Abstract
Using granzyme B–deficient mice obtained by gene targeting, we previously demonstrated that granzyme B is required for the rapid induction of apoptotic target cell death by cytotoxic T lymphocytes (CTLs); however, CTLs are also equipped with additional effector mechanisms. In the present study, we examined the mechanisms responsible for granzyme B–independent cytotoxicity using in vitro lytic assays with CTLs derived from mice deficient for both granzyme B and Fas ligand (FasL) (granzyme B−/− × gld/gld) or for perforin and FasL (perforin × gld/gld). Our results show that primary mixed lymphocyte reaction (MLR)-derived CTLs from granzyme B−/− × gld/gld mice induce apoptosis of allogeneic targets with less efficiency and a longer delay than CTLs deficient for granzyme B alone. The residual cytotoxicity in granzyme B−/− × gld/gld CTLs is primarily accounted for by a perforin-dependent mechanism, since perforin−/− × gld/gld CTLs have virtually no residual cytotoxic activity in our assays. Granzyme B–independent cytotoxicity is therefore partially accounted for by the Fas pathway and partially by another perforin-dependent mechanism.
CYTOTOXIC LYMPHOCYTES, which include cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells, are immune effector cells that are involved in host responses against tumors, viruses, and other intracellular pathogens; they are also involved in graft-versus-host disease (GVHD) and graft rejection. The granule exocytosis model for lymphocyte-mediated cytotoxicity postulates that after cytotoxic effector cells recognize and conjugate with their targets, they secrete the contents of their cytoplasmic granules into the intercellular space between themselves and the target cells.1 These cytoplasmic granules contain perforin (a 70-kD protein with similarity to the C9 component of complement proteins),2-4 granzymes, a family of highly homologous serine proteases,5-9 and additional proteins that have not yet been well characterized. According to the granule exocytosis model, perforin undergoes Ca2+-dependent polymerization and forms large functional pores that allow for the entry of granzymes into the target cell, leading to target cell apoptosis. Pore formation induced by perforin does not cause target cell death10; however, granzymes, in conjunction with perforin, can trigger apoptotic nuclear damage and cell death.11-13
A considerable amount of data now support the hypothesis that the perforin-granzyme pathway is a major effector mechanism for CD8+ cytotoxic lymphocytes. The recent production of mice with null mutations of perforin14-17 and of granzyme B18-19 has provided direct evidence for the role of these two molecules in CTL- and NK-mediated cytotoxicity both in vivo and in vitro. As measured by the in vitro 51Cr-release assay (an indicator of membrane damage), CTLs from mice deficient for perforin have only 10% to 30% as much cytotoxicity against susceptible targets as wild-type CTLs14-17; perforin-deficient NK cells appear to have no cytotoxicity when tested against the NK-susceptible YAC-1 targets.14,17 In vivo, perforin-deficient mice succumb to lymphocytic choriomeningitis virus challenges14,16 and cannot mount an effective immune response against the intracellular pathogen Listeria monocytogenes20 or against fibrosarcoma tumor cells.14 Similarly, studies from our laboratory have demonstrated the importance of granzyme B for CTL function both in vitro and in vivo.18,21 We have shown that granzyme B–deficient CTLs generated in primary MLR cultures are unable to rapidly induce the apoptosis of allogeneic target cells in vitro.18 These observations have been confirmed in vivo using an acute GVHD model in which granzyme B–deficient CD8+ CTLs directed against major histocompatibility complex class I–mismatched hosts caused significantly less death from acute GVHD.21
In addition to the perforin/granzyme pathway, CTLs are equipped with another mechanism of cell-mediated cytotoxicity that involves the Fas receptor (FasR) and its ligand (FasL).22,23 CTLs expressing FasL on their surface can induce apoptosis in target cells displaying FasRs. Studies with the naturally occurring loss-of-function models of FasR (lpr/lpr mice) and FasL (gld/gld mice) have demonstrated a central role of these molecules in the clearance of activated T cells in the periphery.24 25 However, recent studies with perforin-deficient mice have shown that most of the residual cytotoxicity in perforin−/− CTLs is due to Fas,16, 26-27 providing direct evidence for the role of this pathway in cell-mediated cytotoxicity.
Although the perforin/granzyme and Fas pathways appear to be the only mechanisms of lymphocyte cytotoxicity against most target cells in vitro, other lytic pathways may exist in some effector cells against certain target cells under special circumstances. For example, CTLs that are deficient for both perforin and FasL have been demonstrated to induce 51Cr release from tumor necrosis factor-α–sensitive targets after long incubation times28 (J. Spielman, R. K. Lee, E. R. Podack, personal communication). However, the importance of these additional effector mechanisms has not yet been fully defined in vivo.
Studies in our laboratory have been focused primarily on the perforin/granzyme pathway, with a particular emphasis on the role of granzyme B in CTL-mediated cytotoxicity. We have previously demonstrated the existence of a late pathway(s) of cytotoxicity in granzyme B−/− CTLs when tested against allotargets in in vitro lytic assays.18,19 Granzyme B–deficient CTLs cannot induce apoptosis in susceptible targets for up to 2 hours of incubation; however, after 2 hours, a granzyme B–independent mechanism slowly induces target cell apoptosis. In the present study, we examined the mechanisms that account for this delayed cytotoxicity. Our data demonstrate that granzyme B–independent cytotoxicity is accounted for by the Fas pathway and a perforin-dependent mechanism that has yet to be identified.
MATERIALS AND METHODS
Animals.Mice doubly deficient for granzyme B and FasL were produced by crossing H-2b granzyme B−/− (C57B1/6 × 129/SvJ) mice18 with B6 Smn. C3H-FasLgld (Jackson Laboratory, Bar Harbor, ME) followed by intercrossing of heterozygous F1 mice. Similarly, perforin-deficient gld/gld mice were generated by matings of perforin−/− mice16 (C57B1/6 × 129/SvJ; kindly provided by Dr William Clark, UCLA) and C57B1/6 gld/gld mice. All animals used in this study were young F2 or F3 mice aged 5 to 8 weeks.
Detection of mutations.Granzyme B and perforin mutations were identified by Southern blot analysis of mouse tail DNA, as described previously. The FasL-gld mutation was detected by polymerase chain reaction (PCR) amplification of tail DNA using primer sets that specifically detect the single base pair (bp) difference in the gld mutation in which amino acid 272 is changed from Phe (wild-type) to Leu (gld ).29 30 Primers 5′-GAACCCCCACTCAAG-3′ and 5′-CCGAAAAAGGTCTTA-3′ were designed to amplify a wild-type FasL product of 377 bp, whereas primers 5′-TAAGACCCTTTTCGG-3′ and 5′-TGGAGGTATGCATGC-3′ detect a 650-bp gld product. To amplify the wild-type product, the PCR was performed using a buffer containing 10 mmol/L Tris HCl/pH 9.2, 3.5 mmol/L MgCl2 , and 75 mmol/L KCl, Ampli-Taq DNA polymerase (Perkin-Elmer Cetus, Norwalk, CT), 100 to 200 ng primers, and 2 μg DNA for 29 cycles (94°C for 1 minute, 51°C for 1 minute, and 70°C for 1 minute). The gld product was detected using a buffer composed of 10 mmol/L Tris-HCl/pH 8.8, 15 mmol/L MgCl2 , and 75 mmol/L KCl, Ampli-Taq polymerase, 100 to 200 ng primers, and 3 μg DNA for 32 cycles (94°C for 1 minute, 50°C for 1 minute, and 70°C for 1 minute). PCR products were electrophoresed on 1.5% agarose gels and visualized by ethidium bromide staining.
Production of CTLs and target cells.Alloreactive CTLs (H-2b anti–H-2d) from splenocytes of various H-2b mice (both C57B1/6 and 129/Sv) were produced in mixed lymphocyte cultures using irradiated BALB/c splenocytes (H-2d) as stimulators, as previously described.18 EL4 (H-2b), TA3 (H-2d), P815 (H-2d), and YAC-1 (H-2a [KkDdLdIk]) target cell lines were maintained in complete medium (RPMI 1640 medium supplemented with 5% fetal bovine serum, 1% nonessential amino acids, 1% sodium pyruvate, 1% glutamine, 1% penicillin/streptomycin, 50 μmol/L 2-mercaptoethanol, and 10 mmol/L HEPES, pH 7.0).
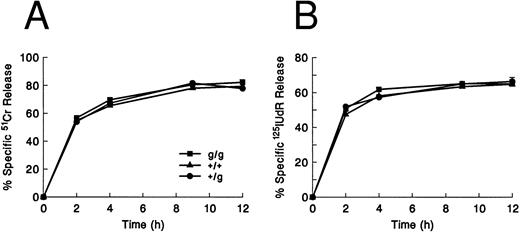
Cytotoxic activity of gld/gld CTLs. MLR-produced CTLs (H-2b anti–H-2d) from wild-type (+/+), +/gld (+/g), and gld/gld (g/g) mouse splenocytes tested against TA3 lymphoma cells at a fixed E:T ratio of 10:1 in 51Cr release (A) and 125IUdR release (B) assays. Both gld/+ and gld/gld CTLs have no detectable defect in the ability to induce 51Cr or 125I-labeled DNA release at all time points tested. Error bars represent the range of values at each point. These are representative data from one of three similar experiments.
Cytotoxic activity of gld/gld CTLs. MLR-produced CTLs (H-2b anti–H-2d) from wild-type (+/+), +/gld (+/g), and gld/gld (g/g) mouse splenocytes tested against TA3 lymphoma cells at a fixed E:T ratio of 10:1 in 51Cr release (A) and 125IUdR release (B) assays. Both gld/+ and gld/gld CTLs have no detectable defect in the ability to induce 51Cr or 125I-labeled DNA release at all time points tested. Error bars represent the range of values at each point. These are representative data from one of three similar experiments.
Flow cytometric analysis.Day 5 MLR-produced CTLs were stained with phycoerythrin-labeled monoclonal antibodies specific for murine CD3, CD4, CD8, B220, and NK1.1 (PharMingen, San Diego, CA) and analyzed on a FACScan using CellQuest software (Becton Dickinson, Mountain View, CA), essentially as previously described.18
RESULTS
Cytotoxic activity of gld/gld CTLs.Allospecific gld/gld CTLs (H-2b anti–H-2d) were produced from spleens of gld/gld mice and tested in lytic assays against both syngeneic (EL4) and allogeneic (TA3) targets (the ability of gld/gld splenocytes to proliferate and activate in MLR cultures was confirmed via flow cytometric analysis; data not shown). In an experiment measuring both 51Cr and 125I-labeled DNA release as a function of time, no differences in cytotoxicity were observed among the FasL+/+, gld/+, or gld/gld CTLs against TA3 targets (Fig 1). The killing of TA3 cells was allospecific, since these same CTLs induced no significant 51Cr or 125I-labeled DNA release against syngeneic EL4 cells (data not shown). These results indicate that MLR-induced CTLs (predominantly CD8+ cells) derived from gld/gld mice have no measurable defect in cytotoxicity against allogeneic targets.
Characterization of granzyme B−/− × gld/gld CTLs.Mice with granzyme B and FasL deficiency were produced by intercrossing granzyme B−/− mice18 to gld/gld mice29-31 and found to be viable. Gross analysis of young (5- to 8-week-old) mice showed normal development, fertility, and hematopoiesis in the doubly deficient animals (data not shown). We used only young animals (5 to 8 weeks of age) in our experiments to avoid studying mice with defects in peripheral T cells (CD4−CD8−T cells accumulate in the periphery of these animals as a function of time). A flow cytometric analysis of primary day 5 MLR cultures obtained from granzyme B+/+, granzyme B−/−, or granzyme B−/− × gld/gld spleens showed normal percentages of CD3+, CD4+, and CD8+ cells in the doubly deficient MLR effector populations (Fig 2). Furthermore, microscopic examination and cell counts of these effector cells showed similar numbers of blast-like cells in MLR cultures derived from the spleens of all three types of animals (data not shown). These results indicate that granzyme B−/− × gld/gld CTLs derived from young animals have normal proliferation and activation.
Flow cytometric characterization of day 5 MLR-generated CTLs (H-2b anti–H-2d) derived from granzyme B+/+, granzyme B−/−, and granzyme B−/− × gld/gld spleens. CTLs were analyzed for CD3, CD4, and CD8 expression by flow cytometry. The relative number of live-gated cells (Y-axis) and logarithmic fluorescence intensity (X-axis) are plotted. All three types of CTLs have a similar composition of T-cell subsets, showing the normal ability of granzyme B−/− × gld/gld effectors to proliferate and activate in response to allogeneic stimuli in vitro.
Flow cytometric characterization of day 5 MLR-generated CTLs (H-2b anti–H-2d) derived from granzyme B+/+, granzyme B−/−, and granzyme B−/− × gld/gld spleens. CTLs were analyzed for CD3, CD4, and CD8 expression by flow cytometry. The relative number of live-gated cells (Y-axis) and logarithmic fluorescence intensity (X-axis) are plotted. All three types of CTLs have a similar composition of T-cell subsets, showing the normal ability of granzyme B−/− × gld/gld effectors to proliferate and activate in response to allogeneic stimuli in vitro.
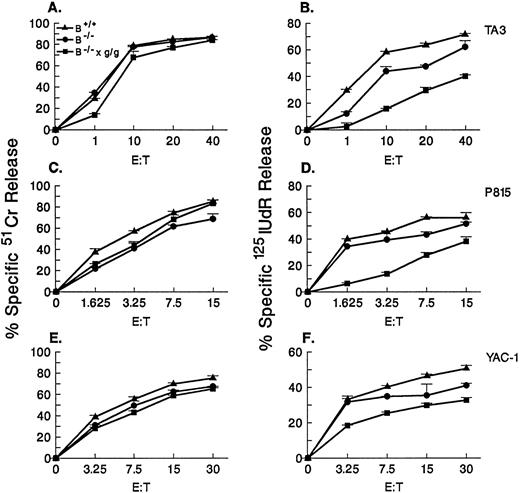
Cytotoxicity of granzyme B−/− × gld/gld CTLs.We next tested the cytolytic activities of these CTLs against syngeneic EL4 (data not shown) or allogeneic TA3, P815, or YAC-1 targets (Figs 3 and 4). Figure 3 shows the results of a lytic assay against TA3 allotargets as a function of time. At all time points tested (from 0 to 24 hours) at a fixed effector:target (E:T) ratio of 10:1, no significant difference was observed in the ability of granzyme B+/+, granzyme B−/−, or granzyme B−/− × gld/gld CTLs to induce 51Cr release from TA3 cells. In contrast, the 125I-labeled DNA release assay showed major differences in these three effector cell populations. As previously shown, granzyme B–deficient CTLs are severely defective in the ability to induce 125I-labeled DNA release for at least 2 hours of incubation; after that, these cells induce increasing amounts of 125I-labeled DNA release as a function of time. The addition of FasL deficiency further compromises the ability of granzyme B−/− CTLs to mediate 125I-labeled DNA release, since the doubly deficient cells cannot mediate any 125I-labeled DNA release for at least 4 to 6 hours of incubation. However, as seen in granzyme B–deficient CTLs, granzyme B−/− × gld/gld CTLs are able to trigger 125I-labeled DNA release after 6 hours of incubation, albeit with reduced efficiency compared with granzyme B−/− cells. These observations were extended in lytic assays performed at 10 hours using three different target cell lines (TA3, P815, and YAC-1; Fig 4). Granzyme B × gld/gld CTLs exhibit a decreased ability to induce 125I-labeled DNA release from all three target lines tested at 10 hours. Together, the results shown in Figs 3 and 4 indicate that granzyme B−/− × gld/gld CTLs have a more severe defect in the induction of allogeneic target cell apoptosis than CTLs lacking granzyme B alone.
One mechanism of granzyme B–independent cytotoxicity involving the Fas pathway. Allospecific day 5 MLR-derived CTLs (H-2b anti–H-2d) from granzyme B+/+ (B+/+), granzyme B−/− (B−/−), and granzyme B−/− × gld/gld (B−/− × g/g) spleens were used against allogeneic TA3 (H-2d) lymphoma cells at a fixed E:T ratio of 10:1 in 51Cr release (A) and 125IUdR release (B) assays. Note that granzyme B−/− and granzyme B−/− × gld/gld CTLs induce essentially normal levels of 51Cr release, whereas both effector populations are unable to mediate any 125I-labeled DNA release at early time points. However, the defective 125I-labeled DNA release is more severe in granzyme B−/− × gld/gld cells than in CTLs deficient for granzyme B alone. Error bars show the range of values at each point. This experiment represents one of four with similar results.
One mechanism of granzyme B–independent cytotoxicity involving the Fas pathway. Allospecific day 5 MLR-derived CTLs (H-2b anti–H-2d) from granzyme B+/+ (B+/+), granzyme B−/− (B−/−), and granzyme B−/− × gld/gld (B−/− × g/g) spleens were used against allogeneic TA3 (H-2d) lymphoma cells at a fixed E:T ratio of 10:1 in 51Cr release (A) and 125IUdR release (B) assays. Note that granzyme B−/− and granzyme B−/− × gld/gld CTLs induce essentially normal levels of 51Cr release, whereas both effector populations are unable to mediate any 125I-labeled DNA release at early time points. However, the defective 125I-labeled DNA release is more severe in granzyme B−/− × gld/gld cells than in CTLs deficient for granzyme B alone. Error bars show the range of values at each point. This experiment represents one of four with similar results.
Allogeneic cytotoxicity of granzyme B−/− × gld/gld CTLs against three different tumor cell lines. H-2b anti–H-2d CTLs produced in day 5 MCR cultures from granzyme B+/+ (B+/+), granzyme B−/− (B−/−), and granzyme B−/− × gld/gld (B−/− × g/g) spleens were tested against TA3 (A and B), P815 (C and D), and YAC-1 (E and F) cells at varying E:T ratios after 10 hours of incubation in a 51Cr release (A, C, and E) and 125IUdR release (B, D, and F) assays. All three types of effector cells induce similar levels of 51Cr release from TA3, P815, or YAC-1 targets. Granzyme B−/− × gld/gld CTLs exhibit reduced amounts of 125I-labeled DNA release at all E:T ratios tested against all three lines.
Allogeneic cytotoxicity of granzyme B−/− × gld/gld CTLs against three different tumor cell lines. H-2b anti–H-2d CTLs produced in day 5 MCR cultures from granzyme B+/+ (B+/+), granzyme B−/− (B−/−), and granzyme B−/− × gld/gld (B−/− × g/g) spleens were tested against TA3 (A and B), P815 (C and D), and YAC-1 (E and F) cells at varying E:T ratios after 10 hours of incubation in a 51Cr release (A, C, and E) and 125IUdR release (B, D, and F) assays. All three types of effector cells induce similar levels of 51Cr release from TA3, P815, or YAC-1 targets. Granzyme B−/− × gld/gld CTLs exhibit reduced amounts of 125I-labeled DNA release at all E:T ratios tested against all three lines.
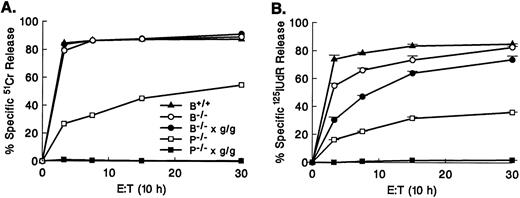
Cytotoxic activities of granzyme B−/− CTLs versus perforin−/− or perforin−/− × gld/gld CTLs.We next wished to determine whether the residual cytotoxicity in granzyme B−/− × gld/gld CTLs is due to a perforin-dependent mechanism. We therefore compared the cytotoxic activities of granzyme B–deficient CTLs versus perforin−/− CTLs to examine the contribution of granzyme B to the perforin/granzyme pathway (Fig 5). As predicted, 51Cr release assays at 4 hours (Fig 5A) and 10 hours (Fig 5C) of incubation showed the presence of an intact 51Cr release pathway in granzyme B−/− CTLs, whereas perforin−/− CTLs were unable to mediate any 51Cr release at 4 hours. However, at 10 hours of incubation, they induced at least 15% of the level of wild-type 51Cr release against allogeneic TA3 targets. Granzyme B accounts for virtually all DNA fragmentation mediated by CTLs in a 4-hour 125I-labeled DNA release assay (Fig 5B). In contrast, the 10-hour 125I-labeled DNA release assay (Fig 5D) confirms that granzyme B−/− CTLs possess a significant ability to induce DNA fragmentation after prolonged incubation. Furthermore, this granzyme B–independent cytotoxicity clearly involves a perforin-dependent mechanism (as represented by the difference between granzyme B−/− and perforin−/− levels) and a perforin-independent mechanism (the difference between perforin−/− and no cytotoxicity). Recent studies from several laboratories have indicated that the residual cytotoxicity in perforin−/− CTLs is mediated at least in part by Fas. We therefore compared cytotoxic activities of granzyme B−/− CTLs, granzyme B−/− × gld/gld CTLs, perforin−/− CTLs, and perforin−/− × gld/gld CTLs in the same experiments. Perforin−/− × gld/gld CTLs cannot trigger 51Cr release even after prolonged incubation (Fig 6A). Similarly, the 10-hour 125I-labeled DNA release assay demonstrates that perforin−/− × gld/gld CTLs do not induce apoptosis in TA3 targets (Fig 6B). The complete lack of cytotoxic activity of perforin−/− × gld/gld CTLs at 10 hours has also been observed against other allotargets, including P815 and YAC-1 (data not shown).
Perforin-dependent mechanism of granzyme B–independent cytotoxicity. Granzyme B+/−, granzyme B−/−, or perforin (−/−) splenocytes were mixed with irradiated Balb/c splenocytes to generate H-2b anti–H-2d CTLs in day 5 MLR cultures. CTLs were tested against TA3 allotargets at various E:T ratios in 4-hour (A and B) and 10-hour (C and D) 51Cr release (A and C) and 125IUdR release (B and D) assays. Granzyme B−/− and perforin−/− CTLs are equally unable to induce DNA fragmentation at 4 hours of incubation. Prolonged incubation of effectors and targets shows that the cytotoxicity remaining in granzyme B−/−CTLs is due to both perforin-dependent and perforin-independent mechanisms. Error bars show the range of values at each point. This experiment represents one of four with essentially identical data.
Perforin-dependent mechanism of granzyme B–independent cytotoxicity. Granzyme B+/−, granzyme B−/−, or perforin (−/−) splenocytes were mixed with irradiated Balb/c splenocytes to generate H-2b anti–H-2d CTLs in day 5 MLR cultures. CTLs were tested against TA3 allotargets at various E:T ratios in 4-hour (A and B) and 10-hour (C and D) 51Cr release (A and C) and 125IUdR release (B and D) assays. Granzyme B−/− and perforin−/− CTLs are equally unable to induce DNA fragmentation at 4 hours of incubation. Prolonged incubation of effectors and targets shows that the cytotoxicity remaining in granzyme B−/−CTLs is due to both perforin-dependent and perforin-independent mechanisms. Error bars show the range of values at each point. This experiment represents one of four with essentially identical data.
Granzyme B–independent cytotoxicity is accounted for by the Fas pathway and a distinct perforin-dependent mechanism. CTLs (H-2b anti–H-2d) produced in day 5 MLR splenocyte cultures from wild-type (B+/+), granzyme B−/−, granzyme B−/− × gld/gld, perforin (P) −/−, and P−/− × gld/gld were tested against allogeneic TA3 cells at various E:T ratios after prolonged incubation (10 hours) in 51Cr release (A) and 125IUdR release (B) assays. P−/− × gld/gld CTLs do not exhibit any cytotoxicity even after prolonged incubation. Similar results were obtained using P815 and YAC-1 target cell lines (data not shown). Error bars show the range of values at each point. This experiment represents one of three with similar results.
Granzyme B–independent cytotoxicity is accounted for by the Fas pathway and a distinct perforin-dependent mechanism. CTLs (H-2b anti–H-2d) produced in day 5 MLR splenocyte cultures from wild-type (B+/+), granzyme B−/−, granzyme B−/− × gld/gld, perforin (P) −/−, and P−/− × gld/gld were tested against allogeneic TA3 cells at various E:T ratios after prolonged incubation (10 hours) in 51Cr release (A) and 125IUdR release (B) assays. P−/− × gld/gld CTLs do not exhibit any cytotoxicity even after prolonged incubation. Similar results were obtained using P815 and YAC-1 target cell lines (data not shown). Error bars show the range of values at each point. This experiment represents one of three with similar results.
DISCUSSION
By using CTLs derived from loss-of-function mouse models, we have demonstrated in this study that the delayed cytotoxicity of granzyme B−/− CTLs is due to Fas- and perforin-dependent mechanisms. Compared with CTLs deficient for granzyme B alone, granzyme B−/− × gld/gld CTLs have a longer delay in the ability to induce DNA fragmentation, and they have less 125I-labeled DNA release with long incubation times. Nevertheless, granzyme B−/− × gld/gld CTLs are still able to induce a substantial amount of target cell apoptosis, suggesting the presence of additional cytotoxic effector mechanisms. Our analysis of perforin−/− and perforin−/− × gld/gld CTLs indicates that most of the residual cytotoxicity in granzyme B−/− × gld/gld CTLs is provided by a perforin-dependent mechanism.
The finding that the Fas system accounts for some granzyme B–independent cytotoxicity in CTLs is not surprising, in light of accumulating data on perforin−/− and perforin−/− × gld/gld mice16,26 (and J. Spielman, R. K. Lee, E. R. Podack, personal communication, November 1996). We have confirmed that the perforin/granzyme and Fas pathways represent the two primary mechanisms of cytotoxicity in primary MLR-derived CTLs directed against a limited variety of allogeneic hematopoietic target cell lines. Although MLR-produced CTLs lacking FasL have normal cytotoxicity in our assay systems, the role of Fas in CTL cytotoxicity becomes apparent using granzyme B−/− × gld/gld or perforin−/− × gld/gld CTLs, indicating that deficiency for either molecule is sufficient to unmask the involvement of Fas in CTL-mediated cytotoxicity. The importance of the Fas pathway for cell-mediated cytotoxicity is still unclear, since the primary defect in lpr and gld mice appears to be the lack of activated T-cell death in the periphery.24,25,32-34 However, studies with perforin−/− × gld/gld and granzyme B−/− × gld/gld mice are beginning to corroborate the importance of this pathway for lymphocyte cytotoxicity in vivo. Recently, Braun et al28 demonstrated that in an acute GVHD model, 100% of mice receiving perforin−/− × gld/gld splenocytes survived, whereas recipients of either perforin−/− or gld/gld splenocytes had only delayed onset of GVHD. Similarly, our own studies on CD4+ or CD8+ CTL-mediated acute GVHD have shown that the Fas pathway accounts for some of the cytotoxicity exhibited by CD4+ CTLs (T.A. Graubert, J.H. Russell, and T. J. Ley, submitted), whereas the perforin/granzyme pathway is the most important for CD8+ CTL-mediated acute GVHD. These in vivo results suggest that different subsets of CTLs may use different primary effector mechanisms. Further studies with these doubly deficient mice should help to answer questions regarding the contribution(s) of the two pathways to different types of cytolytic effector activities in different clinical settings.
Granzyme B−/− × gld/gld CTLs (as well as CTLs deficient for granzyme B alone) have an intact 51Cr release pathway despite a severe inability to rapidly mediate DNA fragmentation. The membrane-damage pathway is therefore clearly separate from that of DNA fragmentation. Since an intact Fas pathway only minimally compensates for the inability of perforin−/− CTLs to induce 51Cr or 125I-labeled DNA release, our results with granzyme B−/− × gld/gld CTLs lend further support to the fact that CD8+ CTLs cause membrane damage (51Cr release) mainly via perforin and induce DNA damage (125I-labeled DNA release) primarily through perforin-dependent molecules (granzyme B and others).
Some of the late cytotoxicity manifest in granzyme B−/− mice is perforin-dependent, suggesting that additional granule proteins may be responsible for this activity. One candidate is granzyme A. Granzyme A purified from mouse CTL granules can induce 125I-labeled DNA release from target cells that have been permeabilized with detergent or perforin.35,36 Additionally, Shi et al36 have shown that granzyme A purified from rat NK granules mediates 125I-labeled DNA release from perforin-permeabilized target cells with slower kinetics than granzyme B. Similarly, studies with noncytotoxic rat basophilic leukemia (RBL) cells transfected with perforin, granzyme A, and/or granzyme B cDNA have shown that (1) RBL lines must express both perforin and either granzyme A or B for induction of DNA fragmentation in targets, and (2) triple-transfected RBL cells expressing perforin, granzyme A, and granzyme B trigger much higher levels of 125I-labeled DNA release, suggesting synergy between the two proteases.13 Finally, CTLs transfected with antisense granzyme A cDNA have a decreased ability to induce 51Cr release.37 Despite these observations, mice homozygous for an insertional mutation in the granzyme A gene have recently been shown to have no clear-cut defect in cell-mediated cytotoxicity.38 However, granzyme B (or some other molecule) could potentially be masking or compensating for the lack of granzyme A in these mice. Studies with mice deficient for both granzymes should clarify the role of granzyme A as a possible effector of granzyme B–independent cytotoxicity.
Another candidate for granzyme B–independent, perforin-dependent cytotoxicity is granzyme K. Although the mouse ortholog of human and rat granzyme K has not yet been described, the rat NK granule-purified granzyme (also called “fragmentin 1”36 or “tryptase 2”39) has been shown to induce DNA fragmentation of perforin-permeabilized target cells with slow kinetics (similar to granzyme A).36 In addition to the granzymes mentioned thus far, murine cytotoxic lymphocyte granules also contain variable amounts of granzymes C, D, E, F, and G9,40-42 and additional proteins, any of which could potentially be involved in granzyme B–independent cytotoxicity. Systematic characterization of these other molecules will be required to define their potential roles in cell-mediated cytotoxicity.
ACKNOWLEDGMENT
We thank Robin Wesselschmidt and Pam Goda for their expertise in animal care, and Nancy Reidelberger for excellent assistance in preparation of the manuscript.
Supported by the National Institutes of Health (CA-49712) and the Washington University-Monsanto Agreement.
Address reprint requests to Timothy J. Ley, MD, Washington University Medical School, Division of Bone Marrow Transplantation and Stem Cell Biology, Box 8007, 660 S Euclid Ave, St Louis, MO 63110-1093.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal