Abstract
Chronic infection with Schistosoma mansoni induces in humans and mice a Th2-dominant immune response in which eosinophils and IgE are conspicuously elevated. Human eosinophils express IgE receptors that participate in an IgE-dependent eosinophil-mediated ADCC reaction against Schistosomula larvae in vitro. To investigate the expression of IgE receptors on murine eosinophils, they were purified (<95% pure by Giemsa-stained cytospin preparations) from liver granulomas of Schistosoma-infected mice. Flow cytometric analysis showed the absence of the low-affinity IgE receptor Fc-ε RII (CD23) and Mac-2 and the absence of binding of murine IgE. Reverse transcription-polymerase chain reaction (RT-PCR) analysis of granuloma eosinophil mRNA did not detect transcripts for Fc-ε RII or the α-chain of the high-affinity IgE receptor Fc-ε RI, but did detect transcripts that encode Mac-2 and the low-affinity IgG receptors Fc-γ RIIb2, Fc-γ RIII, and the FcR-associated γ-chain. In vitro stimulation of granuloma eosinophils with interleukin-4 (IL-4) did not induce IgE binding, surface expression of Mac-2, or the transcription of Fc-ε receptors (Fc-ε RI, Fc-ε RII/CD23). To investigate normal murine eosinophils, we cultured normal mouse bone marrow cells with recombinant IL-3, recombinant IL-5, and recombinant granulocyte-macrophage colony-stimulating factor, conditions that promote eosinophil differentiation. Flow cytometric analysis of bone marrow-derived eosinophils failed to detect IgE binding or cell surface expression of Fc-ε RII and Mac-2, and RT-PCR analysis of fluorescence-activated cell sorted bone marrow-derived eosinophils failed to detect transcripts that encode Fc-ε RI or Fc-ε RII. These findings show that, in contrast to human eosinophils, murine eosinophils do not express cell surface receptors that bind IgE. However, because IgG receptors (Fc-γ RIIb2, Fc-γ RIII) were present on eosinophils purified from granulomas, we investigated whether they might be involved in eosinophil activation. We found that an oxidative burst in eosinophils could be triggered through their IgG receptors.
ELEVATED LEVELS OF serum IgE and eosinophils are characteristic of most parasitic infections and are hallmark features of Schistosoma mansoni infection in both humans1 and in an actively investigated murine model.2-6 Information about the role of IgE and eosinophils in the pathophysiology of schistosomiasis has come from investigations in humans and mice, but with divergent findings. It has been reported that human eosinophils purified from patients infected with S mansoni express three different types of IgE-receptors: the high-affinity Fc-ε receptor (Fc-ε RI), the low-affinity Fc-ε receptor (Fc-ε RII/CD23), and Mac-2/ ε BP.7-10 Although all three have been reported to be capable of mediating IgE-dependent eosinophil cytotoxicity directed to target antigens expressed on Schistosomula,10 there is still some controversy about the functional role of CD23.11 Two different groups have reported that Fc-γ receptors are involved in human eosinophil activation by allergen-antibody immune-complexes and in the IgG-dependent cytotoxic activity against parasites.11 12 Comparable studies have not been reported in the murine model in which a functional role for IgE and eosinophils remains unclear.
After infection with Schistosoma cercariae, mice develop a Th1-dominant, γ-interferon (γ-IFN)/interleukin-2 (IL-2)–rich immune response directed toward antigens expressed on the larval forms of the parasite. It has been shown that this initial immune response imparts a degree of host resistance to the parasite.3,13 As the larvae mature to adult worms that inhabit the hepatic portal venous system, egg production ensues and entrapment of eggs in the liver induces a granulomatous inflammatory response in which eosinophils account for more than 50% of the cellular infiltrate. The onset of egg production induces a shift in the character of the host T-cell response to the parasite and the initial Th1-dominant response is replaced by a progressive dominance of CD4+ Th2 cells. This shift is reflected by the production of IL-4 and IL-5, and, through their actions, the development of high levels of serum IgE and eosinophils and the production of IL-10 that downregulates the activity of the Th1 cells.2,6,13-16 Whether the IgE and eosinophils are detrimental to the parasite is unknown. Studies in humans previously identified an antiparasite role for IgE and IgE-receptor expressing eosinophils through an antibody dependent cellular cytotoxicity (ADCC) mechanism,9,10 but comparable studies have not been performed in the murine model. Therefore, our first goal was to investigate the expression of IgE receptors on murine eosinophils purified from liver granulomas of schistosome-infected mice. We pursued this to explore the in situ pathophysiology of the mouse model and to compare the rodent model to the findings that had been established in the human studies.1 Our second goal was to analyze normal murine eosinophils for the expression of IgE receptors using eosinophils derived from in vitro cultures of normal murine bone marrow as described by other groups.17 18 We report here that murine eosinophils do not bind IgE, but they do have the functional capacity to produce reactive metabolites that are involved in the immune response against parasites, and that this activity can be triggered through IgG Fc-receptors on the surface of the eosinophil.
MATERIALS AND METHODS
Eosinophil purification from liver granulomas infected with S mansoni. Liver granulomas were obtained as previously described.19 Briefly, CBA mice (7 to 8 weeks old) were infected with 35 cercariae of S mansoni and livers were harvested 8 to 10 weeks later. After homogenization of the livers, the intact granulomas were allowed to sediment, treated with collagenase, physically dispersed, and passed through sterile gauze. Inflammatory cells from hepatic granulomas were passed through a nylon wool column to remove macrophages, and nonadherent cells were eluted with RPMI-1640. The nonadherent cell population contained approximately 60% eosinophils, 35% lymphocytes, and 5% macrophages. Murine granuloma eosinophils were purified by centrifugation in a discontinuous Percoll gradient harvesting eosinophils at the interface of 60% and 55% Percoll layers as described.20 Eosinophils were suspended in RPMI 1640 supplemented with 10% bovine calf serum (Hyclone, Logan, UT) and 5 × 10−5 mol/L 2-β-Mercaptoethanol (2-βME) under sterile conditions. Viability was greater than 95% as measured by Trypan blue exclusion.
List of the Primers
| Receptor Product . | Sequence (5′-3′) . | Reference . | PCR . | |
|---|---|---|---|---|
| Fc-γ RI | ||||
| Forward | CTG CAG GAG TGT CCA TCA CGG TGA AAG A | 350 bp | ||
| Reverse | GGA TGT GAA ACC AGA CAG GAG CTG ATG A | |||
| Fc-γ RII | ||||
| Forward (4) | GCT GGA GGA ACA AAC TAC TGA ACA G | 24 | β1 | 477 bp |
| Reverse (2) | GCA GCT TCT TCC AGA TCA GGA GGA | β2 | 339 bp | |
| Fc-γ RIII | ||||
| Forward (1) | AGT CAC AGT GGG GAC TAC TAC TGC A | 24 | 268 bp | |
| Reverse (3) | CAC TTG TCT TGA GGA GCC TGG TGC T | |||
| γ-Chain | ||||
| Forward | CTC AGC CGT GAT CTT GTT CTT GC | 24 | 238 bp | |
| Reverse | GCT TCA GAG TCT CAT ATG TCT CC | |||
| Fc-ɛ RII/CD23 | ||||
| Forward | GCA CGC CTC ATC ACT GAA AGG | 44 | 1,033 bp | |
| Reverse | GGG TTC ACT TTT TGG GGT | |||
| Mac-2 | ||||
| Forward | GCC CCG CAT GCT GAT CAC AAT C | 288 bp | ||
| Reverse | GTT GTA CTG CAG TAG GTG AGC ATC GT | |||
| Fc-ɛ RIα | ||||
| Forward | GAT CCA CAA TGG TAC CGT CTC TGA GG | 358 bp | ||
| Reverse | CCA CCT GCC TAA GAT AGC CCT TGC | |||
| CD2 | ||||
| Forward | GCG GAC TGC AGA GAC AAT GAG AC | 491 bp | ||
| Reverse | CGC CTC ACA CTT GAA TGG TGC | |||
| B29 | ||||
| Forward | TAA GTC TAG AAG TTC CGT GCC ACA GCT GTC | 24 | 320 bp | |
| Reverse | CAC TGA ATT CCC AAG GAA GCC CTT GTT CCC | |||
| MBP | ||||
| Forward | GAG CGT CTG CTC TTC ATC TGA | 387 bp | ||
| Reverse | CAC TGA AAC TGT GAA TGG AGG C | |||
| β-actin | ||||
| Forward | ATG GAT GAC GAT ATC GCT | 44 | 587 bp | |
| Reverse | ATG AGG TAG TCT GTC AGG T |
| Receptor Product . | Sequence (5′-3′) . | Reference . | PCR . | |
|---|---|---|---|---|
| Fc-γ RI | ||||
| Forward | CTG CAG GAG TGT CCA TCA CGG TGA AAG A | 350 bp | ||
| Reverse | GGA TGT GAA ACC AGA CAG GAG CTG ATG A | |||
| Fc-γ RII | ||||
| Forward (4) | GCT GGA GGA ACA AAC TAC TGA ACA G | 24 | β1 | 477 bp |
| Reverse (2) | GCA GCT TCT TCC AGA TCA GGA GGA | β2 | 339 bp | |
| Fc-γ RIII | ||||
| Forward (1) | AGT CAC AGT GGG GAC TAC TAC TGC A | 24 | 268 bp | |
| Reverse (3) | CAC TTG TCT TGA GGA GCC TGG TGC T | |||
| γ-Chain | ||||
| Forward | CTC AGC CGT GAT CTT GTT CTT GC | 24 | 238 bp | |
| Reverse | GCT TCA GAG TCT CAT ATG TCT CC | |||
| Fc-ɛ RII/CD23 | ||||
| Forward | GCA CGC CTC ATC ACT GAA AGG | 44 | 1,033 bp | |
| Reverse | GGG TTC ACT TTT TGG GGT | |||
| Mac-2 | ||||
| Forward | GCC CCG CAT GCT GAT CAC AAT C | 288 bp | ||
| Reverse | GTT GTA CTG CAG TAG GTG AGC ATC GT | |||
| Fc-ɛ RIα | ||||
| Forward | GAT CCA CAA TGG TAC CGT CTC TGA GG | 358 bp | ||
| Reverse | CCA CCT GCC TAA GAT AGC CCT TGC | |||
| CD2 | ||||
| Forward | GCG GAC TGC AGA GAC AAT GAG AC | 491 bp | ||
| Reverse | CGC CTC ACA CTT GAA TGG TGC | |||
| B29 | ||||
| Forward | TAA GTC TAG AAG TTC CGT GCC ACA GCT GTC | 24 | 320 bp | |
| Reverse | CAC TGA ATT CCC AAG GAA GCC CTT GTT CCC | |||
| MBP | ||||
| Forward | GAG CGT CTG CTC TTC ATC TGA | 387 bp | ||
| Reverse | CAC TGA AAC TGT GAA TGG AGG C | |||
| β-actin | ||||
| Forward | ATG GAT GAC GAT ATC GCT | 44 | 587 bp | |
| Reverse | ATG AGG TAG TCT GTC AGG T |
Bone marrow-derived eosinophils from normal mice.Balb/c male mice (6 to 9 weeks old) were maintained in a horizontal laminar flow cabinet and provided with sterile food and water. Femoral and tibial bone marrows of 6- to 9-week-old BALB/c mice (Harlan Sprague-Dawley, Indianapolis, IN) were obtained, and a single-cell suspension was prepared in Hanks' balanced salt solution (HBSS) following a previously described procedure17 with modifications. After three washes, 2 × 105 cells/mL were resuspended in RPMI 1640 supplemented with 10% bovine calf serum, 0.2 mmol/L L-glutamine, 0.1 mmol/L essential and nonessential amino acids, 0.1 mmol/L Na-pyruvate, 5 × 10−5 mol/L 2-βME, and an antibiotic-antimycotic (GIBCO/BRL, Grand Island, NY) and incubated in 24-well tissue culture plates (Costar, Cambridge, MA) in the presence of 10 U/mL recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF ), 20 U/mL rIL-5, and 10 U/mL rIL-3 (Genzyme, Cambridge, MA).17
Monoclonal antibodies (MoAbs).The MoAbs used in this study were labeled with fluorescein isothiocyanate (FITC), phycoerythin (PE), or cyanine 5.18 (Cy5). They included B3B4 (a rat IgG2a antimurine Fc-ε RII/CD23), 2.4G2 (a rat IgG2b antimurine Fc-γ RII/RIII), RB6-8C5 (a rat IgG2b for a granulocyte marker, Gr-1) from Pharmingen (San Diego, CA), 6B2 (a rat IgG2a antimurine CD45R/B220) from Pharmingen, 30-H12 (a rat IgG2b antimurine Thy-1.2) from Pharmingen, M3/38 (a rat IgG2a antimurine Mac-2), and 7-A3B1 (an IgE murine hybridoma). F(ab′)2 fragments of 2.4G2 were obtained by pepsin treatment at pH 4.0 using standard protocol, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels were performed to check the molecular weight. F(ab′)2 fragment was dialyzed against phosphate-buffered saline (pH 7.0) before use.
Flow cytometric analysis.Cells were suspended in HBSS buffer containing 10% bovine calf serum, 10 mmol/L HEPES, and 0.02% Na-azide (fluorescence-activated cell sorting [FACS] staining buffer) at a concentration of 107 cells/mL. Cells were stained with conjugated antibodies in 50 μL for 40 minutes at 4°C and then washed three times, fixed in 2% paraformaldehyde in phosphate-buffered saline (pH 7.3), and analyzed on a Becton Dickinson FACS 440 (Becton Dickinson, Mountain View, CA) equipped with four decade logarithmic amplifiers. Forward and side scatter and two or three fluorescence parameters were collected on 10,000 cells and the data were analyzed on a VAX 4000 computer equipped with DESK software. For sort purification, cells were stained at a concentration of 1.5 to 2 × 107/ mL in FACS staining buffer. Cells were collected and analyzed on a Coulter Epics 753 flow cytometer and Coulter Elite software (University of Iowa Flow Cytometry Facility). A minimum of 3 to 4 × 105 cells were collected.
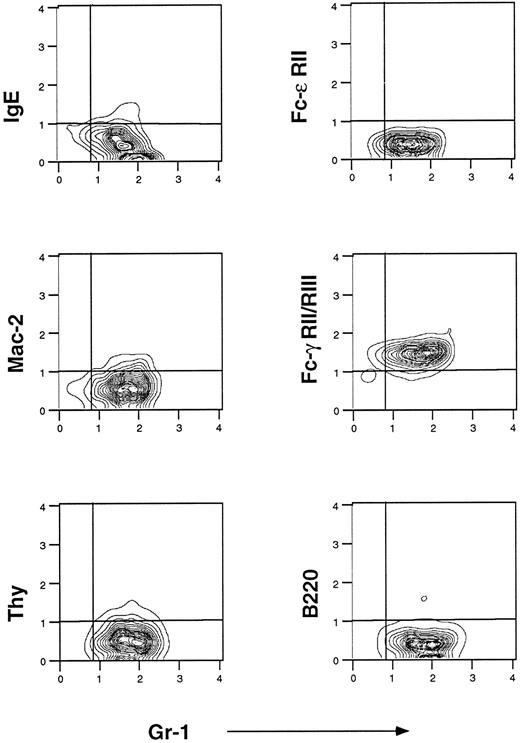
Flow cytometric analysis of murine eosinophils isolated from liver granulomas of S mansoni-infected mice. Cells were prepared as described in Materials and Methods. They were stained with fluorescein-conjugated IgE, fluorescein-conjugated anti–Fc-ε RII/CD23 (B3B4), Cy5-conjugated anti Mac-2 (M3/38), and fluorescein-conjugated anti–Fc-γ RII/RIII (2.4G2). Anti-B220 PE-conjugated and anti-Thy 1 Cy5-conjugated were used to control for B- and T-lymphocyte contamination, respectively. Gr-1 is a marker of mature granulocytes. One representative experiment of seven is shown.
Flow cytometric analysis of murine eosinophils isolated from liver granulomas of S mansoni-infected mice. Cells were prepared as described in Materials and Methods. They were stained with fluorescein-conjugated IgE, fluorescein-conjugated anti–Fc-ε RII/CD23 (B3B4), Cy5-conjugated anti Mac-2 (M3/38), and fluorescein-conjugated anti–Fc-γ RII/RIII (2.4G2). Anti-B220 PE-conjugated and anti-Thy 1 Cy5-conjugated were used to control for B- and T-lymphocyte contamination, respectively. Gr-1 is a marker of mature granulocytes. One representative experiment of seven is shown.
Reverse transcription-polymerase chain reaction (RT-PCR).Oligonucleotides for PCR were synthesized by standard procedures. The sequences of the primers used are listed in Table 1. Poly(A) mRNA was prepared using the Micro-FastTrack kit (Invitrogen, San Diego, CA) and was reverse transcribed according to the manufacturer's protocol. The 40 μL reaction mixture contained enzyme buffer, 40 U/μL of RNasin, 0.5 mmol/L dNTP, 0.5 μg oligo dT/18mer, and 400 U of Moloney murine leukemia virus (M-MLV) reverse transcriptase. The reaction mixture was incubated for 1 hour at 42°C. After incubation, the mixture was diluted to 250 μL with diethylpyrocarbonate (DEPC) water. The reverse-transcribed products were then amplified with Taq-Polymerase (Boehringer Mannheim Corp, Indianapolis, IN). The 50 μL PCR reaction mixture consisted of PCR buffer (10 mmol/L Tris-HCl, pH 8.3, 100 mmol/L KCl, 1.5 mmol/L MgCl2 ), 1.25 mmol/L of each dNTP, 10 μL of the reverse-transcribed product, 1.25 U of DNA polymerase, and 150 nmol/L of each primer. Thirty cycles of PCR were performed using a DNA thermal cycler (PTC-100; M.J. Research Inc, Watertown, MA). Each cycle consisted of 1 minute of denaturation at 94°C, 1 minute of annealing at 60°C, and 1 minute of extension at 72°C. After the final cycle, the temperature was held at 72°C for 8 minutes to allow reannealing of the amplified products.
Eosinophil peroxidase (EPO) activity.EPO activity was measured by the oxidation of o-phenylenediamine in the presence of hydrogen peroxide.18 21 Cultured bone marrow cells (2 × 105/mL) were centrifuged and resuspended in 100 μL of 1 mmol/L o-phenylenediamine (Sigma Chemical Co, St Louis, MO) in 0.05 mol/L Tris-HCl buffer (pH 8) containing 0.1% Triton X-100 and 1 mmol/L hydrogen peroxide (Sigma Chemical Co). After 30 minutes at room temperature, the color reaction was stopped by the addition of 50 μL of 4 mol/L sulfuric acid. Absorbance was measured at 490 and 630 nm with a Microplate EL309 (Bio-Tek Instruments, Winooski, VT). Results are expressed as the ratio of optical density (OD) at 490/630 nm.
Spin trapping.Electron paramagnetic resonance (EPR) assays were performed on purified eosinophils from liver granulomas from S mansoni-infected mice to measure the amount of reactive O−⋅2 produced by these cells. Cells were suspended (2 × 106/mL) in HBSS containing 100 mmol/L diethylenetriaminepentaacetic acid (DTPA) and 100 mmol/L 5,5-dimethyl-1-pyrroline-N-oxide (DMPO; Aldrich, Milwaukee, WI). After the addition of zymosan (1 mg/mL) or opsonized zymosan (1 mg/mL) prepared with serum from S mansoni-infected mice, the reaction mixture was transferred to a flat quartz EPR cell that was in turn placed in the cavity of a Bruker model ESP 300 Electron Paramagnetic Resonance spectrometer (Bruker Instruments, Karlsruhe, Germany). EPRl spectra were then recorded at room temperature. Instrument settings were as follows: microwave power, 20.0 mW; modulation frequency, 100 kHz; modulation amplitude, 0.892 G; sweep time 0.238 G/min; and response time, 0.655 seconds. With this system, the DMPO/⋅OOH spin aduct (aN = 14.3 G and aH = 11.7 G) and the DMPO/⋅OH spin adduct (aN = aH = 15.0 G) are generated during O−⋅2 formation, and the magnitude of this species reflects the relative amount of O−⋅2 generated.22 At lower rates of cellular O−⋅2 production, DMPO/⋅OH is often the predominant or sole species detected.23
RESULTS
Flow cytometric analysis of eosinophils isolated from S mansoni liver granulomas.IgE receptors were sought using three different MoAbs: 7-A3B1 (a murine IgE-producing hybridoma), B3B4 (anti–Fc-ε RII/CD23), and M3/38 (anti–Mac-2). Flow cytometric analysis for IgE receptors showed a phenotype that was consistent over time. For the results in Fig 1, we used preparations of enriched eosinophils after Percoll gradient purification in which a predominant population of Gr-1+ (a granulocyte marker) was found. To avoid macrophage contamination in the eosinophil preparations, dispersed cell granulomas were passed through a nylon column to remove adherent cells before the Percoll gradient. Purity of the eosinophil preparation was greater than 95% as assessed by Wright Giemsa staining and flow cytometric analysis showed that Gr-1 is expressed on 95% of these cells. Murine eosinophils from hepatic granulomas did not bind IgE and did not express CD23; however, very low levels of Mac-2 expression were detected in all experiments (Fig 1). Anti-IgE–FITC did not detect pre-existing IgE coupled to eosinophil IgE receptors (data not shown). In contrast, purified eosinophils did bind 2.4G2 (anti–Fc-γ RII and RIII, Fig 1, right middle panel). Finally, purity of the eosinophil preparations was further confirmed by the absence of staining with MoAbs against Thy-1.2 and B220. The absence of detectable CD23 and IgE binding on murine eosinophils from liver granulomas differs from results reported with eosinophils from human peripheral blood of patients infected with S mansoni in which CD23, Mac-2, and high-affinity Fc-ε RI were detected.8-10
Flow cytometric analysis of enriched eosinophil preparations from spleens and peripheral blood of mice infected with S mansoni showed a similar Fc-phenotype, ie, IgE−, CD23−, Mac-2−, and 2.4G2+, which is consistent with the absence of IgE receptors, but the presence of IgG Fc receptors in this model of parasitic infection (data not shown).
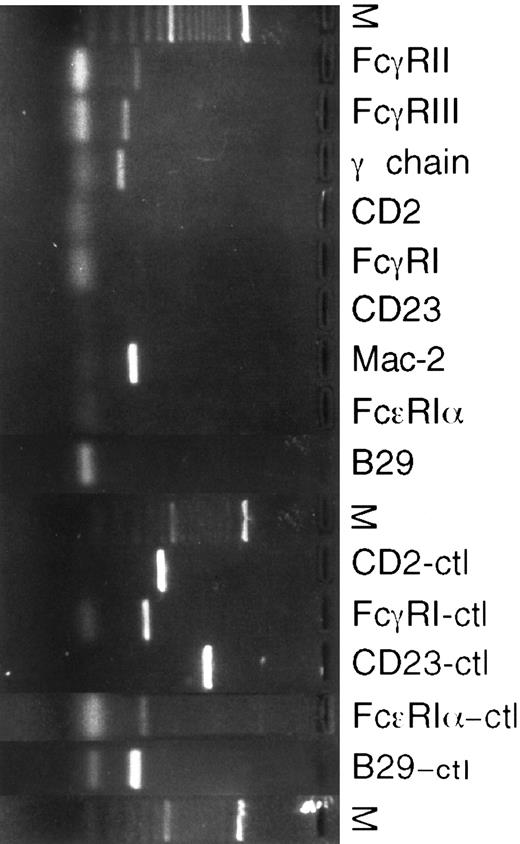
RT-PCR of eosinophils isolated from S mansoni liver granulomas.To further analyze the results obtained by flow cytometric analysis, mRNA expression for various surface receptors was studied using RT-PCR (Fig 2). mRNA for Fc-γ RII, RIII, and the associated γ-chain were sought using appropriate primer pairs.24 All eosinophil preparations analyzed had Fc-γ RIIβ2 , Fc-γ RIII, and γ-chain mRNA transcripts, demonstrating that these transcripts were constitutively expressed. Macrophage contamination was controlled for by testing for the presence of mRNA specific for the high-affinity Fc-γ RI receptor, which is only expressed on macrophages. mRNA for CD23 was not detected, although lymphocytes from hepatic granulomas were positive for CD23 (data not shown). A PCR product for Mac-2 was amplified (Fig 2). To assess for expression of high-affinity IgE receptor mRNA, primers coding for the α subunit of the tetracomplex αβγ2 were designed. No product using these primers was detected. Also, CD2 and B29 primers were used as described25 to detect possible T- and B-lymphocyte contamination. All eosinophil preparations were free of the CD2 and B29 PCR products. D10.3M.24, a murine T-helper clone transfected with CD23; J774, a peritoneal macrophage cell line; P815, a mastocytoma cell line; and BAL17, a B-cell line were used as positive controls for CD2 and CD23, Fc-γ RI, Fc-ε RI, and B29, respectively. To test for truncated forms of CD23 lacking the intracytoplasmic or transmembrane portions of the molecule,26 different CD23 primer pairs were used and all gave negative results (data not shown).
RT-PCR of cDNA from murine eosinophils isolated from liver granulomas of S mansoni-infected mice. Different positive controls to check the PCR reactions were used. D10.3M.24, a murine Th2 clone transfected with Fc-ε RII/CD23, was a positive control for CD2 and CD23 (CD2-ctl, CD23-ctl). J774, a murine peritoneal macrophage cell line, was a positive control for Fc-γ RI. P815, a murine mastocytoma cell line, was a positive control of the Fc-ε RIα subunit. BAL 17, a murine B-cell line, was a positive control for B29.
RT-PCR of cDNA from murine eosinophils isolated from liver granulomas of S mansoni-infected mice. Different positive controls to check the PCR reactions were used. D10.3M.24, a murine Th2 clone transfected with Fc-ε RII/CD23, was a positive control for CD2 and CD23 (CD2-ctl, CD23-ctl). J774, a murine peritoneal macrophage cell line, was a positive control for Fc-γ RI. P815, a murine mastocytoma cell line, was a positive control of the Fc-ε RIα subunit. BAL 17, a murine B-cell line, was a positive control for B29.
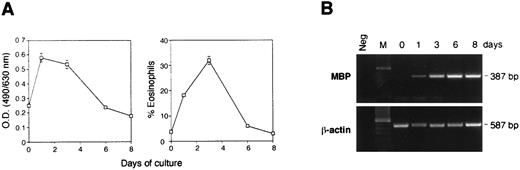
Culture of bone marrow-derived eosinophils in vitro. Bone marrow cells (2 × 105/mL) from normal uninfected mice were cultured for up to 8 days in the presence of rIL-3, rIL-5, and rGM-CSF. (A) On the left, cultured cells were assayed for EPO activity at different times as described in Materials and Methods. On the right is the percentage of eosinophils counted on cytospin preparations with Wright Giemsa staining. Data are the mean ± standard error of at least three separate experiments. (B) Extraction of RNA from bone marrow cells was performed by using 1 × 106 cells and RT-PCR of the MBP and β-actin were performed at different times.
Culture of bone marrow-derived eosinophils in vitro. Bone marrow cells (2 × 105/mL) from normal uninfected mice were cultured for up to 8 days in the presence of rIL-3, rIL-5, and rGM-CSF. (A) On the left, cultured cells were assayed for EPO activity at different times as described in Materials and Methods. On the right is the percentage of eosinophils counted on cytospin preparations with Wright Giemsa staining. Data are the mean ± standard error of at least three separate experiments. (B) Extraction of RNA from bone marrow cells was performed by using 1 × 106 cells and RT-PCR of the MBP and β-actin were performed at different times.
In vitro cultures of purified hepatic eosinophils with IL-4.In additional experiments, purified hepatic granuloma eosinophils and spleen cells were cultured with rIL-4 to assess whether this cytokine could induce expression of Fc-ε RII/CD23, as has been reported with human eosinophils.27,28 Purified hepatic granuloma eosinophils were activated with rIL-4 (600 U/mL) to assess an in vitro stimulation of Fc-ε RII/CD23. Murine spleen cells were cultured overnight with and without rIL-4 as a positive control of IgE receptor induction because it is well described that IL-4 upregulates CD23 on murine splenic B cells.29 Flow cytometric analysis showed that CD23 expression and IgE binding were increased on splenic B cells after incubation with IL-4. Murine eosinophils incubated overnight with IL-4 did not exhibit an expression of CD23 or Mac-2 and did not bind IgE (data not shown). Viability of the cultures was greater than 90%, as tested by Trypan blue exclusion. No CD23 PCR product was detected after incubation with IL-4, although a CD23-amplified product was detected in the control spleen cells and in the B-cell line WEHI 279 stimulated with IL-4 as positive controls of the CD23-PCR reaction.
Differentiation of bone marrow-derived eosinophils.To address whether the lack of IgE binding on murine granuloma eosinophils was possibly related to the S mansoni infection or whether it was a characteristic of murine eosinophils, bone marrow cultures from Balb/c and from CBA mice infected with S mansoni were grown in the presence of the three eosinophil-differentiation cytokines (GM-CSF, IL-3, and IL-5) as a model to induce eosinophil maturation.17,30 Bone marrow cells cultured with these differentiation cytokines for 3 days produced EPO, an eosinophil marker, which was highly expressed at day 3 of culture but absent by day 6 (Fig 3A). Wright Giemsa staining of cytospin preparations confirmed that, on day 3, the cultured bone marrow cells had 32% ± 2% of eosinophils (n = 4) and that this had decreased to 6% ± 1% (n = 3) by day 6. No neutrophils were found in the cultures. To analyze the degree of maturation in bone marrow-derived eosinophils, primers for the major basic protein (MBP) were used to perform RT-PCR (Fig 3B). MBP is stored in the crystalloid core of the eosinophil granule and, when released, is a potent toxin for parasites and mammalian cells.31 32 EPO and MBP are stored inside the granules of eosinophils until they are released during degranulation as part of the ADCC response. In our studies, expression of transcripts that encode MBP was detected in eosinophils from days 1 to 8. The results in Fig 3 show a possible inconsistency because the MBP transcript detection by PCR does not show a decrease, whereas the percentage of eosinophils clearly is decreasing after day 3. Because the PCR is not quantitative and involved 30 cycles, the differences in the intensity of the amplification product at different days is not informative with regards to the amount of original transcript present.
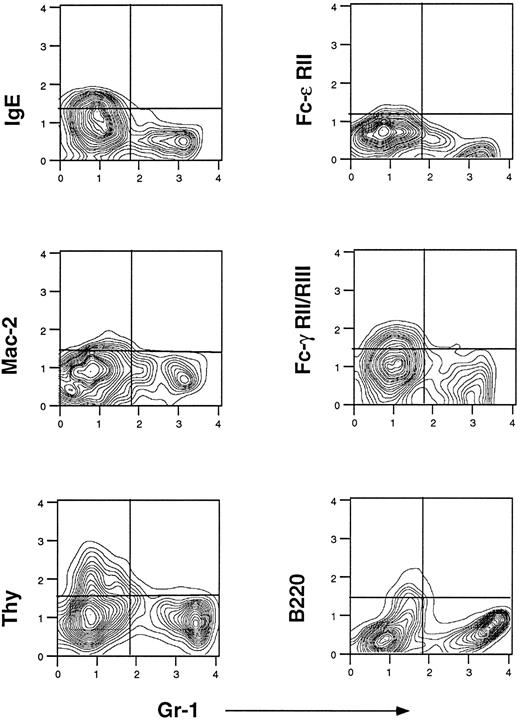
Flow cytometric analysis of bone marrow-derived eosinophils.Wright Giemsa-stained cytospin preparations showed the following composition after 3 days of culture: 32% ± 2% eosinophils, 33% ± 6% macrophages, 15% ± 2% lymphocytes, and 18% ± 10% other cells (n = 4). Figure 4 shows the flow cytometric profile of bone marrow cells after 3 days of culture in the presence of the cytokines that promote eosinophil differentiation. Both Gr-1+ and Gr-1− populations are present and, using cytospin/Wright Giemsa analysis, virtually all the eosinophils were in the Gr-1+ fraction. Staining with 2.4G2 detected no Fc-γ receptors in the Gr-1+ cells. The finding that the majority of Gr-1+ cells did not bind 2.4G2 constitutes a major difference between eosinophils derived from normal bone marrow (Fc-γR−) and eosinophils derived from hepatic granulomas (Fc-γR+). As shown in Fig 4, the Gr-1+ fraction did not contain cells that expressed IgE receptors as defined by the 3 antibodies cited before: 7-A3B1, B3B4, and M3/38. Thus, the bone marrow-derived eosinophils do not express IgE receptors, a finding in agreement with granuloma eosinophils.
Flow cytometric analysis of bone marrow cells from normal uninfected mice cultured for 3 days in medium containing rIL-3, rIL-5, and rGM-CSF. Cells were stained with different MoAbs as described in Fig 1. One representative experiment of five is shown.
Flow cytometric analysis of bone marrow cells from normal uninfected mice cultured for 3 days in medium containing rIL-3, rIL-5, and rGM-CSF. Cells were stained with different MoAbs as described in Fig 1. One representative experiment of five is shown.
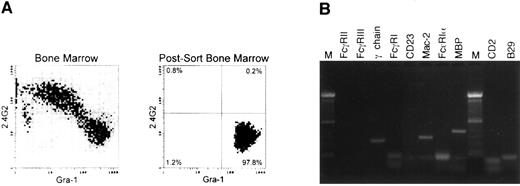
RT-PCR of bone marrow-derived eosinophils from normal mice.Three days of in vitro culture with rIL-3, rIL-5, and rGM-CSF greatly enriched the amount of eosinophils, but other myeloid and nonmyeloid lineages were also present. To further enrich the eosinophil population, sorting was performed with two markers. A granulocyte gate was performed based on the orthogonal and forward scatter, and Gr-1hi, 2.4G2− cells were sorted to avoid any possible contamination of macrophages because those cell types that are present in the Gr-1low and Gr-1− fractions express Fc-γ receptors (2.4G2+; Fig 5A). The composition of the sorted population was quantitated with Wright Giemsa staining and it was routinely found that, in the postsort population, greater than 98% of the cells were eosinophils. Gr-1− cells consisted of immature granulocytes, with less than 3% of this fraction being eosinophils. Figure 5 shows one representative experiment of four performed.
RT-PCR on Gr-1+, Fc-γ R− cells from normal bone marrow cells cultured with rIL-3, rIL-5, and rGM-CSF. (A) Flow cytometric analysis of the initial population and of the postsort Gr-1+ Fc-γ R− cells used for further RT-PCR analysis. (B) RT-PCR analysis of sorted cells using the same primers and conditions as described in Fig 2. Similar results were obtained on four different experiments.
RT-PCR on Gr-1+, Fc-γ R− cells from normal bone marrow cells cultured with rIL-3, rIL-5, and rGM-CSF. (A) Flow cytometric analysis of the initial population and of the postsort Gr-1+ Fc-γ R− cells used for further RT-PCR analysis. (B) RT-PCR analysis of sorted cells using the same primers and conditions as described in Fig 2. Similar results were obtained on four different experiments.
RT-PCR of the sorted population showed the expression of mRNA for the FcR-associated γ-chain (Fig 5B), but not for Fc-γ RII or Fc-γ RIII transcripts, being consistent with the flow cytometric data shown in Fig 4. No transcripts coding for the Fc-ε RIα subunit or CD23 were detected, although expression of Mac-2 message was detected. These results provided further evidence for the complete absence of Fc-ε receptors in bone marrow-derived eosinophils from normal mice. MBP was detected as an eosinophil marker on the sorted population. No CD2 or B29 transcripts were detected, showing the absence of contaminant lymphocytes in the sorted population. Bone marrow-derived eosinophils on day 3 that were subsequently cultured with rIL-4 failed to show induction of Fc-ε RII/CD23 (data not shown).
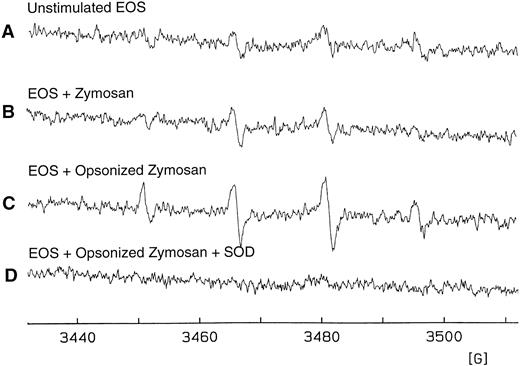
Detection of O−⋅2formation in eosinophils purified from S mansoni-infected mice.To address the question of whether murine eosinophils can be functionally activated in the absence of IgE receptors, purified liver granuloma eosinophils from S mansoni-infected mice were stimulated and O−⋅2 generation was assessed, the magnitude of which was determined using the DMPO spin trapping technique. Opsonized zymosan was used as a known activator to induce the aggregation and phagocytosis of Fc-γ receptors and the complement receptor for C3b (CR1), which leads to activation of a respiratory burst in neutrophils, eosinophils, and macrophages.33 34 A basal level of O−⋅2 was detected on unstimulated eosinophils and on eosinophils stimulated with unopsonized zymosan (Fig 6A and B, respectively). The spectra observed are characteristic of the DMPO/⋅OH spin adduct, a product of the reaction of DMPO and O−⋅2. However, in the presence of opsonized zymosan and DMPO, purified eosinophils consistently produced a greater than 50% increase in O−⋅2 formation (Fig 6C). No signal was observed when superoxide dismutase was present during the incubation period (Fig 6D), demonstrating that the presence of the DMPO/⋅OH adduct observed was due to O−⋅2 formation. Figure 6 shows one representative experiment of four, all with similar results.
Detection of eosinophil O−⋅2 production by the DMPO spin trapping technique. (A) EPR spectrum on isolated, unstimulated hepatic granuloma eosinophils (2 × 106 cells/mL) suspended in HBSS in the presence of DTPA (0.1 mmol/L) and DMPO (100 mmol/L) after 25 minutes of culture at 37°C. (B) EPR spectrum 25 minutes after addition of zymosan (1 mg/mL). (C) The same as (B) except in the presence of opsonized zymosan (1 mg/mL). (D) The same as (C) except in the presence of superoxide dismutase (CuZnSOD; 30 U/mL). Results are representative of five separate experiments. The spectra seen in tracings (A) through (C) are those of DMPO/⋅OH (AN = AH = 15.0 G).
Detection of eosinophil O−⋅2 production by the DMPO spin trapping technique. (A) EPR spectrum on isolated, unstimulated hepatic granuloma eosinophils (2 × 106 cells/mL) suspended in HBSS in the presence of DTPA (0.1 mmol/L) and DMPO (100 mmol/L) after 25 minutes of culture at 37°C. (B) EPR spectrum 25 minutes after addition of zymosan (1 mg/mL). (C) The same as (B) except in the presence of opsonized zymosan (1 mg/mL). (D) The same as (C) except in the presence of superoxide dismutase (CuZnSOD; 30 U/mL). Results are representative of five separate experiments. The spectra seen in tracings (A) through (C) are those of DMPO/⋅OH (AN = AH = 15.0 G).
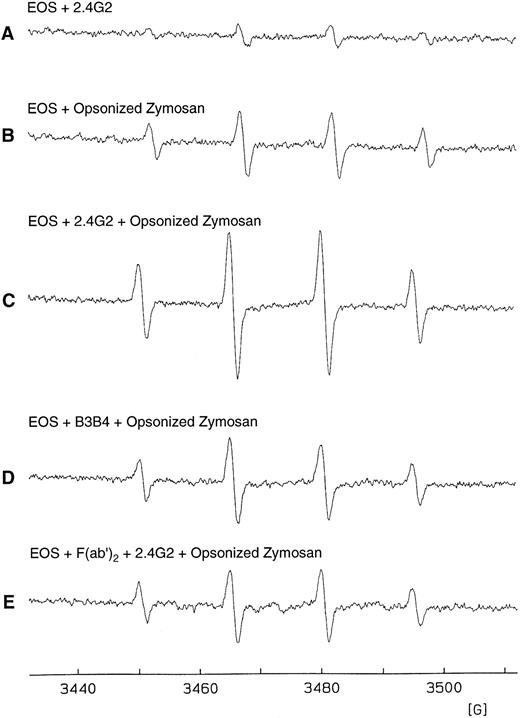
We next addressed whether the Fc-γ receptors on eosinophils could influence the respiratory burst triggered by opsonized zymosan. As shown in Fig 7A, the presence of 2.4G2 at 75 μg and at other concentrations (data not shown) showed only basal levels of superoxide production by the eosinophils. Figure 7C showed that the presence of 2.4G2 and opsonized zymosan enhanced by approximately 70% the magnitude of the superoxide production produced by opsonized zymosan alone (Fig 7B). The effect of 2.4G2 was specific because enhanced superoxide production was not observed when an irrelevant rat IgG antibody was added (B3B4, anti-CD23) to the opsonized zymosan (Fig 7D). Furthermore, the effect of 2.4G2 appears to require cross-linking the FcγR, because enhancement with 2.4G2 was blocked by an excess of the F(ab′)2 of 2.4G2 (Fig 7E). Figure 7 shows one representative experiment of three with similar results.
Production of eosinophil O−⋅2 by triggering Fc-γ receptors using the spin trapping technique. (A) EPR spectrum of isolated hepatic granuloma eosinophils (2 × 106 cells/mL) suspended in HBSS in the presence of DTPA (0.1 mmol/L) and DMPO (100 mmol/L) after incubation for 45 minutes at 37°C with 75 μg of 2.4G2. (B) EPR spectrum after 25 minutes of addition of opsonized zymosan (1 mg/mL). (C) EPR spectrum of purified eosinophils incubated during 20 minutes with 75 μg of 2.4G2 and then added opsonized zymosan (1 mg/mL) for 25 minutes. (D) EPR spectrum of purified eosinophils incubated for 20 minutes with 75 μg of B3B4 followed by the addition of opsonized zymosan (1 mg/mL) for 25 minutes. (E) EPR spectrum of purified eosinophils preincubated with 40 μg of F(ab′)2 fragments of 2.4G2 and then incubated with 2.4G2 and opsonized zymosan in the same conditions as described in (C).
Production of eosinophil O−⋅2 by triggering Fc-γ receptors using the spin trapping technique. (A) EPR spectrum of isolated hepatic granuloma eosinophils (2 × 106 cells/mL) suspended in HBSS in the presence of DTPA (0.1 mmol/L) and DMPO (100 mmol/L) after incubation for 45 minutes at 37°C with 75 μg of 2.4G2. (B) EPR spectrum after 25 minutes of addition of opsonized zymosan (1 mg/mL). (C) EPR spectrum of purified eosinophils incubated during 20 minutes with 75 μg of 2.4G2 and then added opsonized zymosan (1 mg/mL) for 25 minutes. (D) EPR spectrum of purified eosinophils incubated for 20 minutes with 75 μg of B3B4 followed by the addition of opsonized zymosan (1 mg/mL) for 25 minutes. (E) EPR spectrum of purified eosinophils preincubated with 40 μg of F(ab′)2 fragments of 2.4G2 and then incubated with 2.4G2 and opsonized zymosan in the same conditions as described in (C).
DISCUSSION
There is a large body of literature describing the characteristics and functional implications of host T-cell responses to parasitic infections.3 It is well established that Th2 responses generate a pattern of cytokine production that leads to increased IgE antibodies and high numbers of peripheral eosinophils. In humans with S mansoni parasitosis, a prominent Th2 response occurs, and it has been reported that IgE-specific antibodies against S mansoni can bind to IgE receptors on eosinophils and mediate an ADCC reaction.7-10 Whether this occurs in mice has not previously been investigated. In the mouse, an association has been reported between a CD4+/Th2-type response and a poor clinical outcome.2-6,13 14
The present investigations generated three major observations. (1) Murine eosinophils from hepatic granulomas and bone marrow cultures do not bind IgE or express IgE receptors (the high-affinity Fc-ε RI, the low-affinity Fc-ε RII/CD23, and Mac-2/εBP). (2) Murine eosinophils from hepatic granulomas express two isoforms of the low-affinity IgG Fc-receptors, RIII/CD16 and RII/CD32, whereas the majority of eosinophils generated by in vitro culture of normal bone marrow do not express cell surface Fc-γ receptors or, if they do, it is present at levels below our limits of detection. (3) Signaling through Fc-γ receptors on granuloma-derived eosinophils enhances the oxidative burst induced by opsonized zymosan.
Our conclusion that IgE receptors are not expressed on murine eosinophils is based on several types of experiments. Flow cytometric analysis of eosinophils purified from liver granulomas failed to detect IgE binding or the presence of Fc-ε RII/CD23 and Mac-2. These results were corroborated by RT-PCR, which failed to visualize mRNA for Fc-ε RI or Fc-ε RII/CD23. Mac-2 mRNA was present, a result that could be explained by a predominantly cytosolic expression of this protein.35 In vitro cultures with rIL-4 showed that CD23 expression and IgE binding to murine spleen cells increased after incubation with IL-4. This showed that IL-4 could induce IgE receptors on murine B lymphocytes. However, purified hepatic eosinophils and bone marrow-derived eosinophils remained negative for IgE receptor expression (CD23−, IgE binding−, Mac-2−) as tested by flow cytometry and RT-PCR analysis (data not shown). Thus, unlike the human situation, IL-4 does not induce CD23 expression on murine eosinophils.
We were surprised when we found a complete absence of IgE receptors from the granuloma-derived eosinophils, because it has been reported in humans that eosinophils do have IgE receptors and that they can function in IgE-dependent ADCC.7-10 However, there is some disagreement in the literature because there are some reports describing the absence of Fc-ε RI and RII on human eosinophils.36,37 Because the eosinophils were from mice infected with S mansoni, we considered the possibility that the absence of IgE receptors was in some way connected to the disease. We therefore conducted experiments using cells from normal mice. Because eosinophils are uncommon in normal mice, we used a bone marrow culture system17 to generate a source of normal murine eosinophils. Bone marrow cells from normal mice cultured with IL-3, IL-5, and GM-CSF differentiate into eosinophils that express eosinophil peroxidase, a marker of eosinophil differentiation.17 The kinetics of eosinophil development were monitored by (1) EPO activity, (2) the production of MBP transcript, and (3) the number of eosinophils detected by Wright Giemsa staining in cytospin preparations. Flow cytometric analysis of these cells showed no IgE or Fc-γ receptors in the Gr-1+ population, where most of the eosinophils are located. To purify bone marrow-derived eosinophils, sort experiments were performed based on Gr-1 and Fc-γ R expression. The Gr-1+ and 2.4G2− (Fc-γ RII/RIII) cells were sorted and cytospin showed greater than 98% eosinophils. The sorted cells were negative for the Fc-ε RI and Fc-ε RII/CD23 by PCR analysis, but did contain transcripts for Mac-2 and γ-chain. Furthermore, the fact that Mac-2 transcript was detected in hepatic granuloma eosinophils and in bone marrow-derived eosinophils, but that no binding by M3/38 was detected raises two possible explanations: Mac-2 may not be expressed on the cell surface and has a predominant intracellular distribution, or, if it were expressed on the surface, the epitope bound by M3/38 may be altered on the Mac-2 present in murine eosinophils. However, surface expression of Mac-2 is not likely because it did not mediate IgE binding sufficient to detect by flow cytometry.
We failed to detect any IgE binding to murine eosinophils in two strains of mice Balb/c (H-2d) and CBA (H-2k), using hepatic granulomas and bone marrow cultures as sources of murine eosinophils, even after stimulation with rIL-4. Interestingly, in a previous study, murine eosinophils failed to bind IgE in the Toxocara canis model of parasitosis.38 Additionally, it has been shown that ablation of IgE did not affect immunity against Schistosomiasis.5 All of these data lead us to conclude that IgE receptors are not present on mouse eosinophils, a major difference between the rodent and what has been reported for the human immunologic systems. The absence of IgE receptors on murine eosinophils prohibits an IgE-dependent eosinophil ADCC reaction, a mechanism that occurs with human eosinophils and that has been proposed to have a protective function.10 Because mice express IgE receptors on lymphocytes, mast cells, and macrophages, an interesting unsettled issue is the functional significance and the relationship, if any, between the occurrences of eosinophilia and high IgE responses in mice with parasitic infections.
Purified eosinophils from hepatic S mansoni granulomas did express Fc-γ receptors on the surface as detected by flow cytometric studies and did express mRNA encoding Fc-γ RII, RIII, and the associated γ-chain, showing that murine eosinophils have the receptor elements necessary for IgG ADCC responses. The expression of Fc-γ receptors, especially Fc-γ R III and the associated γ-chain, in the absence of experimental stimulation, agrees with previous findings in peritoneal eosinophils from mice infected with Mesocestoides corti, in which both of these transcripts were detected without any in vitro stimulation.39 The significance of the finding that Fc-γ RIII/CD16 is present on murine eosinophils is that, in contrast with Fc-γ RII/CD32, it is well established that activation signals can be transmitted through Fc-γ RIII/CD16. An interesting and highly reproducible finding in our studies is that bone marrow-derived eosinophils did not bind to 2.4G2 and that only transcripts for the associated γ-chain were detected in these cells, implying that the expression of Fc-γ receptors on murine eosinophils is an inducible event. Interestingly, it has been reported that IFN-γ induces the expression of Fc-γ R III/CD16 on human peripheral blood eosinophils.40
A surprising finding was that the cells with the highest level of Gr-1, corresponding by cytospin examination with the eosinophil population, did not express Fc-γ receptors. However, among the cells expressing lower levels of Gr-1, Fc-γ receptors were detected. This population consists of a mixture of eosinophils and other cells. Because there is no MoAb specific for murine eosinophils, it is not possible to determine by FACS analysis whether any of these eosinophils do or do not express Fc-γ receptors. Thus, in cultures from normal mice it is possible that some bone marrow-derived eosinophils express Fc-γ receptors, but it is clear that the vast majority of bone marrow-derived eosinophils do not express Fc-γ receptors.
Because the murine eosinophils differed from human eosinophils in IgE receptor expression, we sought to determine if other elements of eosinophil cytotoxic activity also were different. Eosinophils and other inflammatory cells have the ability to release cytotoxic proteins and oxygen metabolites involved in the immune response against parasites and this also occurs in the hyperresponsiveness of airway smooth muscle in asthmatic processes. Human eosinophils generate O−⋅2 when activated with phorbol esters or opsonized particles.41-43 In our studies, purified hepatic granuloma eosinophils generated O−⋅2 in the complete absence of expression of Fc-ε RI and RII/CD23, implying that the oxidative burst is functional. This raised the possibility that Fc-γ receptors might be important because opsonized zymosan activation of O−⋅2 formation in phagocytic cells is known to involve aggregation and phagocytosis of Fc-γ receptors and the complement receptor for C3b (CR1).41,42 In our studies, complement was inactivated by incubation of serum at 56°C for 30 minutes, which did not influence our results, a finding that indicates complement does not play a role in the enhancement mechanism. Support for a role for Fc-γ receptors comes from our finding that, when the oxidative burst was induced with opsonized zymosan, the production of O−⋅2 can be enhanced by ligation of Fc-γ receptors by 2.4G2. Because, under the conditions we tested, 2.4G2 alone was not capable of inducing a respiratory burst, we infer that the Fc-γ receptors act in concert with other participants in the respiratory burst pathway to regulate the level of activity. In humans, it has been reported that CD32 and C3R act synergistically in effecting cytokine release but not in the activation of the respiratory burst.33 Perhaps the constitutive presence of Fc-γ RIII/CD16 may contribute to the activation of the respiratory burst on murine eosinophils.
These major differences have implications for the use of the mouse as a model for atopic disorders. Many human atopic pathologies are characterized by specific IgE antibodies and a prominent tissue eosinophilia. Because human eosinophils have been reported to express IgE receptors, it is generally assumed that an IgE-receptor-dependent element contributes to the pathology of the atopic disorder. Perhaps the absence of IgE receptors from murine eosinophils is one of the reasons that the mouse has historically not been a useful model for many allergic reactions.
ACKNOWLEDGMENT
The authors are grateful to T. Duling and J. Fishbaugh for their expert technical assistance in the flow cytometric studies and to Dr Garry P. Buettner for helping with the spin trapping experiments.
Supported in part by Grants No. DK38327, DK07663, and DK02428 from the National Institutes of Health.
Address reprint requests to Belen de Andres, PhD, University of Iowa College of Medicine, Department of Pathology, MRC 375, Iowa City, IA 52242.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal