Abstract
Flt-3 ligand (FL) shares many features with stem cell factor (SCF), a widely documented cofactor for peripheral blood progenitor cell (PBPC) mobilization. We investigated the mobilization of PBPCs by FL in combination with granulocyte colony-stimulating factor (G-CSF). As a single agent, FL was a relatively modest mobilizer of PBPCs, resulting in 360 granulocyte/macrophage colony-forming cells (GM-CFCs)/mL blood (control, 155 GM-CFCs/mL blood) and no advantage in leukocyte recovery when these PBPCs were transplanted to irradiated recipient mice. G-CSF, on the other hand, mobilized over 20,000 GM-CFCs/mL blood, and the combination of G-CSF + FL resulted in over 100,000 GM-CFCs/mL blood. The combination of G-CSF + FL stimulated increased levels of monocytes and basophils in the peripheral blood. The performance of the mobilized PBPC product in irradiated hosts correlated with progenitor numbers resulting in long-term engraftment in association with accelerated short-term recovery of both leukocytes and platelets. These data demonstrate the potential of FL to synergize with G-CSF to mobilize PBPCs with both short- and long-term engraftment potential. The effect is similar to the synergistic interaction of G-CSF and SCF on PBPC mobilization. The use of FL as opposed to SCF may elicit a different spectrum of toxicities including lymphoid proliferation effects, in contrast to the mast cell degranulation effects of SCF. Clinical studies of FL are needed to evaluate its usefulness in man.
CLONING OF THE LIGAND for the receptor protein tyrosine kinase flt-3/flk-21 has recently allowed investigation of its potential as a therapeutic agent. The flt-3/flk-2 receptor tyrosine kinase shares a significant degree of homology with other receptor tyrosine kinases such as platelet-derived growth factor (PDGF) receptor (PDGFR), c-fms and c-kit (the receptors for macrophage colony-stimulating factor [M-CSF, CSF-1]), and stem cell factor ([SCF], KL, MGF), respectively.2,3 It was perhaps not surprising therefore that many of the documented in vitro biologic activities of flt-3 ligand (FL) overlapped with those previously reported for SCF. Thus, FL has been shown to synergize with SCF, interleukin-3 (IL-3) and IL-6,4 and IL-3 and GM-CSF5 on myeloid progenitor colony formation and to be a useful component of ex vivo hematopoietic progenitor expansion mixtures.6-8 The major difference between these two primitive-acting hematopoietic growth factors (or cofactors) would appear to be the SCF predilection for cells of the erythroid lineage,9-11 whereas FL would appear to preferentially affect lymphoid cells6,12 and the development of a dysregulated B-lymphocyte or dendritic cell pathology.13
The therapeutic potential of SCF has been widely investigated, and its utility in the area of peripheral blood progenitor cell (PBPC) mobilization, particularly with simultaneous G-CSF, has been demonstrated in animals14-17 and clinical trials in patients.18,19 Preliminary data have been recently presented to indicate that FL may also share with SCF the ability to mobilize transplantable hematopoietic progenitor cells to the blood of mice20-22 and primates.23 Despite a similar demonstrated efficacy for both FL and SCF as single agents in preclinical mouse models,14,16,20 it was shown in clinical studies that SCF was most useful as a cofactor with G-CSF in the area of PBPC mobilization. We therefore reasoned that FL, too, may be more efficacious as a supplement to G-CSF rather than as a single agent, a line of reasoning supported by preliminary published data.24 25 To investigate this, we treated mice with recombinant human FL as a single agent and also in simultaneous combination with G-CSF. Data are presented that indicate a synergistic interaction between FL and G-CSF upon neutrophil formation and the mobilization of PBPCs. These PBPCs have been used to transplant lethally irradiated mice and have been shown to support accelerated blood cell recovery in the short term. Cells mobilized with FL in combination with G-CSF have also been shown to facilitate long-term hematopoietic engraftment in sex-mismatched donor-recipient pairings despite the failure of FL-mobilized PBPCs to support such protracted engraftment.
MATERIALS AND METHODS
Mice.Male BDF1 mice aged 12 to 20 weeks were used as donors, and female BDF1 mice aged 8 to 12 weeks were used as transplant recipients. Preparative irradiation was 1,200 cGy (137Cs; dose rate, 106.7 cGy/min) in a split dose of two times 600 cGy 4 hours apart.
Donor treatment.Mice were implanted with Alzet Micro-osmotic (1007D) pumps (Alza Corp, Palo Alto, CA). The pump, which was handled and filled under aseptic conditions, was inserted through a small incision made through the skin between the scapulae of anesthetized (Aerrane; Ohmeda Carbide Inc, Guayama, Puerto Rico) mice. Seven days later, mice were euthanized by carbon dioxide inhalation, and blood was withdrawn via cardiac puncture and collected in evacuated glass tubes containing 50 μL 15% EDTA (Vacutainer 6536; Becton Dickinson, Rutherford, NJ). Between 800 and 1,200 μL was collected from each donor, and typically groups of five donors were used. Complete blood cell counts were performed on individual blood samples on a Technicon H-1E (Technicon Instruments Corp, Tarrytown, NY) calibrated for the analysis of mouse blood. Blood from individual donors was assayed for granulocyte/macrophage colony-forming cell (GM-CFC) content. Five hundred microliters of blood from each donor was then pooled and diluted with phosphate-buffered saline (PBS), and 200 μL was injected intravenously into irradiated recipients.
Recipient treatment.To monitor short-term recovery, groups of 10 recipients were divided into two groups of five, and blood was withdrawn from the retro-orbital sinus of anesthetized animals via heparinized glass capillaries into tubes containing 50 μL 30% EDTA. This blood was analyzed on a Technicon H-1E. Blood samples were collected from either group of five mice alternately; thus, one cohort of five mice was sampled on days 5, 9, 12, 16, and 21 and the other cohort on days 7, 10, 14, and 19 after transplant.
CSI Versus Subcutaneous Injection for Treatment With FL Alone or in Combination With G-CSF (7-day counts)
| . | Carrier . | FL (100 μg/kg/d) . | G-CSF (100 μg/kg/d) . | FL + G-CSF . | ||||
|---|---|---|---|---|---|---|---|---|
| . | INJ . | CSI . | INJ . | CSI . | INJ . | CSI . | INJ . | CSI . |
| WBC (× 106/mL) | 6.4 ± 1.7 | |||||||
| Neutrophils (× 106/mL) | ||||||||
| GM-CGC/mL blood | ||||||||
| 0.61 ± 0.1 | ||||||||
| 159 ± 63.6 | 8.6 ± 0.5* | |||||||
| 0.79 ± 0.1* | ||||||||
| 155 ± 41.5 | 7.9 ± 1.8 | |||||||
| 0.60 ± 0.60 | ||||||||
| 262 ± 90.7 | 8.3 ± 0.9 | |||||||
| 0.82 ± 0.25 | ||||||||
| 360 ± 193 | 10.3 ± 2.8 | |||||||
| 2.04 ± 1.1 | ||||||||
| 2,620 ± 1,324 | 30.3 ± 5.6* | |||||||
| 19.0 ± 4.4* | ||||||||
| 21,645 ± 3,339* | 10.14 ± 0.8 | |||||||
| 2.6 ± 0.56 | ||||||||
| 3,308 ± 1,358 | 78.7 ± 7.5* | |||||||
| 59.1 ± 6.1* | ||||||||
| 111,132 ± 30,759* | ||||||||
| . | Carrier . | FL (100 μg/kg/d) . | G-CSF (100 μg/kg/d) . | FL + G-CSF . | ||||
|---|---|---|---|---|---|---|---|---|
| . | INJ . | CSI . | INJ . | CSI . | INJ . | CSI . | INJ . | CSI . |
| WBC (× 106/mL) | 6.4 ± 1.7 | |||||||
| Neutrophils (× 106/mL) | ||||||||
| GM-CGC/mL blood | ||||||||
| 0.61 ± 0.1 | ||||||||
| 159 ± 63.6 | 8.6 ± 0.5* | |||||||
| 0.79 ± 0.1* | ||||||||
| 155 ± 41.5 | 7.9 ± 1.8 | |||||||
| 0.60 ± 0.60 | ||||||||
| 262 ± 90.7 | 8.3 ± 0.9 | |||||||
| 0.82 ± 0.25 | ||||||||
| 360 ± 193 | 10.3 ± 2.8 | |||||||
| 2.04 ± 1.1 | ||||||||
| 2,620 ± 1,324 | 30.3 ± 5.6* | |||||||
| 19.0 ± 4.4* | ||||||||
| 21,645 ± 3,339* | 10.14 ± 0.8 | |||||||
| 2.6 ± 0.56 | ||||||||
| 3,308 ± 1,358 | 78.7 ± 7.5* | |||||||
| 59.1 ± 6.1* | ||||||||
| 111,132 ± 30,759* | ||||||||
Data presented are the mean of 2 experiments ± 1 SD, each from 5 donors.
Abbreviations: INJ, injection; CSI, continuous subcutaneous infusion.
P < .005; INJ v CSI.
Long-term engraftment.Blood was removed from the retro-orbital sinus of transplanted mice 1 to 1.5 years after transplantation with FL-, G-CSF–, or FL + G-CSF–mobilized PBPCs. The samples were treated as outlined in a previous report26 and analyzed for the presence of donor cells. Two years after transplant, some mice were euthanized and bone marrow cells were explanted in a soft agar gel system (described later). DNA was extracted and amplified by polymerase chain reaction (PCR) using previously published techniques.26 Briefly, colonies were picked from the agar gel, and the cells were lysed with water. DNA was extracted and amplified using primers for PDGFR and a Y chromosome–specific sequence. Mice were assessed individually, and 44 bone marrow–derived colonies were processed from each mouse. Only PCR products that were positive for PDGFR were processed for the Y-specific sequence. The percentage of colonies that were positive for Y (ie, donor-derived) was calculated.
Growth factors.Recombinant human FL was expressed in Escherichia coli. All growth factors were prepared at appropriate dilutions to yield the dose levels indicated in the results. Doses of between 10 and 100 μg/kg/d rhG-CSF were used. FL was administered at doses of 10 to 100 μg/kg/d. Treatment was administered by continuous subcutaneous infusion (CSI) via Alzet micro-osmotic pumps. The carrier solution was PBS + 0.1% bovine serum albumin and was used to dilute growth factors for infusion or given alone to carrier-treated animals.
Colony-forming assay.Aliquots of between 5 and 50 μL whole blood were removed from the individual blood samples. This volume was added to 3 mL plating mix. Aliquots of 1 mL were then immobilized with 0.33% agar noble (Difco, Detroit, MI) in triplicate Falcon 35-mm petri dishes (Becton Dickinson, Lincoln Park, NJ) in the presence of Iscove's modified Dulbecco's medium (Life Technologies, Grand Island, NJ) supplemented with 20% preselected fetal bovine serum (Cansera, Rexdale, Ontario, Canada), 20 ng/mL SCF (Amgen), and 2.5 ng/mL rmIL-3 (Amgen). Cultures were maintained at 37°C for 7 days in fully humidified N2 plus 5% O2 and 5% CO2 . Remaining erythrocytes in the plates were lysed at the end of the culture period by careful addition of 300 μL 0.3% acetic acid to the surface of the gel, and colonies of greater than 50 cells were counted under a dissecting microscope.
RESULTS
PBPC Donor Mice Treated With FL + G-CSF
FL scheduling.Scheduling concerns have been raised in relation to the screening of various cytokines in murine model systems. We therefore injected FL and compared this directly with the same protein at the same daily dose delivered by continuous infusion via osmotic pumps (Table 1). More detailed data are presented in the following sections on the various peripheral blood populations that change in response to infused FL. Briefly, leukocytes showed little change in FL recipients whether the material was delivered by injection or CSI. G-CSF increased leukocyte numbers, primarily neutrophils, from a baseline of less than 1,000/μL blood to 2 × 103/μL in injected recipients and 19,000/μL in CSI-treated mice. Combination (FL + G-CSF)-treated mice had about 10 × 103 neutrophils/μL blood when the materials were injected and almost 80 × 103/μL when the same dose was given by CSI. Parallel changes were seen in GM-CFCs mobilized to the blood. Carrier-treated animals had less than 200 GM-CFCs/mL blood, and this increased to over 100,000 in combination mice treated by CSI; other groups and injected recipients showed smaller increases.
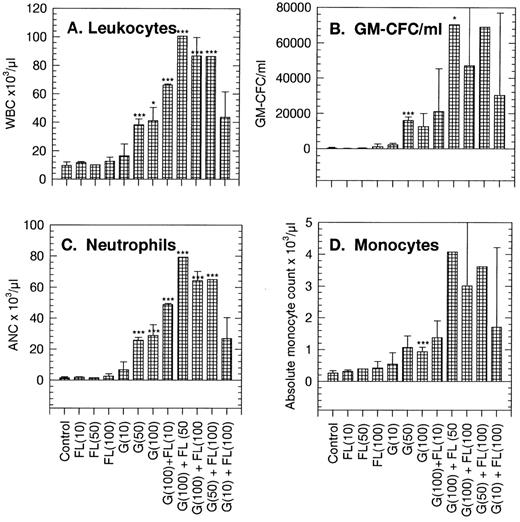
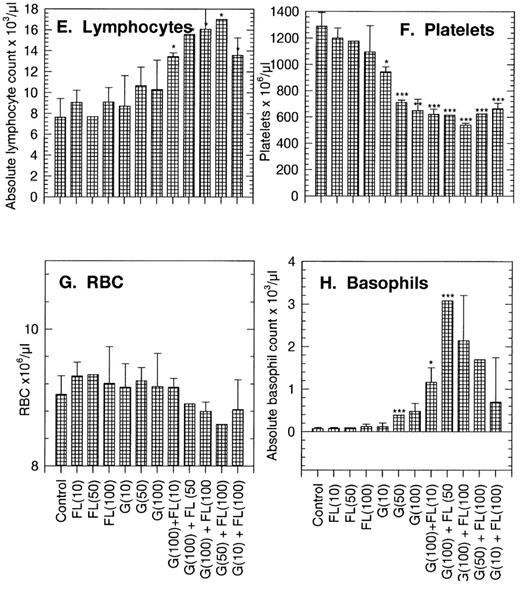
Changes in peripheral blood of PBPC donors.Peripheral blood populations were minimally affected by FL infused alone at doses of 10, 50, or 100 μg/kg/d. White blood cell (WBC), neutrophil, monocyte, lymphocyte, erythrocyte, and basophil counts all were within the normal range (Fig 1A and C to H). Platelet counts were on the lower side of the normal range at 943 ± 40 × 103/μL (compared with a control count of 1,287 ± 104 × 103/μL; Fig 1F). GM-CFC numbers per milliliter of blood were higher than the control, 360/mL compared with 155/mL in the control (Table 1 and Fig 1B). Predictably, G-CSF increased circulating WBCs, neutrophils, monocytes, and GM-CFCs in a dose-dependent manner. Perhaps surprisingly, G-CSF also increased basophil counts from approximately 0.07 × 103/μL in controls to 0.12 × 103/μL and decreased platelet counts to about 648 × 103/μL at 100 μg/kg/d (Fig 1).
Changes in peripheral blood populations in normal mice treated with FL, G-CSF (G), or combinations of the two factors. Doses (in parentheses) are in μg/kg/d. Each bar is the mean of 15 replicate donors. Results are the mean ± SD. Significance of differences from control data: *P < .05, **P < .01, ***P < .005.
Changes in peripheral blood populations in normal mice treated with FL, G-CSF (G), or combinations of the two factors. Doses (in parentheses) are in μg/kg/d. Each bar is the mean of 15 replicate donors. Results are the mean ± SD. Significance of differences from control data: *P < .05, **P < .01, ***P < .005.
Normal mice treated with a standard 100 μg/kg/d G-CSF in combination with incremental doses of FL showed increases above those obtained with G-CSF alone in WBCs, neutrophils, monocytes, basophils, and GM-CFCs per milliliter of blood. A significant lymphocytosis was also apparent in recipients of the combination and a marked depression in platelet numbers to about 520 × 103/μL, or 42% of control. Keeping the dose of FL at the maximum of 100 μg/kg/d and incrementally reducing the dose of G-CSF to 50 and 10 μg/kg/d diminished the response in WBCs, neutrophils, monocytes, basophils, GM-CFCs, and possibly lymphocytes and platelets also.
FL as a single agent thus caused little change if any in the measured parameters. In combination with G-CSF, the effects of FL were to magnify the familiar G-CSF response, increasing in a dose-dependent manner the WBCs (mainly neutrophils, monocytes, and lymphocytes) and myeloid progenitor cells in the circulation.
Bone marrow and spleen response in PBPC donors treated with FL + G-CSF.The bone marrow and spleen of normal mice treated with FL were similar to the same tissues in carrier-treated mice with respect to cellularity and progenitor content. G-CSF or FL + G-CSF recipients showed changes in keeping with the potent PBPC-mobilizing effects of these agents, ie, a reduction in most femoral parameters and a parallel increase in the corresponding spleen populations (Table 2).
Effects on Bone Marrow and Spleen of FL Treatment Alone or in Combination With G-CSF
| Site . | Control . | FL . | G-CSF . | FL + G-CSF . |
|---|---|---|---|---|
| Marrow | ||||
| Cells/femur (× 106/femur) | 27.6 ± 1.1 | 22.0 ± 3.2* | 19.6 ± 0.9* | 18.6 ± 2.1* |
| GM-CFC/femur | 18,000 ± 340 | 18,680 ± 413* | 3,200 ± 173* | 2,400 ± 120* |
| BFU-E/femur | 796 ± 412 | 396 ± 280* | 0 | 0 |
| CFU-mix/femur | 2,792 ± 1,312 | 2,264 ± 865 | 660 ± 245* | 0 |
| CFU-S/femur | 5,405 ± 62 | 4,700 ± 34* | 825 ± 13* | 450 ± 112* |
| Spleen | ||||
| Cells/spleen (× 106/spleen) | 128 ± 23 | 115 ± 16 | 178 ± 21* | 232 ± 59* |
| GM-CFC/spleen | 1,440 ± 321 | 1,880 ± 212* | 74,280 ± 816* | 257,580 ± 2,300* |
| BFU-E/spleen | 0 | 664 ± 54 | 1,464 ± 112 | 16,200 ± 1,800 |
| CFU-mix/spleen | 0 | 396 ± 68 | 12,796 ± 1,402 | 10,680 ± 888 |
| CFU-S/spleen | 7,458 ± 86 | 3,583 ± 321* | 17,050 ± 554* | 22,500 ± 4,590* |
| Site . | Control . | FL . | G-CSF . | FL + G-CSF . |
|---|---|---|---|---|
| Marrow | ||||
| Cells/femur (× 106/femur) | 27.6 ± 1.1 | 22.0 ± 3.2* | 19.6 ± 0.9* | 18.6 ± 2.1* |
| GM-CFC/femur | 18,000 ± 340 | 18,680 ± 413* | 3,200 ± 173* | 2,400 ± 120* |
| BFU-E/femur | 796 ± 412 | 396 ± 280* | 0 | 0 |
| CFU-mix/femur | 2,792 ± 1,312 | 2,264 ± 865 | 660 ± 245* | 0 |
| CFU-S/femur | 5,405 ± 62 | 4,700 ± 34* | 825 ± 13* | 450 ± 112* |
| Spleen | ||||
| Cells/spleen (× 106/spleen) | 128 ± 23 | 115 ± 16 | 178 ± 21* | 232 ± 59* |
| GM-CFC/spleen | 1,440 ± 321 | 1,880 ± 212* | 74,280 ± 816* | 257,580 ± 2,300* |
| BFU-E/spleen | 0 | 664 ± 54 | 1,464 ± 112 | 16,200 ± 1,800 |
| CFU-mix/spleen | 0 | 396 ± 68 | 12,796 ± 1,402 | 10,680 ± 888 |
| CFU-S/spleen | 7,458 ± 86 | 3,583 ± 321* | 17,050 ± 554* | 22,500 ± 4,590* |
Data presented are the mean of 2 experiments ± 1 SD, each from 5 donors.
P < .005 v control.
Mobilization of PBPCs
Graft.The quantitative aspects of PBPC mobilization as assessed by GM-CFCs per milliliter of blood are presented. Various volumes (between 100 and 200 μL) of unmanipulated blood were transferred to irradiated recipients to assess the capacity of the blood-borne progenitors to initiate hematopoietic recovery. The grafts contained WBC and GM-CFC content as outlined in Table 3.
Details of the Graft Obtained From Variously Treated PBPC Donor Groups and Injected Into Lethally Irradiated Mice
| Donor Treatment Group . | WBCs per Graft . | GM-CFCs per Graft . |
|---|---|---|
| Carrier | 1.2 ± 0.12 × 106 | 19 ± 16 |
| FL | 1.2 ± 0.07 × 106 | 175 ± 92 |
| G-CSF | 5.6 ± 0.91 × 106 | 2,280 ± 114 |
| FL + G-CSF | 10.9 ± 0.87 × 106 | 11,307 ± 239 |
| Donor Treatment Group . | WBCs per Graft . | GM-CFCs per Graft . |
|---|---|---|
| Carrier | 1.2 ± 0.12 × 106 | 19 ± 16 |
| FL | 1.2 ± 0.07 × 106 | 175 ± 92 |
| G-CSF | 5.6 ± 0.91 × 106 | 2,280 ± 114 |
| FL + G-CSF | 10.9 ± 0.87 × 106 | 11,307 ± 239 |
Data presented are the mean of 2 experiments ± 1 SD, each from 5 donors.
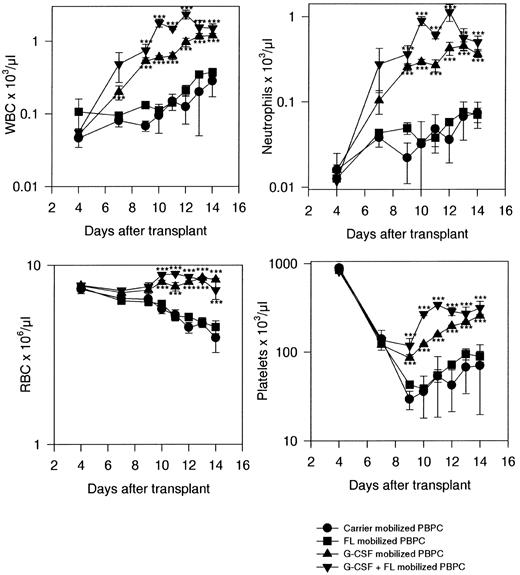
Short-term recovery in transplant recipients.Leukocyte recovery in recipients of carrier-mobilized PBPCs recovered slowly. From a control (pretreatment) value of approximately 10 × 103/μL, WBC counts decreased to a nadir at or slightly less than 0.1 × 103/μL at days 4 to 5. Recovery was negligible before day 10, when numbers began to increase, reaching a maximum of 0.3 × 103/μL by day 14. Mice that received a graft of FL-mobilized PBPCs showed marginal improvement over the leukocyte recovery sustained by carrier-PBPCs. The nadir was slightly better and the final level attained was about 1.5-fold higher than in carrier-PBPC recipients (Fig 2).
Recovery of lethally irradiated mice transplanted with PBPCs from variously treated donors. Results are the mean ± 1 SD per point, with 15 mice per treatment point from three independent experiments. Significance of differences from control data: *P < .05, **P < .01, ***P < .005.
Recovery of lethally irradiated mice transplanted with PBPCs from variously treated donors. Results are the mean ± 1 SD per point, with 15 mice per treatment point from three independent experiments. Significance of differences from control data: *P < .05, **P < .01, ***P < .005.
G-CSF-PBPC-grafted mice showed accelerated WBC recovery compared with carrier-PBPC or FL-PBPC recipients. After a similar nadir in G-CSF-PBPC recipients, recovery started sooner and proceeded at an accelerated rate, reaching a level of about 1 × 103/μL at day 14. However, the fastest recovery rate in WBCs and highest maximum level achieved (2.3 × 103/μL at day 12) were attained in recipients of combination (FL + G-CSF)-mobilized PBPCs.
Neutrophil recovery followed a pattern exactly similar to the recovery in WBCs (Fig 2), reflecting the advantage of using G-CSF-mobilized PBPCs and improvement over even the G-CSF-PBPC recovery rate in recipients of combination-mobilized PBPCs. Erythrocyte numbers declined steadily over the study period in recipients of carrier-PBPCs and FL-PBPCs. In contrast, recipients of G-CSF-PBPCs or FL + G-CSF-PBPCs sustained red blood cell numbers through day 14. Platelets, as is typical of this population, showed a later nadir than most other blood cell populations, and recovery was not significant in carrier-PBPC or FL-PBPC recipients until about day 10 or 12. G-CSF-PBPC and FL + G-CSF-PBPC recipients had a less profound nadir, and recovery also started much sooner. The rate of recovery was the greatest in recipients of combination-mobilized PBPCs, and the maximum numbers attained were also noted in this group (Fig 2).
Long-term engraftment in PBPC recipients.The presence of Y chromosome–specific DNA sequences in peripheral blood cells of recipient mice 1 to 1.5 years after transplant was confirmed in the majority of mice (Table 4). Mice engrafted with PBPCs from carrier-treated animals did not survive in sufficient numbers to allow consideration of the long-term engraftment potential of unmobilized PBPCs. PBPCs mobilized with FL, G-CSF, or the combination all led to the establishment of long-term chimeras as detected in the peripheral blood populations. However, examination of the degree of chimerism in various progenitor populations after 2 to 2.5 years indicated that mice that received a graft mobilized with FL alone did not have substantial donor engraftment at the progenitor cell level. On the other hand, mice that received a PBPC product mobilized with either G-CSF alone or G-CSF + FL had a substantial degree of chimerism at the progenitor cell level (Table 5).
Detection of Donor Cells in the Peripheral Blood of PBPC Recipients 1 Year After Transplant With Cells From the Blood of Donors Treated With FL, G-CSF, or FL + G-CSF
| Parameter . | Carrier Only . | FL . | G-CSF . | FL + G-CSF . |
|---|---|---|---|---|
| Animals showing some donor cells (%) | — 4-150 | 82 | 79 | 90 |
| No. of mice studied | 0 | 17 | 19 | 20 |
| Parameter . | Carrier Only . | FL . | G-CSF . | FL + G-CSF . |
|---|---|---|---|---|
| Animals showing some donor cells (%) | — 4-150 | 82 | 79 | 90 |
| No. of mice studied | 0 | 17 | 19 | 20 |
Lack of surviving animals precluded assessment.
Detection of Donor Progenitor Cells in the Bone Marrow of PBPC Recipients 2 Years After Transplant With Cells From the Blood of Donors Treated With FL, G-CSF, or FL + G-CSF
| Mouse No.5-150 . | FL . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | PDGFR+5-151 . | Y+5-152 . | % Y+ρ . | G-CSF . | |||||||||
| . | No. . | % . | . | . | PDGFR+5-151 . | Y+5-152 . | % Y+ρ . | G-CSF + FL . | |||||
| . | . | . | . | . | No. . | % . | . | . | PDGFR+5-151 . | Y+5-152 . | % Y+ρ . | . | |
| . | . | . | . | . | . | . | . | . | No. . | % . | . | . | . |
| 1 | 15/44 | 34 | 0/15 | 0 | 32/44 | 73 | 29/32 | 91 | 10/27 | 37 | 8/10 | 80 | |
| 2 | 13/44 | 30 | 0/13 | 0 | 22/44 | 50 | 17/22 | 77 | 43/44 | 98 | 40/43 | 93 | |
| 3 | 19/44 | 43 | 0/19 | 0 | 13/44 | 30 | 4/13 | 31 | 20/44 | 46 | 20/20 | 100 | |
| 4 | 16/44 | 36 | 0/16 | 0 | 40/44 | 91 | 39/40 | 98 | 18/42 | 43 | 10/18 | 56 | |
| 5 | 38/44 | 86 | 2/38 | 5.3 | 22/44 | 50 | 15/22 | 68 | |||||
| 6 | 25/44 | 57 | 0/25 | 0 | 38/44 | 86 | 37/38 | 97 | |||||
| Mean5-155 | 48 | 0.9 | 63 | 77 | 56 | 82 | |||||||
| Mouse No.5-150 . | FL . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | PDGFR+5-151 . | Y+5-152 . | % Y+ρ . | G-CSF . | |||||||||
| . | No. . | % . | . | . | PDGFR+5-151 . | Y+5-152 . | % Y+ρ . | G-CSF + FL . | |||||
| . | . | . | . | . | No. . | % . | . | . | PDGFR+5-151 . | Y+5-152 . | % Y+ρ . | . | |
| . | . | . | . | . | . | . | . | . | No. . | % . | . | . | . |
| 1 | 15/44 | 34 | 0/15 | 0 | 32/44 | 73 | 29/32 | 91 | 10/27 | 37 | 8/10 | 80 | |
| 2 | 13/44 | 30 | 0/13 | 0 | 22/44 | 50 | 17/22 | 77 | 43/44 | 98 | 40/43 | 93 | |
| 3 | 19/44 | 43 | 0/19 | 0 | 13/44 | 30 | 4/13 | 31 | 20/44 | 46 | 20/20 | 100 | |
| 4 | 16/44 | 36 | 0/16 | 0 | 40/44 | 91 | 39/40 | 98 | 18/42 | 43 | 10/18 | 56 | |
| 5 | 38/44 | 86 | 2/38 | 5.3 | 22/44 | 50 | 15/22 | 68 | |||||
| 6 | 25/44 | 57 | 0/25 | 0 | 38/44 | 86 | 37/38 | 97 | |||||
| Mean5-155 | 48 | 0.9 | 63 | 77 | 56 | 82 | |||||||
Up to 6 mice that had received a PBPC graft mobilized with FL, G-CSF, or G-CSF + FL were assessed individually.
Forty-four colonies grown from the bone marrow of each mouse were picked from the plates, and only samples that contained sufficient PDGFR to yield a positive signal were assessed for the Y-specific sequence.
Number of colonies that were assessable in which Y-specific sequences were found.
ρ Percentage of progenitor cell colonies that were donor (male)-derived.
Mean percentage of assessable colonies or donor (male) progenitor cells.
DISCUSSION
FL is an effective cofactor for mobilization of PBPCs, with an efficacy of the same order demonstrated in previous studies with SCF at similar doses,14,15 although dose-escalation and mobilization of more PBPCs may be possible. However, as a single agent and at the dose used (100 μg/kg/d), the ability of FL to mobilize significant quantities of PBPCs is limited. In recent studies, Brasel et al22 have escalated the dose of FL to 1,000 μg/kg/d which they administered for 10 days in some studies, and observed a greater degree of mobilization of GM-CFCs, BFU-E, and CFU-GEMM. The magnitude of the FL mobilization effect is, in our hands, on the order of about a twofold increase in circulating progenitors compared with carrier-treated donors. This compares with about a 150-fold increase in circulating GM-CFCs in G-CSF–treated mice and 720-fold in combination-treated (FL + G-CSF) donors. In terms of absolute numbers, FL injected for 7 days at 100 μg/kg/d was found to result in 260 GM-CFCs/mL blood, G-CSF (at 100 μg/kg/d) about 2,600, and the combination 3,300. When infused, these same doses resulted in 360 (FL alone), 22,000 (G-CSF alone), and 111,000 GM-CFCs/mL (G-CSF + FL). This compares with 20,000 GM-CFCs/mL blood when CHO-FL was injected at a fivefold higher dose, 500 μg/kg/d, for a longer period, 10 days, by Brasel et al.22 The time to peak mobilization by FL as opposed to G-CSF is curiously dissimilar, 10 days, compared with 5 to 7 days for G-CSF.
A superior approach to assessing progenitor numbers is to examine the functional capacity of a PBPC product to sustain engraftment after transfer to hematopoietically ablated recipients. In this setting, we have again demonstrated the efficacy of G-CSF as a single agent and also show that FL can magnify the G-CSF response. Recovery in recipients of FL + G-CSF–mobilized PBPCs was more rapid than in recipients of G-CSF-PBPCs or any other group in this study, an observation probably linked to the greater number of progenitor cells transplanted in the G-CSF + FL groups. The advantage obtained in the recovery rate over that seen in G-CSF-PBPC recipients was modest, accelerating recovery in most populations by a single time point (Fig 2) despite receiving about five times the number of progenitors. This observation of diminishing returns on increasing graft size has been reported previously in clinical studies using SCF,27 and may represent a generally applicable rule whereby increasing graft size over the minimum required for survival will not continue indefinitely to result in parallel improvements in performance.
In addition to the ability to mobilize PBPCs with the potential to effect accelerated short-term hematopoietic recovery after transplantation, there is an increased number of cells per milliliter of blood in mice treated with G-CSF + FL with long-term reconstituting capacity, as indicated by the persistence of sex-marked peripheral blood cells 1 year after transplantation. Recipients of carrier-(non)mobilized PBPCs failed to survive in the long-term in these experiments, an observation consistent with low numbers of cells capable of either radioprotection or short- or long-term engraftment in the blood of normal animals. Irradiation controls, which survived in low numbers, did not show any male-specific (donor) DNA in the blood 1 year after irradiation. Surprisingly, given the relatively low numbers of progenitors mobilized by FL alone, cells capable of making a contribution to peripheral blood populations for over 1 year after transplant were present in the blood of FL-treated donors. At this stage, these observations are qualitative — we are unable to comment on the relative quantity of these cells after various mobilization regimens.
Recipient mice were also studied in a more rigorous test of donor engraftment 2 years after transplantation. By growing colonies in agar from myeloid progenitor cells and assessing their origin, it is possible to determine the contribution made by engrafted donor cells at a more fundamental level of hematopoiesis. By this time, the only surviving mice had received grafts of blood from FL-, G-CSF-, or FL + G-CSF–treated donors. There were no 2-year survivors from irradiated control animals or recipients of unmobilized PBPCs. Mice that received grafts from FL-mobilized PBPCs had, on average, 1% donor-type progenitor cells. Those that had received a G-CSF-mobilized PBPC product had 77% donor-type progenitor cells, and G-CSF + FL–mobilized PBPCs established 82% donor-type progenitors after long-term engraftment. From these data, we are able to conclude that sufficient cells capable of radioprotection and short-term engraftment are indeed present in the blood of FL-treated PBPC donors. However, there would not appear to be a significant number of long-term engrafting cells in the blood of these mice. An interesting contrast is presented between the data obtained at 1 year (Table 4) and those obtained at 2 years (Table 5) after transplantation. By using nonquantitative PCR techniques for the detection of donor cells in the blood at 1 year, we drew the misleading conclusion that long-term engraftment had been sustained by PBPCs mobilized with FL alone. One year later, we used a more stringent test of chimerism and found a lower incidence of donor-type cells. The death of some animals in the interim period may influence the data. It is likely that the death of some animals would tend to select mice with superior engraftment in the extreme long term. We assume that recipients of a high-quality graft would have improved hematopoietic status over those that received an inferior graft, and this may increase their chance of survival. The data would thus be skewed in favor of recipients with significant donor engraftment rather than against them. An alternative explanation is that the cells may have persisted through 1 year but are extinct at a later time point in recovery. The individual colony PCR analysis performed at 2 years is a more stringent test to evaluate the presence of donor progenitor cells in the marrow. The earlier and less stringent analysis may detect cells produced from some other tissue, for example, the spleen, in addition to detecting the progeny of marrow-borne hematopoiesis. It is also possible that the PCR technique used is capable of detecting the presence of donor cells in the blood, even when they represent a small minority of the total circulating hematopoietic mass. These data offer a cautionary note for the interpretation of data produced by extremely sensitive PCR techniques applied to the study of transplant chimerism, and suggest the benefits of the use of more stringent methods.
In summary, we have shown that when used in combination with G-CSF, FL can act as a potent cofactor for PBPC mobilization. However, the presence of a modest increment in progenitor cell numbers, the unaltered kinetics of hematopoietic reconstitution after transplant, and the failure of mobilized cells to engraft in the long term imply a relatively modest effect when it is used as a single agent.
These data contrast with preliminary data published elsewhere on progenitor mobilization by FL alone.20-22 The immediate reason for this difference is not clear; perhaps in the cited studies the use of CHO-derived, glycosylated material was critical, and perhaps further scheduling and dosage issues have some influence also. We have shown that there may be scheduling issues associated with the use of FL, since daily injections of pure protein did not result in any marked effects. In contrast, continuous infusion demonstrated the effectiveness of the material. Similar data have been obtained for different growth factors (Molineux, unpublished, 1997) and indicate that continuous delivery of cytokines, at least in the investigatory stage, may offer advantages over the arbitrary definition of dosing schedules that may or may not elucidate the functional capacity of the protein. Other modifications may reproduce this phenomenon; for instance, a glycosylated form may have altered the pharmacokinetics and bioavailability compared with the unmodified E coli–derived protein.
No major side effects of FL treatment were seen in these studies. However, the doses used were relatively low. Recent observations13 indicate that when chronically high levels of FL are produced in mice (after transplantation of bone marrow cells that were engineered to overexpress FL), there may develop a serious pathology associated with dysregulated B- or T-lymphocyte or dendritic cell proliferation. This side effect is manifest as a grossly deformed spleen morphology with increased white pulp and possible development of fibrotic lesions. The significance of these observations awaits clarification. The only other observation of significant side effects made in these studies was that normal mice treated with FL + G-CSF showed marked increases in circulating basophil numbers — from less than 0.07 × 103/μL in controls to about 3.1 × 103/μL — an increase of over 40-fold. This effect is of greater magnitude than the increases seen in mice treated with SCF (McNiece et al, unpublished observations, 1997), and although basophilia has not been reported in mice treated with SCF, degranulation of mature mast cells in patients (although not in lower primates) proved to be the dose-limiting toxicity of SCF in subsequent studies. Confirmation of these observations awaits clinical studies with FL.
Overall, we have shown that FL is a highly effective cofactor in conjunction with G-CSF for the mobilization of PBPCs with good transplantation potential. Many features of FL overlap with those of SCF. The data presented by Fletcher et al13 cause concern over the side effects of persistent high-dose FL treatment. It remains to be seen whether FL will be useful in addition to G-CSF, given the excellent toxicity profile of G-CSF and the unproven clinical advantages of increasing hematopoietic graft size yet further over that attainable with G-CSF alone.
Address reprint requests to Graham Molineux, PhD, Department of Developmental Hematology, Amgen Inc, Mailstop 99-1-A, 1840 DeHavilland Dr, Thousand Oaks, CA 91320.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal