To the Editor:
In a study of considerable practical relevance, Offner et al1 have shown that from 15 days after autologous or allogeneic bone marrow transplantation (BMT), the risk of mortality due to any cause within the first 100 days becomes progressively greater in patients whose absolute neutrophil count is <0.1 × 109/L. This observation could be of clinical significance in the third and fourth week after transplantation, when additional therapeutic interventions may be applied to patients with neutrophil counts of <0.1 × 109/L to decrease early mortality.
There are some limitations to the study: although the investigators mention that causes of death such as relapse and regimen-related toxicity could diminish the relative importance of the degree of neutropenia in autograft recipients,1 these causes of death as well as fatal acute graft-versus-host disease (GVHD), which account for a significant number of deaths in the first 100 days after allogeneic BMT, could decrease the impact of neutropenia after allogeneic transplantation as well. Secondly, although neutropenia is the most important risk factor for death due to bacterial or fungal infections, the use of the absolute neutrophil count may ignore the contribution of monocytes to recovery from infections. Also, in our experience, the absolute neutrophil count is somewhat unreliable and prone to considerable variation when the total leukocyte count is low.2
Because there is no universally accepted definition of delayed engraftment after BMT, and prolongation of pancytopenia is associated with an increasing probability of serious infections and bleeding, we have tried to arrive at an operational definition of graft failure after allogeneic BMT.2 To specifically identify patients who could benefit from early intervention in the third week posttransplant, we limited the events studied to (1) death primarily as a result of infection, hemorrhage, or graft failure within 3 months; and (2) second infusion of allogeneic or autologous marrow as therapy for primary failure of engraftment within 2 months of the first infusion.2 Primary failure of engraftment was usually diagnosed on the basis of an absolute neutrophil count of <0.1 × 109/L on day 21 with a markedly hypocellular marrow.3,4 Detailed information on the mode of death, disease status at death, and principal and contributory causes of death was extracted from a prospective database5 6 to ensure the exclusion of patients dying of infections or hemorrhage secondary to acute GVHD because the latter were classified as dying primarily of GVHD.
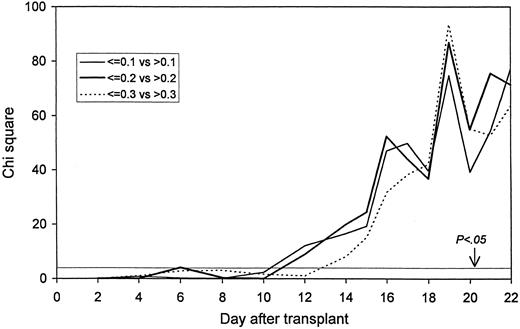
The probability of death: Statistical significance by day after transplant. A chi square value of 3.85 corresponds to a P value of .05.
The probability of death: Statistical significance by day after transplant. A chi square value of 3.85 corresponds to a P value of .05.
Serial total leukocyte counts obtained by automated counters on 712 consecutive patients receiving marrow allografts for hematologic malignancies were analyzed to see if the leukocyte count on days 2-22 after BMT was predictive of death (as defined) or death/second infusion. For each of these posttransplant days, patients were grouped on the basis of the leukocyte counts (109/L) in three ways (≤0.1 v >0.1, ≤0.2 v >0.2, and ≤0.3 v >0.3), and actuarial probabilities of death and death/second infusion calculated. The log-rank test was used to compare the differences. The following factors were analyzed in multivariate fashion to see their effect on the probability of death or death/second infusion: age, sex, diagnosis, conditioning regimen, type of donor, GVHD prophylaxis, nucleated cell dose, prophylactic administration of a growth factor within 2 weeks of BMT, and the total leukocyte count on day 16 after BMT.
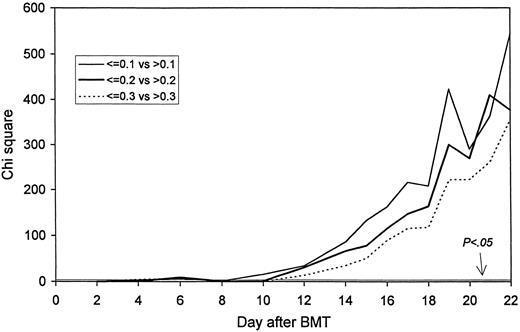
Fifty-two patients fulfilled the definition of death, and 99 the definition of death/second infusion. The leukocyte counts on days 2-10 did not affect the specified outcomes significantly. However, from day 12 onward, the lower leukocyte count for each of the three comparisons on each day was associated with a significantly increased risk of death and death/second infusion. Figures 1 and 2 show the high significance.
The probability of death/second infusion: Statistical significance by day after transplant. A chi square value of 3.85 corresponds to a P value of .05.
The probability of death/second infusion: Statistical significance by day after transplant. A chi square value of 3.85 corresponds to a P value of .05.
In multivariate analysis, a leukocyte count ≤0.2 × 109/L on day 16 was the strongest factor predicting subsequent death (25 of 114 patients v 23 of 546 patients with leukocytes >0.2 × 109/L, relative risk 4.5, P < .0001) and death/second infusion (48 of 114 v 47 of 546, relative risk 5.5, P < .0001). The findings were similar in recipients of non–T-cell depleted marrow from HLA-identical siblings (relative risks 4.5 and 7.6, P = .0001 and <.0001), and after substitution of the day 16 leukocyte counts with day 14 and day 17; with the leukocyte count being the strongest factor in all analyses.2
Although our findings are similar to those of Offner et al, by specifically defining deaths that may be directly attributable to low counts, we have highlighted the importance of early leukocyte recovery after allogeneic BMT. Therefore, we believe that “graft failure” should be defined operationally as early as 2 weeks after BMT when patients are well and more likely to benefit from any intervention rather than at day 21 or day 28 when patients may be too unwell. On the basis of this, an algorithm can be evolved to limit the use of growth factors to patients most likely to benefit from them, to salvage patients at risk of complications of low counts early before their clinical condition deteriorates, and to design studies of growth factors. We suggest that patients with leukocyte counts of 0.2 × 109/L or less 14-16 days after BMT should be started on G-CSF or GM-CSF even if they are clinically well, and consideration should be given for a further infusion of cells, especially if the risk of graft failure is high as in unrelated or mismatched transplants.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal