,24 IL-4–producing CD8+ T-cell clones have been obtained by stimulation of murine CD8+ T cells with anti-CD3 antibody, mitogen or allostimulation, and antigen in the presence of IL-4.13,25-28 Based on these findings, the names Tc1 and Tc2 for cytotoxic CD8+ T cells secreting Th1- and Th2-type cytokines were proposed.13 CD8+ T-cell clones that produce IL-4 have also been generated from the skin of immunologically unresponsive individuals with leprosy,29 KS skin lesions, and PB of HIV-infected patients,15,30 and more recently, from the gengiva of patients with chronic adult periodontitis31 and from normal human peritoneum.32 Of note is that generation of these Tc2 clones does not require T-cell conditioning with exogenous IL-4, suggesting the occurrence in HIV-infected individuals of microenvironmental condition(s) that favor the shift of CD8+ T cells from the Tc1 to the Tc0/Tc2 phenotype.14
In this study, three main questions were addressed. The first question was whether CD8+ T-cell clones, in addition to a different profile of cytokine production, exhibit other distinctive functional properties in comparison to Tc1 clones. It has previously been shown that Tc2 clones generated from HIV-infected individuals express CD3020 and are able to provide optimal B-cell help for immunoglobulin synthesis, including IgE, due to their ability to produce IL-4 and to express the CD40 ligand.15,16 This finding may account for high IgE levels found in some patients with acquired immunodeficiency syndrome (AIDS) despite the virtual absence of circulating CD4+ T helper cells.15,16 Here, we demonstrate that Tc2 clones also exhibit lower cytolytic potential than Tc1 clones following stimulation with anti-CD3 MoAb. These results are apparently at variance with those reported by Sad et al,14 who showed no decrease in the cytolytic activity of murine Tc2 clones generated via IL-4 conditioning in vitro. The reason for this discrepancy is unclear, but it probably reflects the possibility that Tc2 cells spontaneously emerging in some pathological conditions are characterized by major functional changes than those artificially generated by IL-4 conditioning of CD8+ T cells in vitro. It is of note that polyclonal Tc0/Tc2-oriented CD8+ T-cell lines generated from HIV-infected patients showed lower levels of HIV-specific cytolytic activity in comparison to Tc1-oriented CD8+ T-cell lines generated from the same donors. Thus, the decreased cytolytic activity of CD8+ Tc0/Tc2 cells may also account for the reduced protection against both HIV and other viral infections, a feature shared by many seropositive individuals in the advanced phases of HIV infection.4 In contrast to the reduced cytolytic potential, Tc2 clones did not show any significant difference in comparison to Tc1 clones for their ability to produce RANTES and MIP-1α. Nor there was any difference in the production of the same chemokines between Tc2 clones generated from HIV-infected patients or HIV-seronegative donors. This finding is of particular interest in view of the recent demonstration that both RANTES and MIP-1α can contribute to the suppressor activity on HIV replication mediated by CD8+ T cells. Because Tc2 cells seem to be less protective than Tc1 cells in the defense against viruses, the observation that Tc1 and Tc2 CD8+ T cells produce similar amounts of chemokines argue against the exclusive and/or essential role of RANTES and MIP-1α in the protection against HIV.
The second question addressed in this study relates to the mechanism(s) responsible for the shifting of high proportions of CD8+ T cells from the Tc1 to the Tc0/Tc2 phenotype in HIV-infected patients. Several studies have provided evidence that the development of CD4+ Th cells into the Th1 or the Th2 cytokine profile is essentially regulated by the type of cytokines present in the microenvironment at the time of antigen presentation.33-35 The presence of IL-12 released by both macrophages and dendritic cells favors the development of Th1 cells,33-35 whereas the early presence of IL-4 is still more critical for the development of Th2 cells.36-38 Similar mechanisms seem also to be operating in the development of Tc1 and Tc2 cells. Murine CD8+ T cells can indeed be primed by IL-4 in vitro to develop into Tc2 cells.25-27 Furthermore, in mice transgenic for a T-cell receptor (TCR) α/β recognizing defined specificity, activation of CD8+ T cells in the presence of IL-12 generated CD8+ T cells able to produce Th1-type cytokines on restimulation.27 By contrast, the presence of IL-4 promoted the development of IL-4– and IL-5–producing cells and blocked the production of IFN-γ.27 The results of this study show that the addition in bulk culture of IL-4, together with the neutralization of endogenously produced IL-12, shifted the development of normal human CD8+ T cells towards the Tc2 profile. More importantly, they demonstrate that the addition in bulk culture of IL-12 and anti–IL-4 Ab shifted the development of CD8+ T cells from HIV-infected patients into the Tc1 profile. Of note is that the addition of IL-12 or anti–IL-4 antibody alone favored the development of IFN-γ–producing cells, but had no significant inhibitory effect on the development of IL-4 (and IL-5)–producing cells. Only the simultaneous presence of recombinant IL-12 and the neutralization of endogenously produced IL-4 restored the Tc1 profile of CD8+ T cells. These data suggest that the preferential development of CD8+ T cells from HIV-infected patients into Tc2 clones is due to both defective production of IL-12 and early production of IL-4 in bulk culture. Whereas the defective production of IL-12 by macrophages from HIV-infected patients is well established,39 the outcome and the source of the enhanced (and/or earlier) IL-4 production is unclear. A switch from the Th1 to the Th0, or even the Th2, phenotype has been suggested to occur during the progression of HIV infection to full-blown disease.40,41 Thus, activated CD4+ Th0 or Th2 cells may provide IL-4 required for the development of CD8+ T cells into Tc0/Tc2 cells. IL-4 may also be provided by other cell types, such as basophils42,43 or CD4+ natural killer (NK) 1.1+ cells,44 although abnormalities in the function of these cells during HIV infection have not been reported. A final possibility is that IL-4 is provided by CD8+ T cells themselves, due to an intrinsic CD8+ T-cell alteration. An attempt to solve the question of whether the preferential development of CD8+ into Tc2 clones was due to an intrinsic alteration of CD8+ T cells from HIV-infected patients or to the influence of other cell types present within the heterogenous population of feeder cells was made by analyzing the effect of irradiated PBMC from HIV-infected patients on the clonal development of normal CD8+ T cells. The results of these experiments showed that using PBMC from HIV-infected patients as feeder cells increased both the proportions of IL-4– and IL-5–producing CD8+ T-cell clones and the amounts of IL-4 and IL-5 produced, whereas the proportions of IFN-γ–producing CD8+ T-cell clones and the amounts of IFN-γ they produced were decreased in comparison to using feeder cells from HIV-seronegative patients. This suggests that the defective IL-12 production by macrophages from HIV-infected patients and/or the early production of IL-4 by some cell type present in the feeder population may contribute to the preferential development of CD8+ T cells into the Tc0/Tc2 phenotype. However, the fact that CD8+ T cells from HIV-infected patients more easily develop into Tc0/Tc2 clones even under conditions in which allogeneic feeder cells from HIV-seronegative subjects are used,15 supports the possibility that they are already planned to such type of development. The majority of CD8+ T cells in the peripheral blood of HIV-infected patients are indeed memory cells,45 and it is likely that these cells have already been conditioned by microenvironmental in vivo conditions to preferentially develop into Tc0/Tc2 cells.
The final question we asked was whether the shifting of CD8+ T cells to the Tc0/Tc2 phenotype could be prevented by cytokines administered in vivo. To this end, we took the opportunity to study two HIV-infected patients treated with IFN-α because they were affected by KS. KS-infiltrating T cells in HIV-infected patients are prevalently CD8+ and the clones derived from them prevalently show a Tc2 profile.30,46 Moreover, it has been shown that IFN-α is a Th1-inducing agent for both human and murine CD4+ T cells.22 23 When CD8+ T-cell clones were generated from the same skin areas after clearing KS lesions by treatment with IFN-α, a significant reduction in the proportions of IL-4–producing CD8 T-cell clones and a parallel increase in the number of IFN-γ–producing clones were found. On the other hand, when the skin-infiltrating T cells were incubated in the presence of IL-4 before cloning, the switch to the Tc1 profile induced by in vivo treatment with IFN-α was completely blocked. These findings demonstrate that the cytokine profile of CD8+ T cells can be modulated not only in vitro but also in vivo, suggesting that the function of these cells can be manipulated by biological agents and/or drugs.
Supported by grants from Associazione Italiana per la Ricerca sul Cancro, the Istituto Superiore di Sanità (“AIDS” project 1996) and Consiglio Nazionale delle Ricerche (A.C.R.O. and “Cytokines” projects). G.G. was supported by a fellowship from A.I.R.C.
Address reprint requests to Sergio Romagnani, MD, Department of Clinical Immunology, Istituto di Medicina Interna e Immunoallergologia, University of Florence, Viale Morgagni, 85, 50134 Firenze, Italy.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal