Abstract
The recognition of several new types of non-Hodgkin's lymphoma (NHL) in recent years has led to proposals for changing lymphoma classifications, including a new proposal put forth by the International Lymphoma Study Group (ILSG). However, the clinical significance of the new entities and the practical utility of this new proposal have not been studied. Therefore, we performed a clinical evaluation of the ILSG classification. A cohort of 1,403 cases of NHL was organized at nine study sites around the world and consisted of consecutive patients seen between 1988 and 1990 who were previously untreated. A detailed protocol for histologic and clinical analysis was followed at each site, and immunologic characterization as to T- or B-cell phenotype was required. Five expert hematopathologists visited the sites and each classified each case using the ILSG classification. A consensus diagnosis was also reached in each case, and each expert rereviewed a 20% random sample of the cases. Clinical correlations and survival analyses were then performed. A diagnosis of NHL was confirmed in 1,378 (98.2%) of the cases. The most common lymphoma types were diffuse large B-cell lymphoma (31%) and follicular lymphoma (22%), whereas the new entities comprised 21% of the cases. Diagnostic accuracy was at least 85% for most of the major lymphoma types, and reproducibility of the diagnosis was 85%. Immunophenotyping improved the diagnostic accuracy by 10% to 45% for a number of the major types. The clinical features of the new entities were distinctive. Both the histologic types and the patient characteristics as defined by the International Prognostic Index predicted for patient survival. In conclusion we found that the ILSG classification can be readily applied and identifies clinically distinctive types of NHL. However, for clinical application, prognostic factors as defined by the International Prognostic Index must be combined with the histologic diagnosis for appropriate clinical decisions.
BECAUSE OF the increasing incidence of non-Hodgkin's lymphoma (NHL), with approximately 53,000 new cases occurring annually in the United States,1,2 the diagnosis and classification of these disorders is an increasingly important clinical issue. The classification of NHL has evolved steadily throughout the twentieth century. An early system proposed by Gall and Mallory3 used the terms giant follicular lymphoma, lymphosarcoma, and reticulum cell sarcoma, but proved too imprecise for clinical application. In the 1950s, Rappaport et al4 recognized the importance of the growth pattern in some types of NHL and used pattern, in addition to cell size and shape, as the basis of a new and clinically relevant classification. In the 1970s, recognition that NHLs were tumors of the immune system and were derived from T or B cells led to the immunologically based classifications of Lukes and Collins,5 and later Lennert and associates (Kiel classification).6-8 In an attempt to unify terminology and improve the effectiveness of communication between pathologists and clinicians, the Working Formulation was proposed in 1982.9 Over the next two decades, however, the Kiel classification dominated clinical practice in Europe, whereas the Working Formulation became the main classification system used in North America.
In the last two decades, increased understanding of the immune system and the genetic abnormalities associated with NHL have led to the identification of several previously unrecognized types of lymphoma. These include mantle cell lymphoma,10-20 monocytoid B-cell lymphoma,21-28 extranodal lymphoma of mucosa-associated lymphoid tissue (MALT),16,29-34 splenic marginal zone lymphoma,35-38 primary mediastinal large B-cell lymphoma,39-47 and a variety of T-cell lymphomas,48-73 including anaplastic large cell lymphoma.74-83 The recognition of these new and supposedly clinically relevant types of NHL has led to proposals for changing lymphoma classifications. Modifications of the existing classifications, and a new proposal by the International Lymphoma Study Group (ILSG)84 incorporating some aspects of the Kiel classification and Working Formulation, have been put forward. However, the clinical significance of the new lymphoma entities and the practical utility and clinical relevance of this new proposal needed to be tested.
The histologic diagnosis of specific subtypes of NHL is widely believed to be imprecise. Previous studies have identified high rates of diagnostic discrepancy between different pathologists (interobserver variability) and for the same pathologist (reproducibility) when reviewing the same case at different times.85-88 This inaccuracy in diagnosis has made treatment decisions difficult. In the past two decades, the widespread use of immunophenotyping has led to increased insight into the diagnosis and classification of tumors of the immune system. However, the value of immunophenotyping in the day-to-day practice of lymphoma diagnosis and clinical care has not been clearly shown.
With this background, we set out to perform a retrospective clinical evaluation of the newly proposed ILSG classification.84 Although the ILSG classification is a proposal for classifying all lymphoid neoplasms (Table 1), our study was designed to only assess the ILSG classification of NHL. Specific goals of our study were the following: (1) to evaluate the ability of hematopathologists to apply the ILSG classification to a retrospective group of cases collected at sites around the world; (2) to determine the role of immunophenotyping and clinical data in the diagnosis of the various entities; (3) to determine the clinical importance of immunophenotyping; (4) to determine the intraobserver and interobserver reproducibility of diagnosis of the various entities; (5) to determine clinical correlations for the various entities, including clinical features at presentation and survival outcomes; and (6) to determine whether certain entities can be grouped for prognostic or therapeutic purposes.
International Lymphoma Study Group Classification (including provisional categories)
| B-Cell Lymphoma . | T/NK-Cell Lymphoma . | Others . |
|---|---|---|
| Precursor B-lymphoblastic | Precursor T-lymphoblastic | Composite lymphoma (types specified)† |
| Small lymphocytic (CLL) | T-cell chronic lymphocytic leukemia | Malignant lymphoma, unclassifiable low grade |
| Lymphoplasmacytic | Large granular lymphocyte leukemia | Malignant lymphoma unclassifiable high grade |
| Mantle cell | Mycosis fungoides | Malignant lymphoma, unclassifiable |
| Follicle center, follicular | Peripheral T cell, unspecified | |
| Grade 1* | Medium-sized | Hodgkin's disease |
| Grade 2* | Mixed medium and large cell | Diagnosis other than lymphoma |
| Grade 3* | Large cell | Case unclassifiable |
| Follicle center diffuse, small cell | Lymphoepithelioid | |
| Marginal zone B-cell, MALT type | Hepatosplenic | |
| Marginal zone B-cell, nodal | Subcutaneous panniculitic | |
| Marginal zone B-cell, splenic | Angioimmunoblastic | |
| Hairy cell leukemia | Angiocentric, nasal | |
| Plasmacytoma | Intestinal | |
| Diffuse large B-cell | Adult T-cell lymphoma/leukemia | |
| Diffuse mediastinal large B-cell | Anaplastic large cell (including null phenotype) | |
| Burkitt's | Anaplastic large cell, Hodgkin's-like | |
| High grade B-cell, Burkitt-like | Unclassifiable low grade | |
| Unclassifiable low grade | Unclassifiable high grade | |
| Unclassifiable high grade |
| B-Cell Lymphoma . | T/NK-Cell Lymphoma . | Others . |
|---|---|---|
| Precursor B-lymphoblastic | Precursor T-lymphoblastic | Composite lymphoma (types specified)† |
| Small lymphocytic (CLL) | T-cell chronic lymphocytic leukemia | Malignant lymphoma, unclassifiable low grade |
| Lymphoplasmacytic | Large granular lymphocyte leukemia | Malignant lymphoma unclassifiable high grade |
| Mantle cell | Mycosis fungoides | Malignant lymphoma, unclassifiable |
| Follicle center, follicular | Peripheral T cell, unspecified | |
| Grade 1* | Medium-sized | Hodgkin's disease |
| Grade 2* | Mixed medium and large cell | Diagnosis other than lymphoma |
| Grade 3* | Large cell | Case unclassifiable |
| Follicle center diffuse, small cell | Lymphoepithelioid | |
| Marginal zone B-cell, MALT type | Hepatosplenic | |
| Marginal zone B-cell, nodal | Subcutaneous panniculitic | |
| Marginal zone B-cell, splenic | Angioimmunoblastic | |
| Hairy cell leukemia | Angiocentric, nasal | |
| Plasmacytoma | Intestinal | |
| Diffuse large B-cell | Adult T-cell lymphoma/leukemia | |
| Diffuse mediastinal large B-cell | Anaplastic large cell (including null phenotype) | |
| Burkitt's | Anaplastic large cell, Hodgkin's-like | |
| High grade B-cell, Burkitt-like | Unclassifiable low grade | |
| Unclassifiable low grade | Unclassifiable high grade | |
| Unclassifiable high grade |
Provisional categories are indicated in italic type.
Abbreviations: CLL, chronic lymphocytic leukemia; MALT, mucosal-associated lymphoid tissue.
Follicular lymphomas are designated as such and were graded according to the Berard method.90
Composite lymphomas consisted of two distinctly different cytologic subtypes of lymphoma.
Data from Harris et al.84
PATIENTS AND METHODS
Nine institutions in eight countries were chosen to provide up to 200 consecutive cases of previously untreated NHL that were representative of the geographic region during the time between January 1, 1988 and December 31, 1990. The first 200 cases at each site that fulfilled the following criteria were selected for the study. In all cases, tissue biopsy samples that were adequate for diagnosis and classification were required, and all diagnostic pathology materials obtained before initial therapy, including positive bone marrow (BM) specimens, were included in the pathology review. Immunologic characterization as to B- or T-cell origin, by whatever means in use at the institution, was also required in all cases. Leukemias were excluded from the study unless a tissue biopsy, other than BM, was performed before therapy. Clinical characteristics, treatment data, and some follow-up information were also required in all cases. The nine study sites, which provided a total of 1,403 cases, are shown in Table 2.
Number of Cases by Study Site
| Site . | Cases . |
|---|---|
| Omaha, NE | 200 |
| Vancouver, Canada | 202 |
| Cape Town, South Africa | 196 |
| London, UK | 120 |
| Locarno, Switzerland | 80 |
| Lyon, France | 195 |
| Würzburg/Göttingen, Germany | 210 |
| Hong Kong | 200 |
| Site . | Cases . |
|---|---|
| Omaha, NE | 200 |
| Vancouver, Canada | 202 |
| Cape Town, South Africa | 196 |
| London, UK | 120 |
| Locarno, Switzerland | 80 |
| Lyon, France | 195 |
| Würzburg/Göttingen, Germany | 210 |
| Hong Kong | 200 |
The clinical information for each case was abstracted from the medical record by a clinician or data manager and recorded on a standardized form for direct computerized data entry. These data included coded patient and site identifiers; patient sex, ethnic origin, and date of birth; the date and site of the diagnostic biopsy; and a tabulation of nodal and extranodal sites of involvement and Ann Arbor stage at the time of initial diagnosis. Laboratory data were recorded, including the serum lactate dehydrogenase level, absolute lymphocyte count, presence of circulating lymphoma cells, presence of a monoclonal serum Ig, and a history of immunodeficiency and viral (human T-cell leukemia virus-1 [HTLV-1], human immunodeficiency virus [HIV]) status. Also recorded were the performance status and maximum diameter of the largest tumor mass. The initial therapy and therapeutic response, details of remission, progression, or relapse, and subsequent therapies and follow-up were tabulated in each case. For this report, all cases with clinical data were included regardless of the specific therapies given. In 73 of the cases, sufficient data was not available for the clinical and survival analyses.
At each institution, the pathology slides and reports for each case were carefully reviewed by a designated site pathologist. The original stained slides and immunostains were organized for review, and additional sections, immunostains, and other studies were performed if deemed necessary by the site pathologist. The results of the immunologic studies for each case, as well as any available cytogenetic or molecular genetic data, were recorded on a standardized form for direct computerized data entry. Five expert hematopathologists then traveled as a group to each of the nine sites to review and classify each case in each of the three major classifications.9,84,89 The site visits occurred over a period of 8 months beginning in June 1995. All expert pathologists used a standard Nikon Labophot-2 microscope (Nikon, Inc, Melville, NY), including a 10× plan achromat objective (high-power field = 0.159 mm2). The diagnostic categories in each of the three classifications were used according to published criteria.9,84,89 More specific criteria were developed for some of the entities with Nancy L. Harris providing consultation regarding the ILSG classification. The criteria of Mann and Berard90 were used to grade follicular lymphoma in the ILSG classification.
At each site, the diagnostic slides were reviewed and classified independently by each expert hematopathologist. The initial classification was based on examination of the hematoxylin-eosin and/or Giemsa stained slides with only the following clinical information from the time of initial diagnosis: patient age and sex, site of the biopsy, and the major site of disease (ie, diagnosis 1). After recording a diagnosis in each classification, the expert was then presented with the immunophenotypic profile, along with any available cytogenetic and molecular genetic data, and the immunostains and/or flow cytometry report. After review, a second diagnosis was rendered in each classification (ie, diagnosis 2). Then, the expert was presented with all of the pretreatment clinical information and a third diagnosis was made in each classification (ie, diagnosis 3). No previous diagnosis could be changed based on information subsequently revealed. If a case was considered unclassifiable in any of the classifications, the expert was required to give a reason, ie, inadequate material, poor slide preparation, additional phenotyping needed, additional information needed, or other reasons. The expert was allowed to change the phenotype of a case if he interpreted the immunostains and/or phenotype data differently than the site pathologist. For some diagnostic categories, a research protocol was also completed by the expert pathologists. All of this information was recorded on standardized forms for direct computerized data entry. Approximately 40 to 50 cases were reviewed by each pathologist each day.
In addition to the independent diagnoses rendered by each of the expert pathologists, a consensus diagnosis was also reached in each case. A consensus was considered to have been reached if at least four of the five expert pathologists agreed on the third diagnosis (diagnosis 3) in the ILSG classification. A diagnosis of follicular lymphoma of any grade was considered an agreement, and a diagnosis of peripheral T-cell lymphoma of any type was also considered an agreement. In these latter two categories, agreement by three of the five expert pathologists with regard to the specific type was considered the consensus diagnosis; if there was no agreement with regard to the type, the case was arbitrated by D. Weisenburger based on the individual diagnoses and the research protocol. All other cases without a consensus diagnosis were jointly reviewed on a multi-headed microscope and discussed by the five expert pathologists and the site pathologist in a consensus conference at the end of each day, and an attempt was made to reach a consensus of at least four expert pathologists in each case. If additional sections, immunostains, molecular studies, or other information was required, a diagnostic algorithm was developed by the group and the additional materials were obtained, if possible, and reviewed at a subsequent consensus conference at the site. If the additional materials could not be obtained during the site visit, the required materials and information were subsequently sent to D. Weisenburger who arbitrated the case based on the algorithm.
At the end of each site visit, after all cases had been reviewed, each expert pathologist rereviewed 20% of the cases. The cases for rereview were randomly selected by the statisticians. These cases were classified a second time by each expert, without knowledge of his initial interpretation, using all available pathology materials and pretreatment clinical information. Cases in which a consensus diagnosis had not yet been reached were excluded from the rereview.
Completed clinical and pathology forms were reviewed and edited to detect any inconsistencies, and additional information and/or clarification was obtained when needed. After completion of the editing, the clinical and pathology data forms were entered into a computer for data analysis. The International Prognostic Index91 was used to stratify patients within the various disease entities. Treatment outcome was measured using failure-free survival and overall survival. Failure-free survival was defined as the time from diagnosis to the first occurrence of progression, relapse after response or death from any cause. Follow-up of patients not experiencing one of these events was censored at their date of last contact. Overall survival was measured from diagnosis to death from any cause, with surviving patient follow-up censored at the last contact date. Estimates of failure-free survival and overall survival distribution were calculated using the method of Kaplan and Meier.92 Time to event distributions were compared using the log-rank test.93
RESULTS
Twenty-five of the 1,403 cases (1.8%) were found to have a diagnosis other than NHL and, thus, were excluded from further analysis. The types of NHL found in the remaining 1,378 cases are presented in Table 3. Approximately 31% of the cases were forms of diffuse large B-cell lymphoma and approximately 22% of the cases were types of follicular lymphoma. All types of T-cell processes, including natural killer (NK) cell disorders, made up only 12% of the cases. Small lymphocytic lymphoma was observed in 6.7% of the cases, a higher percentage than is sometimes appreciated. The major newly recognized types of lymphoma that occurred most frequently were marginal zone B-cell lymphoma of MALT type (7.6%), mantle cell lymphoma (6.0%), primary mediastinal large B-cell lymphoma (2.4%), and anaplastic large T/null-cell lymphoma (2.4%). Only 2.8% of the 1,378 cases of NHL could not be specifically classified using this system, usually because of technical factors.
Distribution of NHL Cases by the Consensus Diagnosis
| Consensus Diagnosis . | No. of Cases . | % of Total Cases . |
|---|---|---|
| Diffuse large B-cell | 422 | 30.6 |
| Follicular | 304 | 22.1 |
| Grade 1 | 131 | 9.5 |
| Grade 2 | 85 | 6.2 |
| Grade 3 | 88 | 6.4 |
| Marginal zone B-cell, MALT | 105 | 7.6 |
| Peripheral T-cell | 96 | 7.0 |
| Medium-sized, mixed, and large | 51 | 3.7 |
| Angiocentric, nasal | 19 | 1.4 |
| Angioimmunoblastic | 17 | 1.2 |
| Intestinal | 5 | <1 |
| Lymphoepithelioid | 2 | <1 |
| Hepatosplenic | 1 | <1 |
| Adult T-cell leukemia/lymphoma | 1 | <1 |
| Small B-lymphocytic (CLL) | 93 | 6.7 |
| Mantle cell | 83 | 6.0 |
| Primary mediastinal large B-cell | 33 | 2.4 |
| Anaplastic large T/null-cell | 33 | 2.4 |
| High grade B-cell, Burkitt-like | 29 | 2.1 |
| Marginal zone B-cell, nodal | 25 | 1.8 |
| Precursor T-lymphoblastic | 23 | 1.7 |
| Lymphoplasmacytoid | 16 | 1.2 |
| Marginal zone B-cell, splenic | 11 | <1 |
| Mycosis fungoides | 11 | <1 |
| Burkitt's | 10 | <1 |
| All other types | 84 | 6.1 |
| Consensus Diagnosis . | No. of Cases . | % of Total Cases . |
|---|---|---|
| Diffuse large B-cell | 422 | 30.6 |
| Follicular | 304 | 22.1 |
| Grade 1 | 131 | 9.5 |
| Grade 2 | 85 | 6.2 |
| Grade 3 | 88 | 6.4 |
| Marginal zone B-cell, MALT | 105 | 7.6 |
| Peripheral T-cell | 96 | 7.0 |
| Medium-sized, mixed, and large | 51 | 3.7 |
| Angiocentric, nasal | 19 | 1.4 |
| Angioimmunoblastic | 17 | 1.2 |
| Intestinal | 5 | <1 |
| Lymphoepithelioid | 2 | <1 |
| Hepatosplenic | 1 | <1 |
| Adult T-cell leukemia/lymphoma | 1 | <1 |
| Small B-lymphocytic (CLL) | 93 | 6.7 |
| Mantle cell | 83 | 6.0 |
| Primary mediastinal large B-cell | 33 | 2.4 |
| Anaplastic large T/null-cell | 33 | 2.4 |
| High grade B-cell, Burkitt-like | 29 | 2.1 |
| Marginal zone B-cell, nodal | 25 | 1.8 |
| Precursor T-lymphoblastic | 23 | 1.7 |
| Lymphoplasmacytoid | 16 | 1.2 |
| Marginal zone B-cell, splenic | 11 | <1 |
| Mycosis fungoides | 11 | <1 |
| Burkitt's | 10 | <1 |
| All other types | 84 | 6.1 |
Abbreviation: CLL, chronic lymphocytic leukemia.
Three diagnoses were made by each expert pathologist in each case: one based on only histology, the second based on histology and immunophenotype data, and the third based on a combination of histology, immunophenotype, and clinical data. In Table 4, the percentages of the review diagnoses that agreed with the consensus diagnosis are given for each major histologic type. For most of the histologic types, the percentage of third review diagnoses (using all available data) that agreed with the consensus diagnosis equaled or exceeded 85%. The percent agreement was only 53% for high-grade B-cell Burkitt-like tumors, where distinctions between Burkitt's lymphoma and diffuse large B-cell lymphoma often proved difficult. The percent agreement was also below 85% for lymphoplasmacytoid lymphoma and nodal marginal zone B-cell lymphoma, also due at least in part to the imprecise definitions of these entities. Whereas the accuracy of diagnosis of follicular lymphoma was 94%, the percent agreement for the various grades of the follicular lymphoma was only 61% to 73%. However, the agreement in follicular lymphoma, grade 3, increased to 74% if cases with a diffuse component were also considered as an agreement.
Expert Pathologist Agreement With the Consensus Diagnosis
| Consensus Diagnosis . | Dx 14-150 (%) . | Δ Dx 2-1 (%) . | Dx 24-151 (%) . | Δ Dx 3-2 (%) . | Dx 3‡ (%) . |
|---|---|---|---|---|---|
| Follicular, any grade | 93 | 1 | 94 | 0 | 94 |
| Follicular, grade 1 | 72 | 1 | 73 | 0 | 73 |
| Follicular, grade 2 | 61 | 0 | 61 | 0 | 61 |
| Follicular, grade 3 | 60 | 1 | 61 | 0 | 61 |
| Marginal zone B-cell, MALT | 84 | 2 | 86 | 0 | 86 |
| Small lymphocytic (CLL) | 84 | 3 | 87 | 0 | 87 |
| Lymphoplasmacytoid | 53 | 3 | 56 | 0 | 56 |
| High grade B-cell, Burkitt-like | 47 | 6 | 53 | 0 | 53 |
| Primary mediastinal large B-cell | 51 | 7 | 58 | 37 | 85 |
| Marginal zone B-cell, nodal | 55 | 8 | 63 | 0 | 63 |
| Mantle cell | 77 | 10 | 87 | 0 | 87 |
| Diffuse large B-cell | 73 | 14 | 87 | 0 | 87 |
| Precursor T-lymphoblastic | 52 | 35 | 87 | 2 | 89 |
| Anaplastic large T/null-cell | 46 | 39 | 85 | 0 | 85 |
| Peripheral T-cell, all types | 41 | 45 | 86 | 0 | 86 |
| Consensus Diagnosis . | Dx 14-150 (%) . | Δ Dx 2-1 (%) . | Dx 24-151 (%) . | Δ Dx 3-2 (%) . | Dx 3‡ (%) . |
|---|---|---|---|---|---|
| Follicular, any grade | 93 | 1 | 94 | 0 | 94 |
| Follicular, grade 1 | 72 | 1 | 73 | 0 | 73 |
| Follicular, grade 2 | 61 | 0 | 61 | 0 | 61 |
| Follicular, grade 3 | 60 | 1 | 61 | 0 | 61 |
| Marginal zone B-cell, MALT | 84 | 2 | 86 | 0 | 86 |
| Small lymphocytic (CLL) | 84 | 3 | 87 | 0 | 87 |
| Lymphoplasmacytoid | 53 | 3 | 56 | 0 | 56 |
| High grade B-cell, Burkitt-like | 47 | 6 | 53 | 0 | 53 |
| Primary mediastinal large B-cell | 51 | 7 | 58 | 37 | 85 |
| Marginal zone B-cell, nodal | 55 | 8 | 63 | 0 | 63 |
| Mantle cell | 77 | 10 | 87 | 0 | 87 |
| Diffuse large B-cell | 73 | 14 | 87 | 0 | 87 |
| Precursor T-lymphoblastic | 52 | 35 | 87 | 2 | 89 |
| Anaplastic large T/null-cell | 46 | 39 | 85 | 0 | 85 |
| Peripheral T-cell, all types | 41 | 45 | 86 | 0 | 86 |
Abbreviation: CLL, chronic lymphocytic leukemia.
Diagnosis 1 based only on histology.
Diagnosis 2 based on histology and immunophenotype.
Diagnosis 3 based on histology, immunophenotype and clinical data.
The usefulness of immunophenotyping in making the correct diagnosis was dependent on the specific disease (Table 4). For some lymphomas, such as follicular lymphoma, marginal zone B-cell lymphoma of MALT type, and the small lymphocytic and lymphoplasmacytoid lymphomas, information on the immunophenotype did not increase the diagnostic accuracy significantly. However, for the mantle cell, diffuse large B-cell, and the T-cell lymphomas, immunophenotyping was helpful in many cases in reaching the correct diagnosis and improved the diagnostic accuracy by some 10% to 45%. For many of these cases, the initial diagnosis based on histology only was unclassifiable malignant lymphoma. Immunophenotyping allowed the classification of such cases into specific categories. Detailed clinical data was helpful only in distinguishing primary mediastinal large B-cell lymphoma from the other diffuse large B-cell lymphomas, because there were no characteristic histologic or immunologic differences between these two categories.
The expert pathologists' rereview of a 20% sample of the cases at each site showed that they could reproducibly make a diagnosis of NHL (Table 5). Overall, the rereview diagnosis agreed exactly with the pathologist's initial diagnosis 3 or the consensus diagnosis (including the grading of follicular lymphoma) in 85% of the cases (range, 82% to 89%). Because the consensus diagnosis for all of these cases was reached before the time of the rereview, the consensus process may have influenced the assessment of some cases at rereview. Therefore, the pathologists were allowed to agree with either their original diagnosis 3 or the consensus diagnosis at the time of rereview. For an additional 9% of the cases, the rereview diagnosis was nearly the same as the original diagnosis (ie, follicular, grade 1, v follicular, grade 2; or, follicular, grade 3, v follicular, grade 3, plus diffuse large B-cell). Thus, for 94% of the cases rereviewed (range, 92% to 97%), the expert pathologists made a diagnosis consistent with either their original diagnosis 3 or the consensus diagnosis. In only 6% of the cases (range, 3% to 8%) the pathologist's rereview diagnosis would likely have led to a different approach to therapy than the original diagnosis.
Pathologist Agreement Upon Rereview of 20% of the Cases
| . | Dx 3/Consensus5-150 (%) . | Near-miss5-151 (%) . | None (%) . |
|---|---|---|---|
| Overall agreement | 85 | 94 | 6 |
| Expert pathologist | |||
| A | 89 | 97 | 3 |
| B | 87 | 96 | 4 |
| C | 85 | 93 | 7 |
| D | 82 | 93 | 7 |
| E | 82 | 92 | 8 |
| . | Dx 3/Consensus5-150 (%) . | Near-miss5-151 (%) . | None (%) . |
|---|---|---|---|
| Overall agreement | 85 | 94 | 6 |
| Expert pathologist | |||
| A | 89 | 97 | 3 |
| B | 87 | 96 | 4 |
| C | 85 | 93 | 7 |
| D | 82 | 93 | 7 |
| E | 82 | 92 | 8 |
Agreement with either diagnosis 3 or the consensus diagnosis.
Agreement including near-miss diagnoses (see text for explanation).
The clinical characteristics of the more common types of lymphoma are presented in Table 6. It is important to recognize that, although the average results vary between the various types, there was considerable overlap between the types for any particular characteristic. The newly recognized types of lymphoma appear to be distinctive. Marginal zone B-cell lymphoma of MALT type was characterized by a high frequency of localized extranodal disease and a prolonged survival, whereas nodal marginal zone (monocytoid) B-cell lymphoma more often presented with advanced-stage disease and had a worse survival. Mantle cell lymphoma had a striking male predominance, a high frequency of advanced-stage disease with marrow involvement, and the lowest 5-year survival of any type of lymphoma. Primary mediastinal large B-cell lymphoma occurred more frequently in young females and was often of low stage, but the survival was no different from that of other diffuse large B-cell lymphomas. Anaplastic large T/null-cell lymphoma occurred mainly in young patients and had a surprisingly high 5-year survival when compared to other lymphomas with large cell histology or a T-cell phenotype. This was not due to inclusion of a high proportion of patients with only skin involvement, a group that represented just 6% of these patients.
Patient Characteristics by Histologic Type
| Consensus Diagnosis . | % Male . | Median Age . | % Stage 1 or 2 . | % Marrow Positive . | % PI 0/1 . | % PI 4/5 . | % 5-yr OAS . | % 5-yr FFS . |
|---|---|---|---|---|---|---|---|---|
| Follicular, all grades | 42 | 59 | 33 | 42 | 39 | 6 | 72 | 40 |
| Mantle cell | 74 | 63 | 19 | 63 | 19 | 19 | 27 | 11 |
| Marginal zone B-cell, MALT | 45 | 61 | 66 | 14 | 38 | 5 | 74 | 60 |
| Marginal zone B-cell, nodal | 41 | 58 | 18 | 41 | 36 | 9 | 57 | 29 |
| Small lymphocytic (CLL) | 53 | 65 | 6 | 73 | 17 | 10 | 51 | 25 |
| Lymphoplasmacytoid | 53 | 63 | 20 | 73 | 20 | 13 | 59 | 25 |
| Diffuse large B-cell | 55 | 64 | 51 | 17 | 31 | 16 | 46 | 41 |
| Primary mediastinal large B-cell | 34 | 37 | 66 | 3 | 44 | 9 | 50 | 48 |
| Burkitt's | 89 | 31 | 56 | 33 | 44 | 22 | 44 | 44 |
| High-grade B-cell, Burkitt-like | 59 | 55 | 50 | 21 | 25 | 18 | 47 | 43 |
| Precursor T-lymphoblastic | 74 | 25 | 13 | 43 | 35 | 22 | 26 | 24 |
| Peripheral T-cell, all types | 56 | 61 | 18 | 37 | 14 | 27 | 25 | 18 |
| Anaplastic large T/null-cell | 69 | 33 | 50 | 12 | 50 | 19 | 77 | 58 |
| Consensus Diagnosis . | % Male . | Median Age . | % Stage 1 or 2 . | % Marrow Positive . | % PI 0/1 . | % PI 4/5 . | % 5-yr OAS . | % 5-yr FFS . |
|---|---|---|---|---|---|---|---|---|
| Follicular, all grades | 42 | 59 | 33 | 42 | 39 | 6 | 72 | 40 |
| Mantle cell | 74 | 63 | 19 | 63 | 19 | 19 | 27 | 11 |
| Marginal zone B-cell, MALT | 45 | 61 | 66 | 14 | 38 | 5 | 74 | 60 |
| Marginal zone B-cell, nodal | 41 | 58 | 18 | 41 | 36 | 9 | 57 | 29 |
| Small lymphocytic (CLL) | 53 | 65 | 6 | 73 | 17 | 10 | 51 | 25 |
| Lymphoplasmacytoid | 53 | 63 | 20 | 73 | 20 | 13 | 59 | 25 |
| Diffuse large B-cell | 55 | 64 | 51 | 17 | 31 | 16 | 46 | 41 |
| Primary mediastinal large B-cell | 34 | 37 | 66 | 3 | 44 | 9 | 50 | 48 |
| Burkitt's | 89 | 31 | 56 | 33 | 44 | 22 | 44 | 44 |
| High-grade B-cell, Burkitt-like | 59 | 55 | 50 | 21 | 25 | 18 | 47 | 43 |
| Precursor T-lymphoblastic | 74 | 25 | 13 | 43 | 35 | 22 | 26 | 24 |
| Peripheral T-cell, all types | 56 | 61 | 18 | 37 | 14 | 27 | 25 | 18 |
| Anaplastic large T/null-cell | 69 | 33 | 50 | 12 | 50 | 19 | 77 | 58 |
Abbreviations: PI, International Prognostic Index; OAS, overall survival; FFS, failure-free survival; CLL, chronic lymphocytic leukemia.
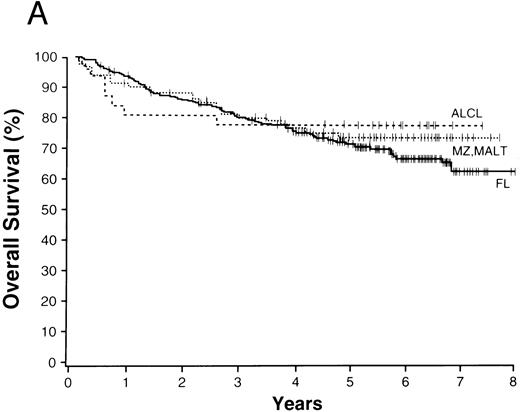
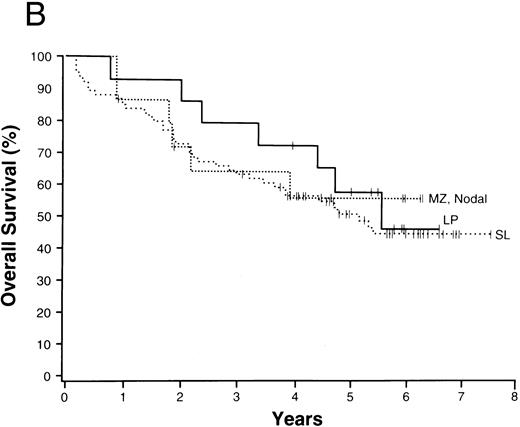
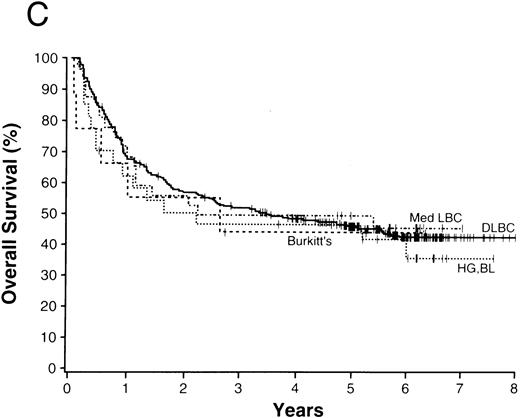
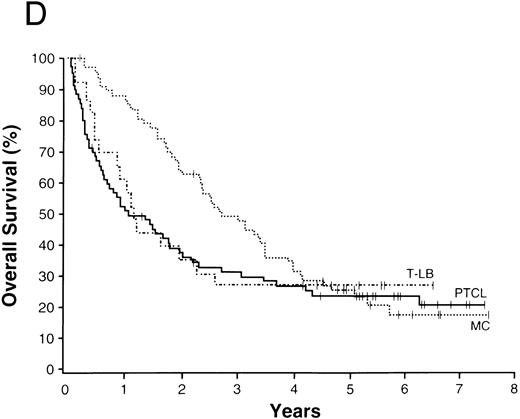
The average overall survival by histologic type allowed for division of the NHLs into four broad groupings (Fig 1). Those with a 5-year overall survival of greater than 70% included follicular lymphoma, marginal zone B-cell lymphoma of MALT type, and anaplastic large T/null-cell lymphoma. Lymphomas within a 50% to 70% 5-year overall survival included the small lymphocytic, lymphoplasmacytoid, and nodal marginal zone B-cell lymphomas. Lymphomas with a 30% to 49% 5-year overall survival included diffuse large B-cell lymphoma, primary mediastinal large B-cell lymphoma, and the high-grade B-cell Burkitt-like and Burkitt lymphomas. Lymphomas with less than a 30% 5-year overall survival included peripheral T-cell lymphoma, precursor T-lymphoblastic lymphoma, and mantle cell lymphoma.
NHLs with a 5-year overall survival of greater than 70% (A), 50% to 70% (B), 30% to 49% (C), and less than 30% (D); ALCL, anaplastic large T/null-cell lymphoma; MZ, MALT, marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue; FL, follicular lymphoma; MZ, nodal, marginal zone B-cell lymphoma of nodal type; LP, lymphoplasmacytoid lymphoma; SL, small lymphocytic lymphoma; Med LBC, primary mediastinal large B-cell lymphoma; DLCBL, diffuse large B-cell lymphoma; HG, BL, high-grade B-cell Burkitt-like lymphoma; T-LB, precursor T-lymphoblastic lymphoma; PTCL, peripheral T-cell lymphoma; MC, mantle cell lymphoma.
NHLs with a 5-year overall survival of greater than 70% (A), 50% to 70% (B), 30% to 49% (C), and less than 30% (D); ALCL, anaplastic large T/null-cell lymphoma; MZ, MALT, marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue; FL, follicular lymphoma; MZ, nodal, marginal zone B-cell lymphoma of nodal type; LP, lymphoplasmacytoid lymphoma; SL, small lymphocytic lymphoma; Med LBC, primary mediastinal large B-cell lymphoma; DLCBL, diffuse large B-cell lymphoma; HG, BL, high-grade B-cell Burkitt-like lymphoma; T-LB, precursor T-lymphoblastic lymphoma; PTCL, peripheral T-cell lymphoma; MC, mantle cell lymphoma.
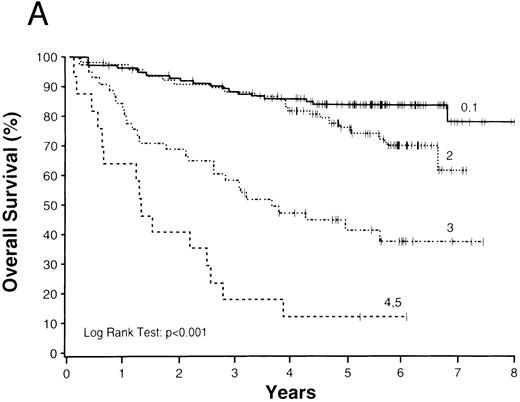
Whereas the histologic diagnosis of a specific type of lymphoma provides clinically important information, equally important prognostic information was obtained from the clinical characteristics of the individual patients. We found considerable variation within any particular histologic type for both overall survival and failure-free survival based on patient clinical characteristics using the International Prognostic Index (Table 7). For example, patients with follicular lymphoma had significantly different outcomes depending on their clinical prognostic characteristics (Fig 2). Moreover, patients with follicular lymphoma with a high (unfavorable) prognostic index had a far worse overall and failure-free survival (ie, 17% and 6%) than patients with a diffuse large B-cell lymphoma and a low (favorable) prognostic index (ie, 73% and 63%). In contrast, the histologic diagnosis of anaplastic large cell lymphoma was important because it was associated with a surprisingly good survival, even with a high prognostic index. In contrast, patients with mantle cell lymphoma had a relatively poor outcome despite apparently good clinical characteristics. The prognostic index also did not predict survival in precursor T-lymphoblastic lymphoma, although the number of cases was small.
Survival by Histologic Type and the International Prognostic Index
| Consensus Diagnosis . | % 5-yr OAS . | % 5-yr FFS . | ||
|---|---|---|---|---|
| . | Index 0/1 . | Index 4/5 . | Index 0/1 . | Index 4/5 . |
| Follicular, all grades | 84 | 17 | 55 | 6 |
| Mantle cell | 57 | 0 | 27 | 0 |
| Marginal zone B-cell, MALT | 89 | 40 | 83 | 0 |
| Marginal zone B-cell, nodal | 76 | 50 | 30 | 0 |
| Small lymphocytic (CLL) | 76 | 38 | 35 | 13 |
| Diffuse large B-cell | 73 | 22 | 63 | 19 |
| Primary mediastinal large B-cell | 77 | 0 | 69 | 0 |
| High grade B-cell, Burkitt-like | 71 | 0 | 71 | 0 |
| Precursor T-lymphoblastic | 29 | 40 | 29 | 40 |
| Peripheral T-cell, all types | 36 | 15 | 27 | 10 |
| Anaplastic large T/null-cell | 81 | 83 | 49 | 83 |
| Consensus Diagnosis . | % 5-yr OAS . | % 5-yr FFS . | ||
|---|---|---|---|---|
| . | Index 0/1 . | Index 4/5 . | Index 0/1 . | Index 4/5 . |
| Follicular, all grades | 84 | 17 | 55 | 6 |
| Mantle cell | 57 | 0 | 27 | 0 |
| Marginal zone B-cell, MALT | 89 | 40 | 83 | 0 |
| Marginal zone B-cell, nodal | 76 | 50 | 30 | 0 |
| Small lymphocytic (CLL) | 76 | 38 | 35 | 13 |
| Diffuse large B-cell | 73 | 22 | 63 | 19 |
| Primary mediastinal large B-cell | 77 | 0 | 69 | 0 |
| High grade B-cell, Burkitt-like | 71 | 0 | 71 | 0 |
| Precursor T-lymphoblastic | 29 | 40 | 29 | 40 |
| Peripheral T-cell, all types | 36 | 15 | 27 | 10 |
| Anaplastic large T/null-cell | 81 | 83 | 49 | 83 |
Abbreviations: PI, International Prognostic Index; OAS, overall survival; FFS, failure-free survival; CLL, chronic lymphocytic leukemia.
Overall (A) and failure-free (B) survivals of patients with follicular lymphoma grouped according to International Prognostic Index scores.
Overall (A) and failure-free (B) survivals of patients with follicular lymphoma grouped according to International Prognostic Index scores.
DISCUSSION
This study shows that, using the definitions proposed in the ILSG classification, it is possible to accurately identify most of the major types of NHL. The major types recognized by this classification are also clinically distinctive, with the possible exception of high-grade B-cell Burkitt-like lymphoma, which appears to be very similar clinically to diffuse large B-cell lymphoma (Table 6). This classification was, in general, easily and accurately applied by the expert hematopathologists. In fact, this study suggests that when expert pathologists work from clear definitions, with the use of immunologic markers, the diagnosis of NHL can be made more accurately than had been thought. Previous studies, using morphology only, found that the diagnosis of specific types of NHL could only be made accurately 50% to 60% of the time.85-88 In contrast, we have shown that, when expert pathologists work from clear and agreed upon criteria, the diagnosis of NHL can be at least 85% accurate for most of the common types. However, the methods used to reach a consensus diagnosis in our study certainly had a positive influence on these agreement rates. Because treatment depends on the diagnosis, it must be made as accurately as possible. We believe that the diagnosis of NHL should always be made by a hematopathologist who is experienced in lymphoma classification.
Immunophenotyping added significantly to the accuracy of diagnosis of many of the lymphoma types, including mantle cell lymphoma, diffuse large B-cell lymphoma, and the T-cell lymphomas. However, immunophenotyping did not add significantly to the accuracy of diagnosis of some lymphomas, such as follicular lymphoma, small lymphocytic lymphoma, and marginal zone B-cell lymphoma of MALT type, all of which have very distinctive histologic features which usually facilitate the diagnosis without a need for immunologic data. For other types, such as the lymphoplasmacytoid, nodal marginal zone B-cell, and high-grade B-cell Burkitt-like lymphomas, imprecise histologic criteria and the lack of specific immunologic markers led to a diagnostic accuracy of only 53% to 65%. Further definition of these entities is clearly needed. Because the need for immunophenotyping cannot be predicted before biopsy, it is vital that each patient have tissue available for immunophenotyping and other special studies to facilitate proper patient care. In many cases, this will require communication between the oncologist, the surgeon, and the pathologist.
The 13 major types of NHL shown in Fig 1 made up over 90% of the cases in our study, with diffuse large B-cell lymphoma and follicular lymphoma comprising over 50% of the cases and the newly recognized entities comprising 21% of the cases (Table 3). The clinical features of the various lymphoma types were remarkably different, as were the survivals (Table 6). Using overall survival, the various lymphoma types could be divided into four broad groups for prognostic purposes (Fig 1). The groups consist of the those with a 5-year survival of greater than 70% (Fig 1A), those with a 5-year survival between 50% and 70% (Fig 1B), those with a 5-year survival of 30% to 49% (Fig 1C), and those with a 5-year survival of less than 30% (Fig 1D). Although such groupings may be useful for planning or interpreting future clinical studies, important differences in the approach to treatment and the potential curability of the various lymphoma types in these broad groups are well known. However, we believe that patient-specific information is also very important for clinical decision making, with the histologic diagnosis being only the first step in “classification” for proper patient management. The prognostic factors used in the International Prognostic Index69 provide important information in most of the major types of NHL. Whereas the pathologic entities are distinctive, the variation in outcome within a particular entity by clinical prognostic characteristics is great. “Good prognosis” pathologic entities contain patients with a poorer outcome than the better patients in the “poor prognosis” entities. Therefore, to make proper clinical decisions, it is necessary to consider both the histologic type and the various prognostic factors present in an individual patient. Any useful clinical grouping of the NHLs must take both types of information into account.
Although the ILSG classification could be accurately applied and appears to be useful clinically, there are a number of areas that could be improved. Changes in organization and terminology have been suggested by others.94-96 In addition, more specific criteria for some of the lymphoma types are clearly needed, such as the lymphoplasmacytoid, nodal marginal zone B-cell, and high-grade B-cell Burkitt-like lymphomas. The cellular origin of so-called splenic “marginal zone” lymphoma, along with diagnostic criteria, also need to be elucidated.97 Subtyping of the diffuse large B-cell lymphomas into immunoblastic and nonimmunoblastic types may be useful, and the clinical and pathologic features of anaplastic large B-cell lymphoma need to be more carefully studied before combining it into the generic category of diffuse large B-cell lymphoma. Precise criteria for grading within the various lymphoma types are clearly needed, such as for the follicular lymphomas, mantle cell lymphomas, and marginal zone B-cell lymphomas of MALT type. Finally, the number of categories of peripheral T-cell lymphoma, for which the diagnostic criteria are imprecise and difficult to apply, seems excessive when there is little evidence to support subdividing for clinical purposes. Hopefully, these issues will be addressed by the working groups of the new World Health Organization classification project.
In conclusion, the ILSG classification was readily applied and identified clinically distinctive types of NHL. Immunophenotyping added significantly to the accuracy of diagnosis in certain major lymphoma types and was clinically important. For clinical application, however, prognostic factors as defined by the International Prognostic Index91 must be combined with the histologic classification for appropriate clinical decisions.
ACKNOWLEDGMENT
The study participants thank Dr Saul A. Rosenberg for his advice regarding the study design and analysis.
APPENDIX
Study Participants: The pathologists and clinicians at each institution were, respectively, Wing C. Chan and James O. Armitage (Omaha, NE), Randy Gascoyne and Joseph Connors (Vancouver, Canada), Pauline Close and Peter Jacobs (Capetown, South Africa), Andrew Norton and T. Andrew Lister (London, UK), Ennio Pedrinis and Franco Cavalli (Locarno, Switzerland), Francoise Berger and Bertrand Coiffier (Lyon, France), Faith Ho and Raymond Liang (Hong Kong), German Ott/Alfred Schauer and Wolfgang Hiddemann (Würzburg/Göttingen, Germany). The five visiting expert hematopathologists were Jacques Diebold (Paris, France), Kenneth A. MacLennan (Leeds, UK), H. Konrad Müller-Hermelink (Würzburg, Germany), Bharat N. Nathwani (Los Angeles, CA), and Dennis D. Weisenburger (Omaha, NE). Nancy L. Harris (Boston, MA) participated as a consultant regarding application of the International Lymphoma Study Group classification. James R. Anderson (Omaha, NE) and Pascal Roy (Lyon, France) provided statistical expertise regarding the study design and data analysis.
Supported in part by US Public Health Service CA36727 awarded by the National Cancer Institute, Department of Health and Human Services, the Foundazione San Salvatore, and the Stacey Greene family.
Address reprint requests to James O. Armitage, MD, Department of Internal Medicine, University of Nebraska Medical Center, 600 S 42nd St, Omaha, NE 68198-3332.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal