Abstract
We investigated the role of humoral factors in lipopolysaccharide (LPS) priming of polymorphonuclear leukocytes (PMN) using cells isolated from adults and from neonates. Plasma from newborn infants had decreased priming activity of adult plasma when mixed with LPS in studies measuring oxidative radical production of PMN after stimulation with a formyl bacterial oligopeptide (fMLP). This marked difference was not caused by LPS binding protein (LBP) because the LBP concentration in newborn and adult plasma were similar (138.4 ± 12.9 U for adults, and 126.9 ± 12.1 U for neonates, P = .53). Therefore, we attempted to identify other plasma factors that may contribute to LPS priming of PMN. We identified an LPS priming factor for PMN that is present in plasma, heat stable (56°C for 30 minutes), enhanced by heparin, and concentrated in cold precipitates of plasma. Because these properties resemble those of plasma fibronectin, we assessed the role of fibronectin in LPS priming of PMN. Although fibronectin in phosphate-buffered saline (PBS) had little effect on LPS priming of PMN, fibronectin in combination with other plasma factors appeared to play a role in LPS priming of PMN because (1) removing fibronectin from adult plasma dramatically decreased LPS priming activity from plasma (P < .005), (2) addition of fibronectin to fibronectin-depleted plasma restored its LPS plasma priming activity (P < .05), and (3) neutralizing fibronectin with antibody decreased the LPS priming activity of plasma (60.3 ± 1.3 v 30.2 ± 2.2, P < .01). Thus, plasma fibronectin plays a role in LPS priming of PMN in the presence of other factors in plasma.
BACTERIAL INFECTION is a leading cause of mortality among newborn infants.1 Increased susceptibility of neonates to bacterial infection is due, in part, to the immaturity of both the cellular and the humoral immune systems.1 Although a number of abnormalities in the neonate's host defense have been described, one of the most consistent is in the deficiency of the polymorphonuclear leukocytes (PMN) to phagocytose and kill bacteria.2-4 PMN from neonates are known to have defective phagocytosis, chemotaxis, and deformability.5,6 In addition, humoral factors required for opsonization of bacteria may be inadequate.7
Because of the high rate of infection in the neonate and the availability of products for replacement, transfusions with PMN,8 intravenous IgG (IGIV), or plasma have been used in an attempt to prevent infection or modify its severity.8-10 Such efforts have met with limited success largely because each of these components has a short half-life or, as with IGIV, a narrow spectrum of activity.
In adults, bacterial products and host cytokines elaborated during bacterial infection can affect PMN activity. Interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and IL-8 enhance PMN activity in vitro and are produced by the host during infection.11-14 In addition, lipopolysaccharide (LPS), which is a component of gram-negative bacteria, may “prime” monocytes and PMN in vitro and in vivo.15-19 Although primed PMN appear no different morphologically from unprimed cells, when exposed to formylized bacterial oligopeptides such as formyl-methionyl-leucyl-phenylalanine (fMLP), they produce reactive oxygen radicals including superoxide (O−2) and hydrogen peroxide (H2O2 ) at a greater rate than unprimed PMN.
Recently, Ulevitch and Tobias have shown that leukocytes interact with LPS through a serum protein which first binds to LPS.20-22 The LPS-protein complex then associates with specific receptors on the cell surface. They identified this factor in the serum of rabbits and termed it LPS-binding-protein (LBP).23 The protein has recently been characterized and cloned.21,24 LBP is a 60-kD protein synthesized in the liver. LBP forms a high-affinity (kd approximately 10−9) complex with LPS. LPS-LBP complexes interacted with membrane-bound CD14 on monocytes, macrophages, and PMN, and with soluble CD14 and endothelial cells to facilitate the activation of a number of intracellular events.25-27 The LPS-LBP interaction with membranes has been studied most extensively on monocytes, where its receptor has been identified as CD14, a myeloid differentiation antigen of monocytes and macrophages.21
The role of other plasma factor(s) in LPS interaction with PMN has not been studied as extensively. Vosbeck et al18 have proposed that LBP serves the same function with PMN as it does with monocytes. In their hands, LBP, when complexed to LPS, enhanced priming induced by LPS on PMN. However, Wright et al28 have shown that two components in plasma interact to produce a factor that opsonizes LPS for recognition by PMN. This factor, termed “septin,” exists in normal plasma and is not neutralized by monoclonal antibody to LBP. Recently, Lee et al29 and Ulevitch and Tobias have proposed that other membrane molecules may be required for the LPS-LBP complex to induce transmembrane signaling.
With PMN taken from adults, priming with LPS is associated with upregulation of fMLP receptors and CD14.25,30,31 We have recently shown that PMN from newborn cord blood incubated with LPS do not upregulate fMLP receptors and CD14, and do not increase O−2 activity when triggered with fMLP.32,33 Adult membrane receptors for LPS also differ from those of newborn cells. As recently reported, 55-kD LPS-binding proteins are predominantly expressed on adult but not on neonatal plasma.34 Because CD14 has a molecular weight of 55 kD, these results support a role for LBP, in plasma, and CD14, on PMN, as important factors in LPS priming of PMN. In the present study we have directed our attention to identify other plasma factor(s) that may contribute to LPS priming. On the basis of the experiments described here we believe that a second humoral factor, fibronectin, is involved in LPS priming of PMN.
MATERIALS AND METHODS
Reagents.fMLP, lucigenin, and LPS (serotype 0111: B4) were obtained from Sigma Chemical Co (St Louis, MO). LPS was diluted in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (PBS/BSA). Sterile water (no preservative, nonpyrogenic) was obtained from Abbott Laboratories. Hanks' balanced salt solution (HBSS) and human fibronectin were obtained from GIBCO Laboratories (Grand Island, NY). All buffers were checked for endotoxin by the limulus amebocyte lysate test (Sigma Chemical Co) to ensure that none was detectable (<12 pg/mL).
Antibodies.Polyclonal antifibronectin was raised in rabbit against adult human plasma fibronectin and has been characterized to be specific for fibronectin; the antiserum yields a single precipitin band against purified human FN, human plasma, and serum in an Ouchterlony assay.35
PMN preparation and priming.Peripheral venous blood from healthy adult volunteers and umbilical cord blood from normal full-term neonates were collected sterilely and anticoagulated with heparin (10 U/mL). PMN were separated using Percoll gradient centrifugation (Pharmacia Chemical Co, Montreal, Quebec, Canada) as described previously, and suspended to a concentration of 2 × 106/mL in HBSS.33 PMN were routinely isolated to greater than 90% purity, and greater than 98% viability in approximately 2 hours and were assayed immediately unless otherwise specified. All solutions used in the separation of PMN were prepared using intravenous-grade, pyrogen-free water. If single-use sterile plasticware was not used, glassware was rendered pyrogen-free by baking at 270°C. Priming of PMN was done in the presence or absence of plasma using 0.2 mL of PMN suspended in HBSS (2 × 106/mL). Unless otherwise stated, final concentration of LPS for priming of PMN was 10 ng/mL. Duplicate samples were incubated at 37°C with shaking (100 rpm). After 45 minutes a chemiluminescence (CL) assay was performed using fMLP to trigger oxidative radical production by PMN. The optimal concentration of LPS (10 ng/mL) and length of time for priming (45 minutes) were used in LPS priming studies unless otherwise stated.32 33
CL assay.A CL assay was used as an indicator of oxidative radical production. Luminol (10−5 mol/L), a CL indicator for oxidative radicals,36 was used initially (see Figs 2 and 3). Lucigenin (8 × 10−5 mol/L), a sensitive CL indicator which is specific for O−2 ,37 was used later to confirm the specificity of oxygen radical release. After adding 0.8 mL of the CL enhancer, background CL activity (measured in millivolts) was determined using a luminometer (LKB, Wallac, Finland). The oxidative reaction was triggered by adding 100 μL of fMLP (final concentration 10−5 mol/L), and the CL reaction was recorded continuously for 4 minutes. Results were expressed as CL units, which were calculated as the area under the curve (AUC) defined by millivolts and time in minutes. Background AUC was subtracted from the AUC after adding fMLP.
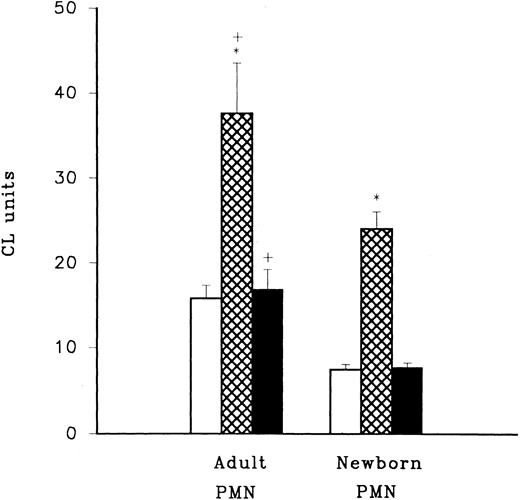
LPS priming of adult and neonatal PMN. LPS priming activity was assessed using LPS mixed with HBSS (□), adult plasma (⊠), and newborn plasma (▪) in combination with adult or newborn PMN. LPS primed PMN were triggered to produce CL activity by the addition of fMLP and oxidative radical production was assessed using luminol (Sigma Chemical Co) with a CL assay. Activity for cells triggered with fMLP in the absence of LPS priming was consistently less than 5 CL units in this experiment. *P < .0005 adult PMN with adult plasma (n = 12) compared to adult PMN with newborn plasma (n = 12), and neonatal PMN with adult plasma (n = 12) compared to neonatal PMN with newborn plasma (n = 12). +P < .05 adult PMN versus newborn PMN.
LPS priming of adult and neonatal PMN. LPS priming activity was assessed using LPS mixed with HBSS (□), adult plasma (⊠), and newborn plasma (▪) in combination with adult or newborn PMN. LPS primed PMN were triggered to produce CL activity by the addition of fMLP and oxidative radical production was assessed using luminol (Sigma Chemical Co) with a CL assay. Activity for cells triggered with fMLP in the absence of LPS priming was consistently less than 5 CL units in this experiment. *P < .0005 adult PMN with adult plasma (n = 12) compared to adult PMN with newborn plasma (n = 12), and neonatal PMN with adult plasma (n = 12) compared to neonatal PMN with newborn plasma (n = 12). +P < .05 adult PMN versus newborn PMN.
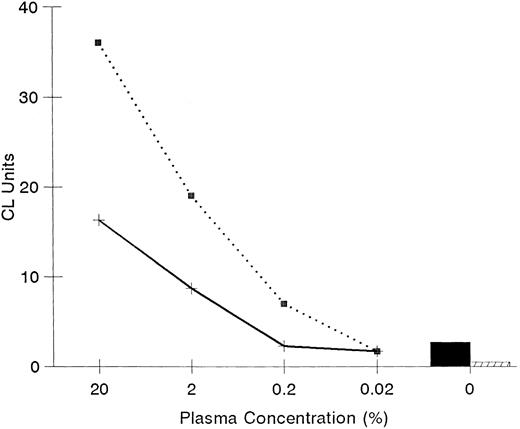
Effect of plasma on LPS priming. Effects of plasma on LPS priming of PMN were assessed using plasma from a single adult (⋅⋅⋅) and a single newborn infant (——) done in triplicate. CL units were determined with luminol as an indicator of oxidative radicals using PMN primed with LPS and plasma (0% to 20%). The priming activity with LPS by itself (▪) or with no LPS (□) are also shown.
Effect of plasma on LPS priming. Effects of plasma on LPS priming of PMN were assessed using plasma from a single adult (⋅⋅⋅) and a single newborn infant (——) done in triplicate. CL units were determined with luminol as an indicator of oxidative radicals using PMN primed with LPS and plasma (0% to 20%). The priming activity with LPS by itself (▪) or with no LPS (□) are also shown.
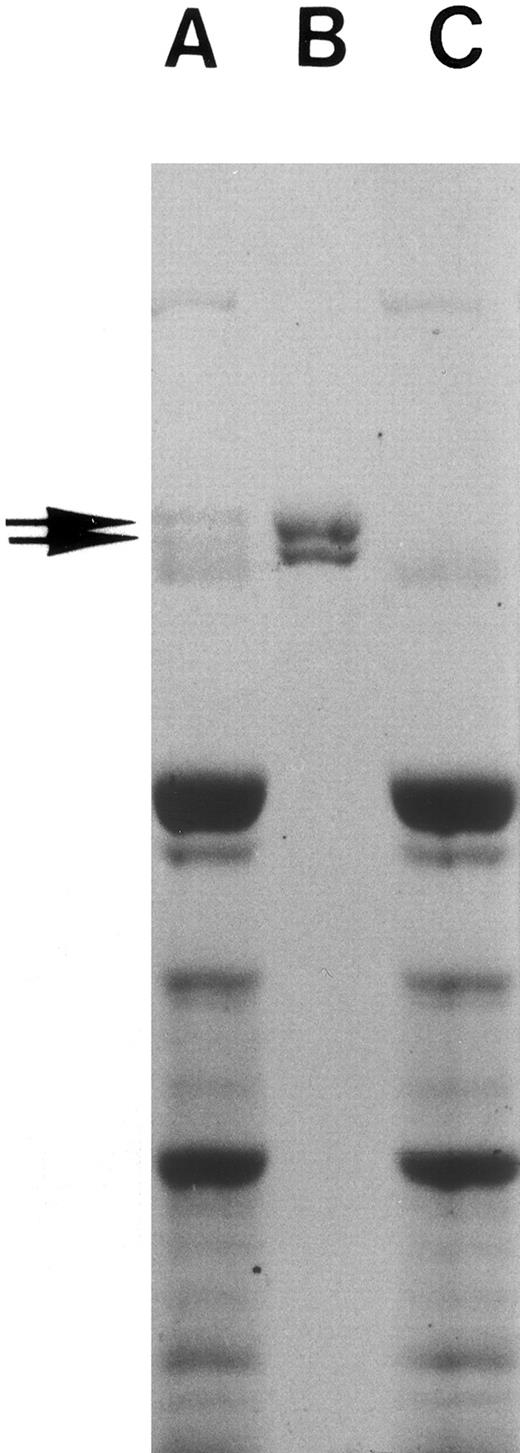
Preparation of fibronectin and fibronectin-free plasma.Fibronectin from human plasma was prepared by the gelatin-Sepharose (Sigma Chemical Co) affinity column38,39 and used as such or further purified by gel filtration40 or obtained from commercial sources (GIBCO-BRL, Bethesda, MD). Purified fibronectin showed two major bands with a molecular weight of about 225 kD and 235 kD in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; Fig 1). Plasma passed through the affinity column three times was free of any residual fibronectin as indicated by SDS-PAGE (Fig 1) and was used as fibronectin-depleted plasma.39,41 Plasma contained no detectable endotoxin (<12 pg/mL) while fibronectin-depleted plasma had less than 0.3 ng/mL, a concentration that does not affect PMN priming.32 Protein concentration was measured with an automated colorimetric spectrophotometer using Bromocresol green (Kodak, Rochester, NY). Fibronectin concentration was 640 ± 20 μg/mL for adult plasma, <5 μg/mL in fibronectin-depleted adult plasma, and 249 ± 100 μg/mL for newborn plasma. Fibronectin was measured using a commercial ELISA (Biomedical Technologies Inc, Stoughton, MA).
Fibronectin preparation. Human whole plasma was passed through gelatin-Sepharose column three times, and fibronectin eluted from the column as described in Materials and Methods. SDS-PAGE of adult human plasma (A), purified plasma fibronectin (10 μg, B), and fibronectin-depleted adult human plasma (C) is shown. The gel was then stained with Coomassie blue. Note the missing fibronectin doublet in lane C and that no other protein bands are missing.
Fibronectin preparation. Human whole plasma was passed through gelatin-Sepharose column three times, and fibronectin eluted from the column as described in Materials and Methods. SDS-PAGE of adult human plasma (A), purified plasma fibronectin (10 μg, B), and fibronectin-depleted adult human plasma (C) is shown. The gel was then stained with Coomassie blue. Note the missing fibronectin doublet in lane C and that no other protein bands are missing.
Statistical analysis.All data are presented as the mean ± SE. For comparison, paired or nonpaired t-test as described in text were used; a P value <.05 was considered significant. Statistical determinations were done using a computer statistics program (Statistix; Analytical Software, Minneapolis, MN).
RESULTS
Adult but not newborn plasma enhances LPS priming of PMN.The pretreatment of PMN with low concentrations of LPS (10 ng/mL) for 45 minutes at 37°C was found to potentiate oxidative radical release triggered by fMLP. The amount of fMLP-triggered oxidative radical released in LPS-pretreated PMNs was significantly greater for adult and newborn cells that were incubated with 10% adult heparinized plasma compared to cells incubated with 10% newborn heparinized plasma or with no plasma (Fig 2, P < .0005). Although newborn PMN activity was significantly greater when plasma from adults was present compared to plasma from newborn infants, PMN from neonates were still significantly less active than adult cells (P < .05). In these experiments newborn plasma did not enhance LPS priming compared with LPS priming in the absence of plasma (P > .05). The greatest LPS priming effect was achieved when adult PMN and adult plasma were used.
The effectiveness of adult plasma in combination with LPS to prime PMN was evident over a range of plasma concentrations (Fig 3). However, plasma from newborn infants in combination with LPS was less effective than plasma from adults at priming adult PMN; newborn plasma at 20% concentration was equivalent to 2% adult plasma. To assess higher concentrations of plasma, the method for PMN preparation was modified by suspending cells in adult or newborn plasma before adding LPS. With this modification plasma concentrations of 40% and 80% were assessed. A difference in priming activity between adult and newborn plasma was evident at higher concentrations of plasma but at reduced amounts. At 40% plasma, newborn plasma showed 39% ± 8% reduced activity compared with adult plasma (P < .05 for adult v newborn plasma). At 80% plasma the difference between adult and newborn plasma was 13% ± 4% (P > .05 for adult v newborn plasma). To further investigate the role of plasma in LPS priming of PMN, we examined if an inhibitor of LPS priming of PMN is present in newborn plasma. As shown by mixing of plasma from adults and newborns, plasma from newborns did not interfere with the priming activity of adult plasma (Table 1). Also, adult plasma partially corrected the priming capacity of plasma from newborns. These experiments indicated that plasma from cord blood does not inhibit LPS priming activity.
Priming Activity of Adult and Newborn Plasma
| Expt. No. . | Adult . | Newborn (NB, 10%) . | Adult . | Newborn (8%) . | AD + NB (2% + 8%) . |
|---|---|---|---|---|---|
| . | (AD, 10%) . | . | (2%) . | . | . |
| 1 | 3.7* | 2.8 | 2.3 | 2.4 | 3.3 |
| 2 | 2.5 | 2.3 | 2.0 | 2.0 | 3.0 |
| 3 | 5.5 | 3.8 | 3.5 | 3.8 | 4.5 |
| 4 | 3.6 | 2.0 | 2.0 | 2.1 | 2.6 |
| Mean ± SE | 4.08 ± 0.45 | 2.73 ± 0.4 | 2.45 ± 0.35 | 2.58 ± 0.4 | 3.36 ± 0.4 |
| Expt. No. . | Adult . | Newborn (NB, 10%) . | Adult . | Newborn (8%) . | AD + NB (2% + 8%) . |
|---|---|---|---|---|---|
| . | (AD, 10%) . | . | (2%) . | . | . |
| 1 | 3.7* | 2.8 | 2.3 | 2.4 | 3.3 |
| 2 | 2.5 | 2.3 | 2.0 | 2.0 | 3.0 |
| 3 | 5.5 | 3.8 | 3.5 | 3.8 | 4.5 |
| 4 | 3.6 | 2.0 | 2.0 | 2.1 | 2.6 |
| Mean ± SE | 4.08 ± 0.45 | 2.73 ± 0.4 | 2.45 ± 0.35 | 2.58 ± 0.4 | 3.36 ± 0.4 |
Plasma priming activity was expressed as fold increase (FI) of PMN fMLP-triggered CL activity for samples containing LPS and plasma divided by CL activity for samples containing LPS and HBSS (no plasma).
Role of LBP in LPS priming of PMN.Because of its known role in priming of PMN, we compared LBP activity in the plasma of newborns and adults. The LBP assay was kindly performed by Dr P. Tobias (Scripps Institute, La Jolla, CA) using an LBP functional assay.42 When this was done, no significant difference was found between LBP content in newborn plasma (126.9 ± 12.1 U) compared with adult plasma (138.4 ± 12.9 U, P = .53). However, plasma priming activity, defined as fold increase (FI) of LPS priming in the presence over the absence of plasma, was significantly greater for adult plasma (1.88 ± .32, FI) compared with newborn plasma (1.02 ± 0.32 FI, P < .00001).
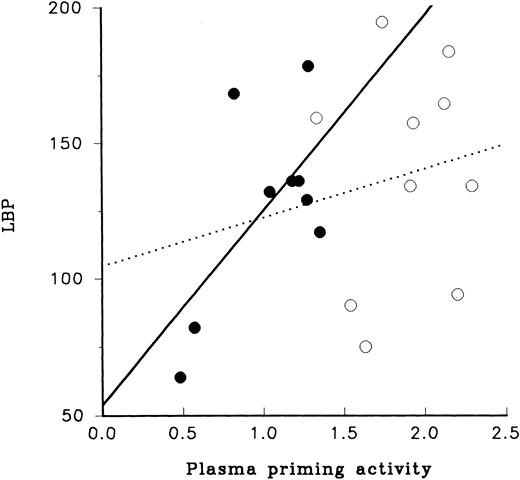
Analysis of the relationship between LBP and plasma priming activity provided contrasting results for adult and newborn samples (Fig 4). For adult plasma the level of LBP had little relationship to its plasma priming activity, whereas plasma priming activity of newborn plasma appeared to have a direct relationship to LBP; samples with low or high levels of LBP also had low or high levels of plasma priming activity, respectively.
LBP and LPS priming of PMN. The relation of LBP to priming activity of plasma obtained from newborn infants (•) and from adults (○) is illustrated. As shown here the range of concentrations of LBP for neonatal and adult plasma were similar, 50 to 200 U. Relationship of LBP to priming activity for newborn plasma is shown by the solid line (r = .40, slope1.4). The relationship of LPS priming activity using adult plasma to LBP is shown with the dotted line (r = .02, slope = 17.9).
LBP and LPS priming of PMN. The relation of LBP to priming activity of plasma obtained from newborn infants (•) and from adults (○) is illustrated. As shown here the range of concentrations of LBP for neonatal and adult plasma were similar, 50 to 200 U. Relationship of LBP to priming activity for newborn plasma is shown by the solid line (r = .40, slope1.4). The relationship of LPS priming activity using adult plasma to LBP is shown with the dotted line (r = .02, slope = 17.9).
Characterization of LPS-plasma priming factor.Because the experiments described so far used heparin as the anticoagulant, we investigated whether heparin and plasma were necessary or if other anticoagulants or serum would enhance priming of PMN. For this we used blood from the same adult donor collected with two different anticoagulants, citrate phosphate dextrose (CPD) or heparin, to prepare plasma or with no anticoagulant to prepare serum (Table 2). We also determined if cold precipitated plasma contained the priming factor because only a limited number of plasma proteins are concentrated by cold precipitation. Cryoprecipitate was prepared by quickly freezing citrated phosphate dextrose plasma then warming it to 4°C over 50 minutes. Precipitated proteins were separated by centrifugation and resolubolized by warming to 37°C. Resolubolized cryoprecipitate was centrifuged to remove any sediment, and then filtered through a 0.2-μm filter to remove any remaining particulate material. We found that solubilized, filtered cryoprecipitate also had high LPS-plasma priming factor (Table 2).
Characterization of LPS Plasma Priming Factor
| LPS-Priming Cofactor . | No. Studies . | Priming Activity . | Significance . | |
|---|---|---|---|---|
| . | . | % of Simultaneously Conducted Heparinized Plasma Control . | v Plasma . | v HBSS . |
| Plasma (heparin)* | — | 100 | — | P < .0001 |
| Plasma (CPD) | 9 | 77.6 ± 13† | NS | P < .001 |
| Serum | 7 | 71.1 ± 12 | P < .05 | P < .05 |
| Cryoprecipitate | 5 | 144.3 ± 17.5 | P < .01 | P < .001 |
| HBSS | 10 | 23.2 ± 5.8 | P < .0001 | — |
| LPS-Priming Cofactor . | No. Studies . | Priming Activity . | Significance . | |
|---|---|---|---|---|
| . | . | % of Simultaneously Conducted Heparinized Plasma Control . | v Plasma . | v HBSS . |
| Plasma (heparin)* | — | 100 | — | P < .0001 |
| Plasma (CPD) | 9 | 77.6 ± 13† | NS | P < .001 |
| Serum | 7 | 71.1 ± 12 | P < .05 | P < .05 |
| Cryoprecipitate | 5 | 144.3 ± 17.5 | P < .01 | P < .001 |
| HBSS | 10 | 23.2 ± 5.8 | P < .0001 | — |
Abbreviation: NS, not significant.
Anticoagulant used in preparing plasma is indicated in brackets.
Percent of simultaneously conducted LPS priming study with plasma (heparin) present as cofactor (mean ± SEM).
The specific role of heparin in enhancing priming activity was studied further. Heparin did not significantly increase LPS priming activity of serum or plasma compared with nonheparin controls at a high concentration of serum or CPD plasma (10% concentration). However, heparin did increase priming activity of CPD-derived plasma at a 0.1% concentration of plasma. The properties of plasma priming factor were assessed further by determining if the factor was tolerant to heat. Warming plasma to 56°C for 30 minutes had no effect on its ability to prime PMN in combination with LPS. Heat-treated plasma and serum had 110% and 103% priming activity of unheated controls, respectively (P > .05, not significant).
Thus, the factor(s) in plasma that contribute to LPS-priming of PMN is(are) relatively heat stable, and may be enhanced by heparin. In addition, LPS-priming activity was present in high concentration in the cryoprecipitates of plasma.
Role of fibronectin in LPS priming of PMN.Because fibronectin is present in high concentration in cryoprecipitate and is known to be in low concentration in newborn cord blood,43 we determined if fibronectin plays a role in LPS priming of PMN. Several experimental strategies were used to explore this hypothesis.
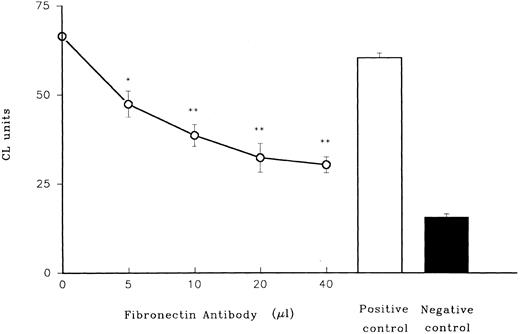
When polyclonal rabbit antibody to fibronectin was added to heparinized plasma, the ability of the plasma in combination with LPS to prime PMN was drastically diminished in a dose-dependent manner (Fig 5), while control preimmune rabbit serum had no effect.
Role of fibronectin in LPS priming of PMN. Effect of rabbit antibody to fibronectin on LPS priming of adult plasma is shown. Antiserum from rabbits was mixed with adult plasma (n = 3) before it was used in combination with LPS to prime adult PMN. In these and subsequent experiments lucigenin, which is more sensitive than luminol as a CL indicator, was used. PMN were triggered with fMLP. A positive control (preimmune rabbit serum together with plasma and LPS) and a negative control (plasma with no LPS) are shown on the right. *P < .05 versus positive control (preimmune rabbit serum). **P < .01 versus positive control.
Role of fibronectin in LPS priming of PMN. Effect of rabbit antibody to fibronectin on LPS priming of adult plasma is shown. Antiserum from rabbits was mixed with adult plasma (n = 3) before it was used in combination with LPS to prime adult PMN. In these and subsequent experiments lucigenin, which is more sensitive than luminol as a CL indicator, was used. PMN were triggered with fMLP. A positive control (preimmune rabbit serum together with plasma and LPS) and a negative control (plasma with no LPS) are shown on the right. *P < .05 versus positive control (preimmune rabbit serum). **P < .01 versus positive control.
In a second experiment, fibronectin-depleted (FN-depleted) plasma was compared to FN-depleted plasma reconstituted with fibronectin (Fig 6). Priming activity of adult FN-depleted plasma was decreased compared with normal adult plasma (56.3 ± 9.5 v 79.5 ± 10.3, P < .005). Priming activity was restored after FN-depleted plasma was reconstituted with purified fibronectin. We also supplemented FN-depleted newborn plasma and PBS with fibronectin purified from adult plasma to determine the effect on LPS priming. We found that the ability of all preparations to prime PMN was increased in a dose-dependent manner by fibronectin. As shown here, fibronectin by itself in PBS had little effect on priming activity (increment of 16.7 CL units as fibronectin increased from 0 to 300 μg/mL) compared with fibronectin added to adult FN-depleted plasma (increment of 39.4 CL units) or newborn FN-depleted plasma (increment of 40.2 CL units). Newborn plasma and adult FN-depleted plasma had similar activity (50.3 and 56.3 CL units, respectively). Also, newborn FN-depleted plasma that was reconstituted with 300 μg/ml adult fibronectin had similar activity as normal adult plasma (79.3 ± 20.6 and 79.5 ± 10.3 CL units, respectively).
Fibronectin supplements and LPS priming of PMN. Purified fibronectin was added to PBS (n = 5, •), adult fibronectin-depleted plasma (n = 5, ▿), or newborn fibronectin-depleted plasma (n = 5, ▾) and the preparations were used in LPS priming experiments. Results are expressed as CL units using lucigenin as the indicator for superoxide production by PMNs after fMLP triggering. Using multivariate repeated measures analysis of variance the overall effect of fibronectin dose was highly significant (P = .001). In addition, for each step increment of fibronectin 0-75, 75-150, and 150-300 significant increases were found (P = .002, P = .0001, and P = .008, respectively). This relationship was also significant for both the adult and newborn FN-depleted samples supplemented with fibronectin. Effect of untreated adult plasma (AD) and untreated newborn plasma (NB) on LPS priming are shown on right of the figure. *P < .05, increment in CL units for sample supplemented with 0 to 300 μg/mL fibronectin versus increment in CL units for PBS supplemented with 0 to 300 μg/mL fibronectin. +P < .05 priming activity (CL units) of newborn FN-depleted plasma reconstituted with 300 μg of fibronectin compared with that of adult plasma. ▿P < .05 priming activity (CL units) of adult plasma compared with that of adult FN-depleted plasma.
Fibronectin supplements and LPS priming of PMN. Purified fibronectin was added to PBS (n = 5, •), adult fibronectin-depleted plasma (n = 5, ▿), or newborn fibronectin-depleted plasma (n = 5, ▾) and the preparations were used in LPS priming experiments. Results are expressed as CL units using lucigenin as the indicator for superoxide production by PMNs after fMLP triggering. Using multivariate repeated measures analysis of variance the overall effect of fibronectin dose was highly significant (P = .001). In addition, for each step increment of fibronectin 0-75, 75-150, and 150-300 significant increases were found (P = .002, P = .0001, and P = .008, respectively). This relationship was also significant for both the adult and newborn FN-depleted samples supplemented with fibronectin. Effect of untreated adult plasma (AD) and untreated newborn plasma (NB) on LPS priming are shown on right of the figure. *P < .05, increment in CL units for sample supplemented with 0 to 300 μg/mL fibronectin versus increment in CL units for PBS supplemented with 0 to 300 μg/mL fibronectin. +P < .05 priming activity (CL units) of newborn FN-depleted plasma reconstituted with 300 μg of fibronectin compared with that of adult plasma. ▿P < .05 priming activity (CL units) of adult plasma compared with that of adult FN-depleted plasma.
DISCUSSION
The major elements identified to date that are crucial for PMN priming are LBP and PMN membrane-bound CD14. In this report we provide evidence that a second humoral element, fibronectin, contributes to the LPS-induced PMN priming process. These studies follow from our recent report showing that LPS fails to prime newborn PMN33 and to upregulate PMN CD14.32 Although this is the first report identifying and characterizing a second plasma factor, our findings are consistent with those of others who have speculated on the existence of a multichain receptor or multiple receptors for LPS priming.27,29 44
In our experiments, heparinized plasma produced a dose-dependent increase in LPS priming of PMN as plasma concentration was increased from 0.02% to 20%. Although a dose-dependent response for priming was found using either adult or newborn plasma, adult plasma primed PMN more effectively. At 40% and 80% plasma, differences between adult and newborn activity remained, but at a lower amount. This suggests that body sites with a low concentration of plasma protein, such as cerebrospinal fluid or respiratory secretions, may offer poor conditions for priming of PMN in newborn infants. When adult and newborn plasma were mixed, small amounts of newborn plasma did not lower the ability of adult plasma to prime PMN, whereas small amounts of adult plasma enhanced the ability of newborn plasma in LPS priming of PMN. These mixing experiments suggested that an inhibitor to LPS priming is not present in newborn plasma.
Because LBP has been characterized as an essential element in the LPS priming of PMN, we assessed newborn and adult LBP content. When this was done we found that newborn and adult LBP concentrations were similar (LBP for adults 138 ± 12.9 U, for newborns 126.9 ± 12.1 U, P = .53). In addition, no correlation was found between LBP content and plasma priming activity of adult plasma. However, a direct relation between LBP and plasma priming activity was suggested for newborn plasma, indicating that LBP in newborn plasma has direct effect on LPS priming. The relationship between LBP and LPS priming may be more complex with adult plasma.
Because physical properties of the plasma factor involved in priming resemble those of plasma fibronectin, we assessed the role of fibronectin in LPS priming of PMN. We concluded that fibronectin plays a role in LPS-priming of PMN for the following reasons: (1) removal of fibronectin from adult plasma decreases its LPS priming activity with a highly significant dose relationship, (2) reconstituting fibronectin to fibronectin-depleted plasma restores LPS plasma priming activity, and (3) antibody to fibronectin neutralizes the LPS priming activity of plasma.
The results of our experiments should be interpreted with caution because impurities present in the fibronectin preparations may affect the results. A synthetic preparation of fibronectin is not yet available. In addition, the epitopes on fibronectin responsible for LPS priming should be identified using monoclonal antibody to determine the site on fibronectin involved in PMN priming. In addition, other mechanisms may exist for priming of PMN with other agents such as TNF-α, IFN-γ, IL-1, and IL-8. The role of fibronectin with these agents should be assessed in the future.
The steps leading to LPS priming of PMN may be more complex than previously thought. LBP and CD14 are clearly essential elements in the LPS priming of PMN, since a number of investigators including our group have shown that antibody to CD14 will block the LPS priming of PMN.25,32 45
The finding that newborn fibronectin is less active than adult fibronectin suggests that LBP may be a more important determinant for priming in neonates than it is in adults. This suggestion is supported by our observation of a closer relationship of LBP to priming activity when neonatal plasma was used compared with that of adult plasma (Fig 4). A role for fibronectin in LPS priming of PMN is consistent with its role in a number of other activation processes. In 1981, Doran et al46 showed that cold insoluble globulin (fibronectin) enhanced macrophage membrane binding to surfaces, particularly in the presence of heparin. In addition, solid-phase fibronectin is thought to prime PMN for a respiratory burst by TNF-α47 and enhance PMN migration to fMLP.48
Fibronectin is a high-molecular-weight glycoprotein present in an insoluble form in the extracellular matrix and in a soluble form in plasma and interstitial fluids.43,49 The molecule is a dimer composed of two similar but not identical subunits linked by two disulfide bonds. Fibronectin is a macromolecule containing eight functional domains including a cell binding domain (domain 6) and an adjacent domain that avidly binds heparin. Heparin increases the binding of soluble fibronectin to macrophage receptors.43,49 The reported concentration of soluble fibronectin of newborn infants ranges from 100 to 250 μg/mL and increases slowly to reach adult levels which are twofold to threefold higher.43,50 Fibronectin plays an important role in enhancing opsonization of the organism and in protecting the host from lethal infection with group B streptococcus.51-53 Fibronectin from neonatal or fetal plasma differs from adult fibronectin in several ways. Fetal fibronectin contains a unique segment (III CS) that is defined by monoclonal antibody FDC-6. In addition, fibronectin in the fetus is more heavily glycosylated than fibronectin from adults, a feature that alters ligand binding.54 In our experiments addition of purified adult plasma fibronectin to FN-depleted newborn plasma or to FN-depleted adult plasma increased priming activity in a dose-related manner (Fig 6). The domain in the fibronectin molecule responsible for this priming activity has not been identified and should be determined in the future.
The effect of insoluble fibronectin on LPS-induced priming of PMN was not assessed in the present experiment. In preliminary experiments we have found that PMN in suspension interact with soluble fibronectin differently than adherent PMN interact with fibronectin-coated surfaces in generating O−2 when triggered with fMLP in the presence or absence of LPS (unpublished data, November 1996). Yang et al51 have observed that fibronectin-adherent PMN have lower response to fMLP in generating O−2 than PMN in suspension. The effects of LPS priming on adherent PMN should be explored further.
Receptors to fibronectin belong to a family of cell adhesion receptors known as integrins. Integrins are a superfamily of heterodimeric transmembrane receptors formed by noncovalent association of the α and the β subunits. The mechanisms of integrin interaction with LPS, CD14, LBP, or fibronectin has not been identified. Future studies should be performed to identify integrin subunits involved in PMN priming, and differences between adult and newborn PMN integrin expression. Integrins are not only involved in cell-matrix interactions, but also play a major role in both inside-out and outside-in signal transduction leading to altered gene expression via tyrosine kinase action.49 Ulevitch and Tobias27 have postulated that the LPS receptor is a multichain receptor that includes CD14 and other transducing molecules that lead to intracellular protein tyrosine kinase activation. Our findings of a role for plasma fibronectin in LPS priming of PMN are consistent with this proposed model. Our study suggests that two cellular proteins may be required for effective LPS priming of PMN in the presence of plasma: one is dependent on LBP and its receptor and a second is dependent on fibronectin.
ACKNOWLEDGMENT
The authors acknowledge the assistance of Kim Barrett in typing this manuscript, Dr John LeBlanc (Dalhousie University, Halifax, Canada) for statistical analysis, and Dr Peter Tobias (Scripps Institute, La Jolla, CA), who kindly performed LBP measurement. Appreciation is also expressed to the Case Room nurses of the Grace Maternity Hospital for their assistance in collecting blood samples from newborn umbilical cords. This work was conducted at the research facilities of the IWK Children's Hospital, Dalhousie University.
Supported by MRC Grant No. MT7610 and The Hospital for Sick Children Foundation.
Address reprint requests to Robert Bortolussi, MD, IWK Children's Hospital, 5850 University Ave, Halifax, Nova Scotia, Canada B3J 3G9.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal