Abstract
Two highly related receptor tyrosine kinases, TIE and TEK, comprise a family of endothelial cell-specific kinase. We established monoclonal antibodies against them and performed detailed analyses on their expression and function in murine hematopoietic stem cells (HSCs). TIE and TEK were expressed on 23.7% and 33.3% of lineage marker-negative, c-Kit+ and Sca-1+ (Lin− c-Kit+ Sca-1+) HSCs that contain the majority of day-12 colony-forming units-spleen (CFU-S) and long-term reconstituting cells, but not committed progenitor cells. Lin− c-Kit+ Sca-1+ cells were further divided by the expression of TIE and TEK. TIE+ and TEK+ HSCs as well as each negative counterpart contained high proliferative potential colony-forming cells and differentiated into lymphoid and myeloid progenies both in vitro and in vivo. However, day-12 CFU-S were enriched in TIE+ and TEK+ HSCs. Our findings define TIE and TEK as novel stem cell marker antigens that segregate day-12 CFU-S, and provide evidence of novel signaling pathways that are involved in the functional regulation of HSCs at a specific stage of differentiation, particularly of day-12 CFU-S.
HEMATOPOIETIC stem cells (HSCs) are defined as the cells that have the capacity for self-renewal and the capability to differentiate into all lineages of hematopoietic cells. The functions of HSCs, including self-renewal, differentiation, and proliferation, are strictly regulated by interactions with their microenvironment, which comprises stromal cells, extracellular matrixes, and various secreted cytokines. However, the precise mechanisms of regulation have not been well-defined.
Recently, a number of cytokines have been shown to regulate the survival and proliferation of HSCs.1 Some of them, such as stem cell factor (SCF ) and FLT3/FLK2 ligand (FL), exert their biological functions via receptor tyrosine kinases (RTKs). RTKs play a critical role in pleiotropic cell functions including cell proliferation and differentiation.2 They comprise a large family, among which c-Kit, the receptor for SCF, and FLT3/FLK2, the receptor for FL, have been shown to be expressed on HSCs and implicated in the HSC functions. The SCF–c-Kit signaling pathway is crucial in the proliferation of HSCs, and mice carrying a loss-of-function mutation at the c-kit locus (W mutation) show a severe reduction of HSCs.3 The FL-FLT3/FLK2 signaling pathway also plays a role in the proliferation of HSCs.4 These findings indicate an important role of RTKs in the regulation of HSC functions. In addition, failure of blood-island formation in FLK1-deficient mice suggests that RTK signals are also essential for the ontogeny of HSCs.5
To elucidate the regulatory mechanism of HSC functions, we previously performed a cloning of RTKs from highly purified murine HSCs (Lin− c-Kit+ Sca-1+ cells) and showed that related RTK genes, TIE (tyrosine kinase with Ig-like loops and epidermal growth factor homology domains) and TEK (tunica interna endothelial cell kinase), are predominantly expressed in the HSC fraction at the mRNA level.6 We also showed that TIE is expressed on human HSCs (CD34+ CD38− cells) using a monoclonal antibody (MoAb) against human TIE.7 They share unique structural properties of coexistent Ig-like loops, epidermal growth factor-like repeats, and fibronectin type III repeats in their extracellular domains and define a distinct subfamily of RTKs. They are also expressed in endothelial cells and play distinct roles in blood vessel formation.8-10 Targeted mutant mice in each gene locus showed that TIE is required for integrity and survival of vascular endothelial cells,11,12 whereas TEK is important for vascular network formation in endothelial cells.11 13 However, because of the embryonal lethality, the role of TIE and TEK in hematopoiesis has not yet been clarified.
To clarify the involvement of TIE and TEK in HSC functions, we established MoAbs against murine TIE and TEK. In this study, we show that these related RTKs are preferentially expressed on murine HSCs. We further show the distinct nature of TIE+ and TEK+ HSCs from each negative counterpart and the possible involvement of these related RTKs in the regulation of HSC functions.
MATERIALS AND METHODS
Animals.The Wistar rats for immunization and C57BL/6 (Ly-5.2) mice were purchased from SLC (Hamamatsu, Japan). C57BL/6 (Ly-5.1) mice were kindly provided by Dr Kazuo Moriwaki (National Institute of Genetics, Shizuoka, Japan).
Antibodies and factors.The antibodies (Abs) used as the lineage markers were anti-B220 (RA3-6B2), Mac-1 (M1/70), Gr-1 (RB6-8C5), CD4 (GK1.5), CD8 (53-6.72), and TER-119 (erythroid lineage marker).14 Phycoerythrin (PE)-conjugated antimouse Sca-1 Ab (Ly-6A/E) was obtained from PharMingen (San Diego, CA). Monoclonal antimouse c-Kit Ab (ACK-2) was provided by Dr Shin-Ichi Nishikawa (Kyoto University, Kyoto, Japan) and was conjugated with fluorescein isothiocyanate (FITC). Polyclonal Abs against TIE and TEK C-terminus were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal antiphosphotyrosine Ab (4G10) was from Upstate Biotechnology Inc (Lake Placid, NY). Allophycocyanin (APC)-conjugated streptavidin (SA-APC) was from Becton Dickinson Immunocytometry System (San Jose, CA). Recombinant mouse interleukin-3 (IL-3) and recombinant human IL-6 were provided by Dr Tetsuo Sudo (Toray Industries Inc, Kamakura, Japan). Recombinant mouse stem cell factor (SCF ) was provided by Chemo-Sero-Therapeutic Co Ltd (Kumamoto, Japan). Recombinant human erythropoietin (Epo) was provided by Snow-Brand Milk Product Co (Tochigi, Japan).
Establishment of stable transfectants.Full-length murine TIE and TEK cDNAs were subcloned into the expression vector, MKITneo (kindly provided by Dr Kazuo Maruyama, Tokyo Medical and Dental University, Tokyo, Japan) and introduced into Ba/F3 cells by electroporation. Among G418-resistant clones, clones with high levels of receptor expression were selected and designated BaF/TIE and BaF/TEK cells, respectively. Ba/F3 cells transfected with vector alone (BaF/Vector cells) served as negative control cells.
Establishment of monoclonal and polyclonal Abs against murine TIE and TEK.The entire extracellular domains of murine TIE and TEK tagged with a FLAG octapeptide (DYKDDDDK) were produced by COS7 cells. Proteins in the conditioned media of COS7 cells were purified using an antiFLAG MoAb M2 affinity gel (Eastman Kodak, New Haven, CT). The Wistar rats were immunized with these purified proteins. Hybridomas were produced by fusion of murine myeloma cells (P3 − X63 − Ag8 − U1; P3U1) with splenic cells from immunized rats. Positive hybridoma clones were screened by fluorescence-activated cell sorter (FACS) analysis using BaF/TIE and BaF/TEK cells as indicators. The MoAbs were purified using a protein G-Sepharose column (Pharmacia, Uppsala, Sweden) and biotinylated. Antisera against TIE and TEK were raised in rats by immunization with the extracellular domains of TIE and TEK, respectively. IgG fractions of each antisera were collected using a protein G-Sepharose column and were used for in vivo phosphorylation assay.
Cell surface biotinylation, immunoprecipitation, and Western blotting.Cell surface proteins of BaF/Vector, BaF/TIE, and BaF/TEK cells were biotinylated using the enhanced chemiluminescence (ECL) protein biotinylation system (Amersham International plc, Buckinghamshire, UK), as previously described.15 After biotinylation, cells were solubilized with lysis buffer (1% Triton X-100, 10 mmol/L Tris [pH 7.8], 150 mmol/L sodium chloride, 1 mmol/L EDTA, 50 μg/mL aprotinin, and 1 mmol/L phenylmethanesulfonyl fluoride [PMSF]), and cell lysates were precleared with Pansorbin Cells (Calbiochem Co, La Jolla, CA) and protein G-Sepharose. Then, proteins in cell lysates were immunoprecipitated with anti-TIE or TEK MoAb. Immunoprecipitates collected on protein G-Sepharose were boiled for 3 minutes at 100°C in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer with 5% 2-mercaptoethanol (2-ME) and resolved by SDS-PAGE with 7.5% polyacrylamide. After electrophoresis, proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Nihon Millipore Ltd, Yonezawa, Japan), which were then probed with the polyclonal Ab against TIE or TEK C-terminus and reprobed with streptavidin-HRP (SA-HRP). Specific binding was detected using the ECL system (Amersham International plc).
In vivo tyrosine phosphorylation assay.BaF/TIE and BaF/TEK cells, deprived of IL-3 for 12 hours, were used for the phosphorylation assay. These cells (2 × 106/mL) were incubated with 100 μg/mL preimmune rat IgGs or various amounts of anti-TIE or TEK IgGs at 37°C for 10 minutes. The cells were then solubilized with lysis buffer (1% Triton X-100, 50 mmol/L HEPES [pH 7.4], 10% glycerol, 10 mmol/L sodium pyrophosphate, 100 mmol/L sodium fluoride, 4 mmol/L EDTA, 2 mmol/L sodium orthovanadate, 50 μg/mL aprotinin, 1 mmol/L PMSF, 100 μmol/L leupeptin, and 25 μmol/L pepstatin A). Proteins were immunoprecipitated with anti-TIE or TEK MoAb. Tyrosine phosphorylation of TIE and TEK was evaluated by Western blotting with antiphosphotyrosine Ab, 4G10.
Preparation of lineage marker-negative cells.Two different density solutions (1.063 and 1.077 g/mL) were prepared by diluting Nycodenz solution (NYCOMED AS, Oslo, Norway) with NaCl diluent. Bone marrow (BM) cells were flushed from femurs and tibiae with α-medium supplemented with 0.1% (wt/vol) bovine serum albumin (BSA/α-MEM) and resuspended in 3 mL of lower density solution. This suspension was layered over 4 mL of higher density solution and then centrifuged for 30 minutes at 1,000g at room temperature. The cells at the interface were collected, washed, and resuspended in 2 mL of BSA/α-MEM. To remove debris and platelets, this suspension was layered over 5 mL of 10% (wt/vol) BSA/phosphate-buffered saline (PBS) (pH 7.5) and centrifuged for 10 minutes at 100g at room temperature. The pellet was collected, washed, and then incubated with a cocktail of rat antimouse lineage (Lin) marker Abs (B220, Gr-1, Mac-1, CD4, CD8, and TER-119) for 30 minutes on ice. After washing with 0.1% (wt/vol) BSA/PBS the cells were mixed with Dynabeads M-450 sheep antirat IgG (DYNAL, Great Neck, NY) at a 1:40 cell:bead ratio and incubated for 40 minutes on ice. The cells and beads mixture was diluted with 7 mL of 0.1% (wt/vol) BSA/PBS, then the beads-bound cells and free beads were removed from medium in a magnetic field. The cells that remained in the medium were used as lineage-marker negative (Lin−) cells in this study.
FACS analysis and cell sorting.BM cells in a staining medium (5% fetal bovine serum [FCS] in PBS) were incubated with biotinylated anti-TIE or TEK MoAb followed by SA-APC, FITC-conjugated anti–c-Kit Ab, and PE-conjugated anti–Sca-1 Ab. FACS analysis and cell sorting were performed on a FACSvantage (Becton Dickinson Immunocytometry System). Transfectants and hematopoietic cell lines were stained with biotinylated anti-TIE or TEK MoAb followed by SA-FITC (GIBCO, Grand Island, NY). In the FACS analysis, specific bindings of anti-TIE and TEK MoAbs to TIE and TEK proteins, respectively, were blocked by incubating the MoAbs with 100-fold excess molar of TIE-FLAG or TEK-FLAG proteins for 1 hour at room temperature before staining.
Reverse transcription-polymerase chain reaction (RT-PCR).To clarify the coexpression of TIE and TEK in HSCs, we examined the TIE expression in sorted TEK+ cells by RT-PCR. Five thousand TEK+ cells and 5,000 TEK− cells among Lin− c-Kit+ cells were sorted by FACS. Total RNA was obtained from these fractionated cells, as described.16 The first-strand cDNA was synthesized from the total RNA using cDNA synthesis system (GIBCO-BRL, Gaithersburg, MD) and random hexamer oligonucleotides. PCR primers for the TIE gene were directed to amplify a portion of the extracellular domain. Cycling parameters were 1 minute at 94°C, 2 minutes at 55°C, and 3 minutes at 72°C for 40 cycles. Primer sequences were as follows: TIE sense, 5′-ACCCACTACCAGCTGGATGT-3′; TIE antisense, 5′-ATCGTGTGCTAGCATTGAGG-3′. Ten percent of this reaction mixture was electrophoresed on a 2% agarose gel.
Progenitor assay in methylcellulose culture.Sorted cells were embedded in 1 mL of α-medium containing 1.3% methylcellulose (1,500 cp; Aldrich Chemical Co, Milwaukee, WI), 30% FCS (CSL Ltd, Victoria, Australia), 1% deionized BSA (Sigma Chemical Co, St Louis, MO), 0.1 mmol/L 2-ME, 100 ng/mL SCF, 200 U/mL recombinant mouse IL-3, 20 ng/mL recombinant human IL-6, and 2 U/mL recombinant human Epo. The cells were cultured in a 35 mm nontissue culture dish and incubated at 37°C in a humidified atmosphere with 5% CO2 . The numbers of colonies were scored on day 10 of culture using an inverted microscope. High proliferative potential colony-forming cells (HPP-CFC) were defined as colonies with a diameter greater than 1 mm.17
Coculture with stromal cells.PA-6 stromal cells established from newborn mouse calvaria18 were cultured in 24-well plates. After reaching confluence, they were irradiated at 0.5 Gy/min to provide a dose of 15 Gy. Sorted cells were cultured on this stromal layer in 1 mL of α-medium containing 10% FCS in the presence or absence of IL-7.
Spleen colony assay.The spleen colony assay of Till and McCulloch19 was used. Sorted cells were injected into lethally irradiated mice (total body irradiation of 9 Gy). The spleens were removed on day 12 after transplantation and fixed in Bouin's solution, and then spleen colonies were counted by macroscopical observation.
Reconstitution assay.Sorted cells from Ly-5.1 donor mice were injected into lethally irradiated Ly-5.2 recipient mice. Several weeks after transplantation, peripheral blood was obtained from the retro-orbital sinus. After hemolysis with 0.17 mol/L ammonium chloride solution, cells were stained with biotinylated anti-Ly-5.1 Ab (PharMingen) and MoAbs specific to lineage marker antigens of myeloid cells (Gr-1 plus Mac-1), B cells (B220), and T cells (CD4 and CD8).
RESULTS
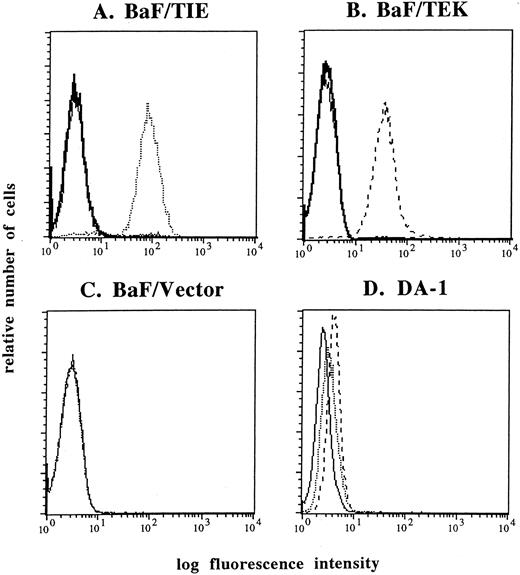
Establishment of MoAbs against TIE and TEK.We established MoAbs against the extracellular domain of murine TIE and TEK. Positive hybridoma clones were selected by FACS using BaF/TIE and BaF/TEK cells as indicator cells. Among several clones, the anti-TIE MoAb, clone 117, and the anti-TEK MoAb, clone 4 were biotinylated and used in this study. FACS analyses showed that these MoAbs reacted with each indicator cell, but not with BaF/Vector cells (Fig 1A through C). The specificity of each MoAb was confirmed by the findings that an excess molar of TIE-FLAG or TEK-FLAG proteins completely blocked the reactivity of each MoAb with indicator cells (Fig 1A and B). Both MoAbs reacted with DA-1 cells that coexpress TIE and TEK mRNAs (Fig 1D).6 Anti-TIE and TEK MoAbs showed no cross-reactivity with BaF/TEK and BaF/TIE cells, respectively (data not shown).
FACS analysis of TIE (dotted lines) and TEK (broken lines) expression. Anti-TIE and TEK MoAbs reacted with each indicator cell, BaF/TIE (A) and BaF/TEK cells (B), respectively, but not with BaF/Vector cells (C). The reactivity of anti-TIE and TEK MoAbs was blocked by an excess molar of TIE-FLAG and TEK-FLAG proteins (thick lines). Both anti-TIE and anti-TEK MoAbs reacted with DA-1 cells (D). Thin lines denote negative control cells stained with FITC-conjugated streptavidin alone.
FACS analysis of TIE (dotted lines) and TEK (broken lines) expression. Anti-TIE and TEK MoAbs reacted with each indicator cell, BaF/TIE (A) and BaF/TEK cells (B), respectively, but not with BaF/Vector cells (C). The reactivity of anti-TIE and TEK MoAbs was blocked by an excess molar of TIE-FLAG and TEK-FLAG proteins (thick lines). Both anti-TIE and anti-TEK MoAbs reacted with DA-1 cells (D). Thin lines denote negative control cells stained with FITC-conjugated streptavidin alone.
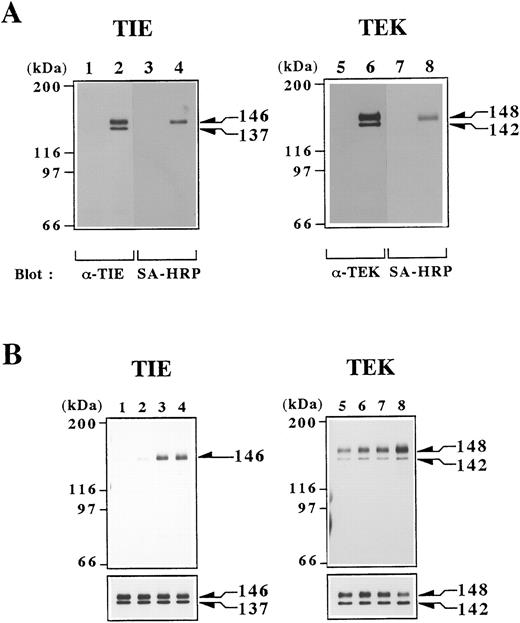
Cell surface proteins of BaF/TIE, BaF/TEK, and BaF/Vector cells were biotinylated, and cell lysates were prepared from these cells. Proteins in lysates were immunoprecipitated with anti-TIE or TEK MoAb and immunoprecipitates were detected by Western blotting. Polyclonal Abs against TIE and TEK C-terminus detected two forms of TIE and TEK proteins that were immunoprecipitated from lysates of each indicator cells (Fig 2A, lanes 2 and 6) and DA-1 cells (data not shown), but not from BaF/Vector cells (Fig 2A, lanes 1 and 5). The molecular weights of TIE and TEK proteins were 146 and 137 kD, and 148 and 142 kD, respectively (Fig 2A, lanes 2 and 6). Reprobing with SA-HRP showed no major proteins except for the 146 kD TIE and 148 kD TEK proteins (Fig 2A, lanes 4 and 8). These data confirmed the specific reactivity of MoAbs to TIE or TEK proteins on the cell surface, and indicated that the proteins with smaller molecular weights may represent cytoplasmic precursor proteins.
(A) Detection of TIE and TEK proteins in transfectants. Cell lysates were obtained from BaF/vector (lanes 1 and 5), BaF/TIE (lane 2), and BaF/TEK (lane 6) cells that were surface-biotinylated. Proteins in lysates were immunoprecipitated with anti-TIE (lanes 1 and 2) or TEK (lanes 5 and 6) MoAb. Immunoprecipitates were separated by SDS-PAGE and Western blotted with polyclonal Ab against C-terminus of TIE (lanes 1 and 2) or TEK (lanes 5 and 6) and then reprobed with SA-HRP (lanes 3, 4, 7, and 8). (B) Induction of autophosphorylation of TIE and TEK by rat postimmune IgGs against the extracellular domain of TIE or TEK. BaF/TIE (lanes 1 through 4) and BaF/TEK cells (lanes 5 through 8) were stimulated with medium alone (lanes 1 and 5), preimmune IgGs (lanes 2 and 6), 1 μg/mL postimmune IgGs (lanes 3 and 7), and 10 μg/mL postimmune IgGs (lanes 4 and 8). After 10 minutes of stimulation, cells were solubilized and proteins in lysates were immunoprecipitated with each MoAb. Immunoprecipitates were Western blotted with antiphosphotyrosine Ab (upper panels) and then reprobed with polyclonal Ab against C-terminus of TIE (lanes 1 through 4) or TEK (lanes 5 through 8; lower panels).
(A) Detection of TIE and TEK proteins in transfectants. Cell lysates were obtained from BaF/vector (lanes 1 and 5), BaF/TIE (lane 2), and BaF/TEK (lane 6) cells that were surface-biotinylated. Proteins in lysates were immunoprecipitated with anti-TIE (lanes 1 and 2) or TEK (lanes 5 and 6) MoAb. Immunoprecipitates were separated by SDS-PAGE and Western blotted with polyclonal Ab against C-terminus of TIE (lanes 1 and 2) or TEK (lanes 5 and 6) and then reprobed with SA-HRP (lanes 3, 4, 7, and 8). (B) Induction of autophosphorylation of TIE and TEK by rat postimmune IgGs against the extracellular domain of TIE or TEK. BaF/TIE (lanes 1 through 4) and BaF/TEK cells (lanes 5 through 8) were stimulated with medium alone (lanes 1 and 5), preimmune IgGs (lanes 2 and 6), 1 μg/mL postimmune IgGs (lanes 3 and 7), and 10 μg/mL postimmune IgGs (lanes 4 and 8). After 10 minutes of stimulation, cells were solubilized and proteins in lysates were immunoprecipitated with each MoAb. Immunoprecipitates were Western blotted with antiphosphotyrosine Ab (upper panels) and then reprobed with polyclonal Ab against C-terminus of TIE (lanes 1 through 4) or TEK (lanes 5 through 8; lower panels).
It is well known that some Abs exert agonistic activity on receptors and activate them. Because the ligands for TIE and TEK have not been identified, we analyzed whether IgGs against the extracellular domain of TIE or TEK have agonistic activities. Postimmune IgGs against TIE or TEK greatly induced tyrosine phosphorylation of the 146-kD TIE or 148-kD TEK proteins, respectively, whereas preimmune IgGs did not alter the basal level of phosphorylation (Fig 2B). These results indicated that TIE and TEK are functional molecules in hematopoietic cells.
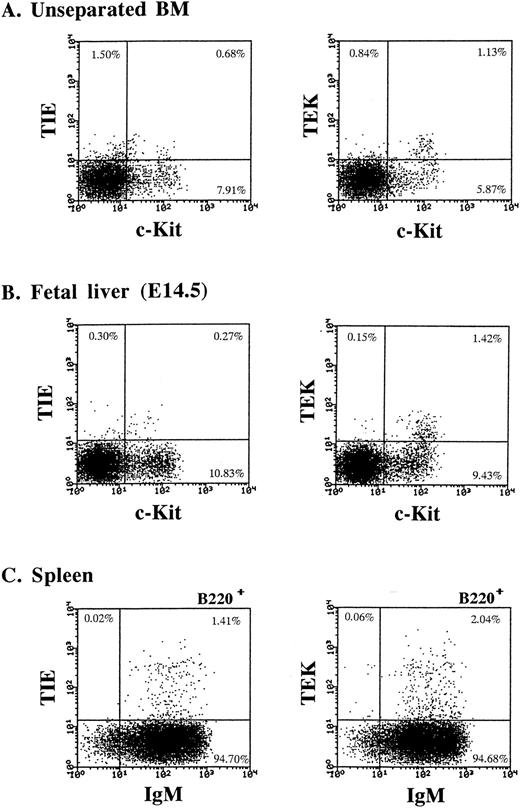
Expression of TIE and TEK on hematopoietic cells.Using these MoAbs, we initially examined the expression of TIE and TEK on unseparated BM hematopoietic cells by FACS analysis. Our previous analysis showed that TIE and TEK mRNAs are predominantly expressed in murine HSC fraction (Lin− c-Kit+ Sca-1+ cells).6 Thus, we examined the expression of TIE and TEK on c-Kit+ cells that contain hematopoietic progenitors. As shown in Fig 3A, TIE and TEK were expressed on 5.4% and 31.7% of c-Kithigh cells, respectively. TIE was also expressed on a subset of c-Kitlow cells. We next examined the expression of TIE and TEK on day-14.5 (E14.5) fetal liver cells. TEK was expressed on c-Kithigh cells, whereas TIE was mainly expressed on c-Kitlow cells and less frequently on c-Kithigh cells (Fig 3B). TIE+ c-Kitlow cells in the BM and E14.5 fetal liver expressed a low level of Mac-1 and contained committed progenitors for macrophages (data not shown). We showed previously that TIE is expressed on a subset of CD19+ or CD20+ B cells of human BM.7 Thus, we analyzed the expression of TIE and TEK on splenic B cells (Fig 3C). TIE and TEK were expressed on 1.4% and 2.1% of B220+ splenic B cells, respectively. These B cells were also surface IgM+ (Fig 3C), heat stable antigen (HSA)+, and CD43− (data not shown), indicating that TIE and TEK are expressed on a subset of mature B cells. TIE and TEK were not expressed on other lineage-committed cells.
TIE and TEK expression on hematopoietic cells. (A) The expression of TIE and TEK on c-Kit+ cells. Both TIE and TEK was expressed on a subset of c-Kithigh cells. (B) The expression of TIE and TEK on c-Kit+ cells of E14.5 fetal liver cells. TEK was expressed on a subset of c-Kithigh cells, whereas TIE was on c-Kitlow cells. (C) The expression of TIE and TEK on a subset of B220+ IgM+ mature B cells of the spleen.
TIE and TEK expression on hematopoietic cells. (A) The expression of TIE and TEK on c-Kit+ cells. Both TIE and TEK was expressed on a subset of c-Kithigh cells. (B) The expression of TIE and TEK on c-Kit+ cells of E14.5 fetal liver cells. TEK was expressed on a subset of c-Kithigh cells, whereas TIE was on c-Kitlow cells. (C) The expression of TIE and TEK on a subset of B220+ IgM+ mature B cells of the spleen.
Expression of TIE and TEK on HSCs.We have previously shown that Lin− c-Kit+ Sca-1+ cells, which comprise 0.08% of BM mononuclear cells, contain the majority of the BM day-12 CFU-S, but not day-8 CFU-S, and that they have long-term repopulating ability (LTRA) and contain very primitive HSCs.20 Recently, Osawa et al21 showed that Lin− c-Kit+ Sca-1+ cells are the only subset of BM Lin− cells that have LTRA. Thus, we further examined the expression of TIE and TEK on this HSC fraction. We prepared Lin− cells by immunomagnetic negative selection, then performed three-color FACS analysis using anti–c-Kit, anti–Sca-1, and anti-TIE or TEK MoAbs. As shown in Fig 4, TIE and TEK were expressed on 23.7% and 33.3% of Lin− c-Kit+ Sca-1+ cells, respectively. The expression of TIE and TEK divided Lin− c-Kit+ Sca-1+ cells into two subpopulations; Lin− c-Kit+ Sca-1+ TIE+/− and Lin− c-Kit+ Sca-1+ TEK+/− cells, respectively. In contrast, Lin− c-Kit+ Sca-1− cells contain only progenitor cells that have lost LTRA, which transiently repopulate peripheral blood cells for the first several weeks.20 21 TIE and TEK were expressed on 7.3% and 33.1% of Lin− c-Kit+ Sca-1− cells, respectively (data not shown), indicating that a significant number of progenitor cells express TEK and less express TIE.
The expression of TIE and TEK on HSCs. Approximately 23.7% and 33.3% of Lin− c-Kit+ Sca-1+ cells expressed TIE and TEK, respectively.
The expression of TIE and TEK on HSCs. Approximately 23.7% and 33.3% of Lin− c-Kit+ Sca-1+ cells expressed TIE and TEK, respectively.
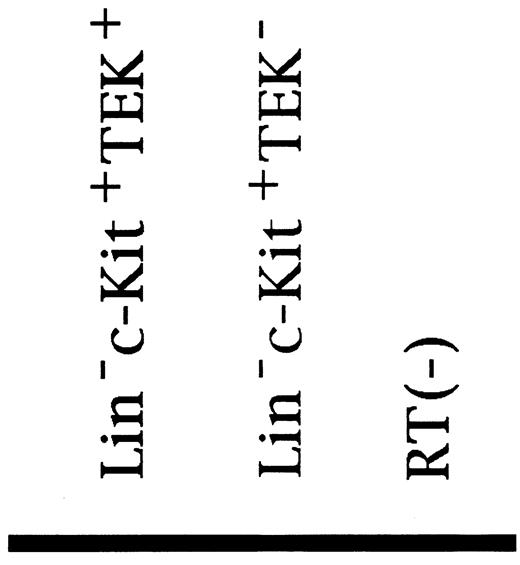
To clarify whether TIE and TEK were coexpressed on HSCs, we performed the RT-PCR method. As shown in Fig 5, TIE was expressed on both TEK+ Lin− c-Kit+ cells and TEK− Lin− c-Kit+ cells at the mRNA level.
RT-PCR analysis of TIE and TEK expression in fractionated HSCs (ethidium bromide staining). TIE (301 bp) was expressed in both TEK+ and TEK− cells, and TEK (456 bp) was expressed on both TIE+ and TIE− cells (data not shown).
RT-PCR analysis of TIE and TEK expression in fractionated HSCs (ethidium bromide staining). TIE (301 bp) was expressed in both TEK+ and TEK− cells, and TEK (456 bp) was expressed on both TIE+ and TIE− cells (data not shown).
In vitro colony formation and multilineage differentiation of TIE+ and TEK+ HSCs.To clarify the nature of each subpopulation of Lin− c-Kit+ Sca-1+ cells defined by the expression of TIE and TEK, we first analyzed the ability of each subpopulation to form hematopoietic colonies in the presence of SCF, IL-3, IL-6, and Epo (Table 1). We previously showed that Lin− c-Kit+ Sca-1+ cells form no or few colonies in the presence of SCF, IL-3, or IL-6 alone; however, the combination of these three factors induces approximately 50% of Lin− c-Kit+ Sca-1+ cells to form HPP-CFC–derived colonies.20 In this study, Lin− c-Kit+ Sca-1+ cells formed 69.3 ± 2.9 colonies per 100 sorted cells and all of them were derived from HPP-CFC. Lin− c-Kit+ Sca-1+ TIE+ and Lin− c-Kit+ Sca-1+ TEK+ HSCs formed 68.9 ± 2.0 and 69.3 ± 2.4 colonies, respectively. On the other hand, their negative counterparts formed slightly less numbers of colonies, but gave no statistical significance. Each subpopulation similarly contained CFU-G, CFU-M, CFU-GM, and CFU-Mix and there was no significant difference in their proportions as well as in the size of colonies formed among subpopulations (data not shown).
Colony Formation by Lin− Sca-1+ Fractions
| Fractions . | No. of Colonies per 100 Sorted Cells* . |
|---|---|
| c-Kit + | 69.3 ± 2.9 |
| c-Kit + TIE− | 62.0 ± 2.2 |
| c-Kit + TIE+ | 68.9 ± 2.0 |
| c-Kit + TEK− | 62.7 ± 0.9 |
| c-Kit + TEK+ | 69.3 ± 2.4 |
| Fractions . | No. of Colonies per 100 Sorted Cells* . |
|---|---|
| c-Kit + | 69.3 ± 2.9 |
| c-Kit + TIE− | 62.0 ± 2.2 |
| c-Kit + TIE+ | 68.9 ± 2.0 |
| c-Kit + TEK− | 62.7 ± 0.9 |
| c-Kit + TEK+ | 69.3 ± 2.4 |
For the progenitor assay, 100 sorted cells were embedded in 1 mL of methylcellulose medium containing SCF, IL-3, IL-6, and Epo. The numbers of colonies were scored on day 10 of culture.
Mean ± SD for triplicates.
Next, HSCs of each subpopulation were analyzed for their ability to give rise to B lymphocytes in vitro. Sorted cells were cultured on a stromal cell line, PA-6, in the presence or absence of IL-7. In all dishes of these four subpopulations, colonies of granulocytes and macrophages were formed within 1 week, and then B220+ cells appeared and proliferated only in dishes containing IL-7 within 2 weeks (Fig 6). Cytospin preparation showed that small lymphocytes dominantly expanded in the presence of IL-7 (data not shown). These findings indicated that TIE+ and TEK+ HSCs, as well as TIE− and TEK− HSCs have the ability of multilineage differentiation in vitro.
B lymphopoiesis from TIE+ and TEK+ HSCs on PA-6 stromal cells. B220+ cells appeared only in the presence of IL-7 (thick line) but not in the absence of IL-7 (thin line) after 2 weeks of culture. B220− cells consisted of mature granulocytes and macrophages.
B lymphopoiesis from TIE+ and TEK+ HSCs on PA-6 stromal cells. B220+ cells appeared only in the presence of IL-7 (thick line) but not in the absence of IL-7 (thin line) after 2 weeks of culture. B220− cells consisted of mature granulocytes and macrophages.
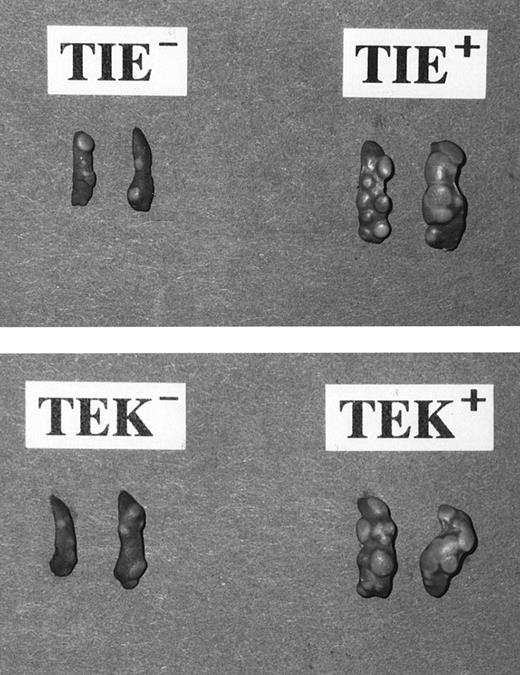
Frequency and size of colonies formed by day-12 CFU-S. The spleen colony assay is an available and simple assay in vivo to estimate the property of transplanted cells as progenitors or stem cells. We injected 200 sorted cells into lethally irradiated mice to define the frequency of CFU-S within each subpopulation of HSCs. Our previous analyses showed that Lin− c-Kit+ Sca-1+ cells contain the majority of the BM day-12 CFU-S, but not day-8 CFU-S.20 In this study, too, we found that all subpopulations form no colonies in the spleen 8 days after injection (data not shown), but at day-12, TIE+, TEK+, TIE−, and TEK− HSCs formed 8.0 ± 1.0, 9.5 ± 0.9, 5.3 ± 0.5, and 4.5 ± 1.1 CFU-S, respectively (Table 2). The frequency of day-12 CFU-S within TIE+ and TEK+ fractions were significantly higher than that within their negative counterparts.
Spleen Colony Formation by Lin− Sca-1+ Fractions
| Fractions . | No. of Day-12 CFU-S per 200 Sorted Cells* . |
|---|---|
| c-Kit + TIE− | 5.3 ± 0.5 ] † |
| c-Kit + TIE+ | 8.0 ± 1.0 |
| c-Kit + TEK− | 4.5 ± 1.1 ] † |
| c-Kit + TEK+ | 9.5 ± 0.9 |
| Fractions . | No. of Day-12 CFU-S per 200 Sorted Cells* . |
|---|---|
| c-Kit + TIE− | 5.3 ± 0.5 ] † |
| c-Kit + TIE+ | 8.0 ± 1.0 |
| c-Kit + TEK− | 4.5 ± 1.1 ] † |
| c-Kit + TEK+ | 9.5 ± 0.9 |
Sorted cells were injected into lethally irradiated mice. Spleen colonies were counted on day 8 or day 12 after transplantation. No colony was observed on day 8 in any fraction (data not shown).
Mean ± SD.
P < .01.
Interestingly, the size of spleen colonies generated from TIE+ and TEK+ HSCs was much larger than that of the colonies from their negative counterparts (Fig 7). Although there was no apparent difference in the frequency and size of colonies in vitro between TIE+ or TEK+ HSCs and their negative counterparts, this difference in vivo is indicative of the different nature of each HSC subpopulation defined by the expression of TIE and TEK.
Day-12 CFU-S formed from fractionated HSCs. Note that colonies from TIE+ and TEK+ HSCs were larger in number as well as in size in comparison to those from their negative counterparts. All HSC fractions formed no day-8 CFU-S.
Day-12 CFU-S formed from fractionated HSCs. Note that colonies from TIE+ and TEK+ HSCs were larger in number as well as in size in comparison to those from their negative counterparts. All HSC fractions formed no day-8 CFU-S.
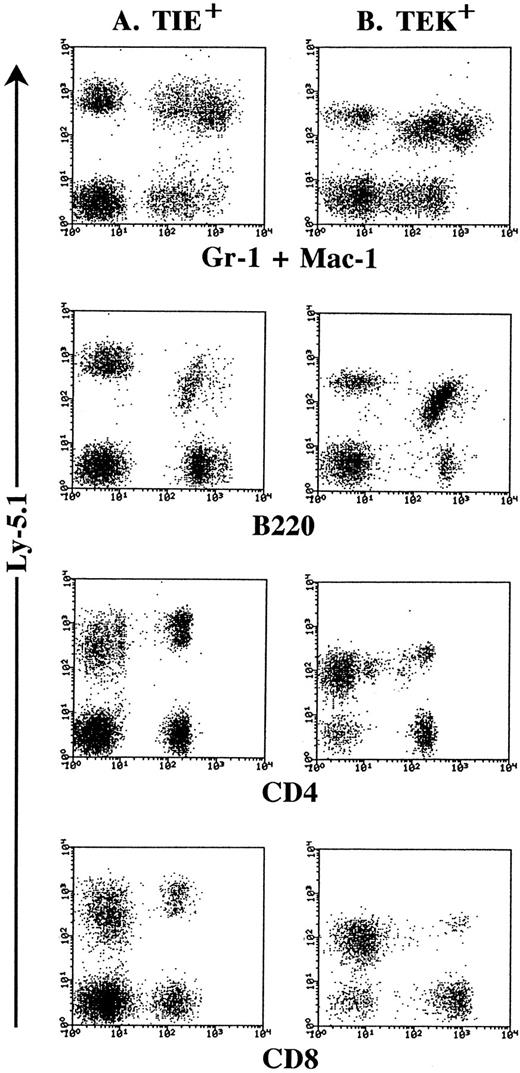
In vivo multilineage repopulating activity of TIE+ and TEK+ HSCs.To clarify whether TIE+ and TEK+ HSCs have multilineage repopulating activity in vivo, we performed a transplantation assay. Six weeks after transplantation, donor type-TIE+ and TEK+ HSCs gave rise to both lymphoid and myeloid progenies in the peripheral blood of recipient mice (Fig 8). TIE− and TEK− HSCs also showed multilineage repopulating activity in vivo (data not shown). These data were also confirmed 10 weeks after transplantation.
In vivo multilineage differentiation by Lin− c-Kit+ Sca-1+ TIE+ (A) and Lin− c-Kit+ Sca-1+ TEK+ cells (B). Two hundred cells of each fraction from Ly-5.1 donor mice were injected intravenously into lethally irradiated Ly-5.2 recipient mice. Six weeks after transplantation, donor-derived (Ly-5.1) cells were detected in the peripheral blood of recipient mice and phenotyped by two-color FACS analysis. These are the representative data of the transplantation experiment of 8 mice in each group.
In vivo multilineage differentiation by Lin− c-Kit+ Sca-1+ TIE+ (A) and Lin− c-Kit+ Sca-1+ TEK+ cells (B). Two hundred cells of each fraction from Ly-5.1 donor mice were injected intravenously into lethally irradiated Ly-5.2 recipient mice. Six weeks after transplantation, donor-derived (Ly-5.1) cells were detected in the peripheral blood of recipient mice and phenotyped by two-color FACS analysis. These are the representative data of the transplantation experiment of 8 mice in each group.
DISCUSSION
TIE and TEK have been shown to be crucial for the formation of blood vessels from the analyses of homozygous mutant mice.11 Embryos deficient in TIE failed to establish structural integrity of vascular endothelial cells, resulting in edema and local hemorrhage. They died at E13.5 to 14.5. In contrast, embryos deficient in TEK died at E9.5 to 10.5 from malformation of vascular network. These findings indicate that the structurally related RTKs TIE and TEK have important but distinct roles in the formation of blood vessels.
We showed here that both TIE and TEK are expressed in murine HSCs and demonstrated that the expression of TIE and TEK defines subpopulations with distinct functional properties within HSCs. Furthermore, as shown in the endothelial cells, we showed the different expression profiles and possible distinct roles of TIE and TEK in HSCs.
The MoAbs we established in this study showed specific reactivity to TIE or TEK (Figs 1 and 2). Using these MoAbs, we analyzed TIE and TEK expression on BM mononuclear cells. In murine BM cells, TIE and TEK were preferentially expressed on a subset of c-Kithigh cells that contain hematopoietic progenitors (Fig 3A). Murine HSCs have been found in a population of adult BM cells that express high levels of Sca-1 and c-Kit, low levels of Thy-1.1, and little to none of the lineage markers for lymphoid, myeloid, and erythroid cells.22,23 We have previously shown that Lin− c-Kit+ Sca-1+ cells contain the majority of the BM day-12 CFU-S and HSCs with LTRA, but not committed progenitor cells.20 Notably, TIE and TEK were expressed on 23.7% and 33.3% of Lin− c-Kit+ Sca-1+ cells. These expression profiles of TIE and TEK were in good agreement with our previous observation by RT-PCR analysis6 and confirmed TIE and TEK as stem cell antigens, the expression of which are highly restricted to primitive progenitor cells. Among RTKs, c-Kit and FLT3/FLK2 have been shown to be expressed on primitive progenitor cells.24 25 The expression of TIE and TEK is relatively more restricted to a subpopulation of primitive progenitor cells than c-Kit and FLT3/FLK2, indicating that TIE and TEK are involved in a specific stem cell function.
As shown in Fig 4, Lin− c-Kit+ Sca-1+ cells were further divided based on the expression of TIE and TEK into TIE+/− and TEK+/− HSCs, respectively. However, TIE+ or TEK+ HSCs did not show any significant differences from each negative counterpart in frequency or profile of colonies formed in vitro (Table 1). These four subsets of HSCs gave rise to HPP-CFC–derived multilineage colonies, including those from CFU-Mix, and differentiated into B cells on stromal cells in the presence of IL-7 (Fig 6). In addition, they contained multilineage repopulating cells in vivo (Fig 8). These findings indicated that each subset of HSCs (TIE+/− and TEK+/−) contain multipotent progenitor cells, that have a high proliferative potential and could differentiate into lymphoid and myeloid progenies both in vitro and in vivo.
On the other hand, TIE+ and TEK+ HSCs contained about twofold larger number of day-12 CFU-S (Table 2) and formed much larger colonies in spleens 12 days after injection (Fig 7) than each negative counterpart. Randall et al recently showed that within Linlow/− c-Kit+ Sca-1+ cells, CD38low/− cells contained most of the day-12 CFU-S, whereas CD38high cells contained few, but significant, day-12 CFU-S and all the long-term reconstitution cells.26 Interestingly, they found that CD38low/− day-12 CFU-S gives rise to large colonies, whereas CD38high gives rise to small colonies. Although the experiments by them and ours are not directly comparable because different parameters (TIE and TEK instead of CD38) were used, their findings are similar to our observation in separating day-12 CFU-S into two subsets that give rise to different sizes of colonies. It is reported that the secondary seeding of CFU-S from more primitive HSCs or pre–CFU-S give rise to small spleen colonies at day-12.26 Thus, this difference in size of spleen colonies is likely due to the timing of their appearance rather than the proliferative ability between the subpopulations. These findings may suggest that TIE+ and TEK+ HSCs contain a majority of day-12 CFU-S, while their negative counterparts contain HSCs at a distinct differential stage, probably more primitive HSCs.
HSCs contain day-12 CFU-S and long-term BM reconstituting cells. Recently, many investigators separated stem cells with LTRA from CFU-S. Jones et al27 separated the long-term reconstituting cells from CFU-S by cell size and density. Morrison et al28 subdivided Thy-1.1low Linlow/− Sca-1+ cells based on the expression of low levels of Mac-1, and showed that the majority of CFU-S were in the Mac-1low cells, whereas the majority of long-term reconstituting cells were Mac-1−. As described above, Randall et al26 showed that within Linlow/− c-Kit+ Sca-1+ cells, CD38low/− cells contain most of the day-12 CFU-S, whereas CD38high cells contain all the long-term reconstituting cells. More recently, Osawa et al29 subdivided Lin− c-Kit+ Sca-1+ cells by the expression degree of CD34, and showed that within Lin− c-Kit+ Sca-1+ cells, CD34+ cells contain all the day-12 CFU-S, whereas CD34− cells contain all the long-term reconstituting cells. We showed that day-12 CFU-S are enriched in the TIE+ and TEK+ HSCs (Table 2 and Fig 7). So far now, we did not find the significant differences in LTRA between TIE+ or TEK+ HSCs and their negative counterparts (Fig 8). More quantitative reconstitution assays will be required.
The precise relationship of TIE and TEK expression on HSCs remains to be determined. The very similar nature of TIE+ and TEK+ HSCs both in vitro and in vivo suggests that the expression of TIE and TEK may be overlapped on HSCs to a large extent, particularly on day-12 CFU-S. From the results of RT-PCR, it was shown that TIE and TEK-coexpressing cells were contained in HSC fraction at mRNA level (Fig 5). Unfortunately, the nature of these double-positive cells has not been revealed in FACS analysis, because our antibodies against TIE or TEK conjugated directly with dye did not detect positive cells brightly.
To date, four vascular endothelial cell-specific RTKs have been identified. Those include TIE, TEK, FLK1, and FLT1. FLK1 and FLT1 are the receptors for vascular endothelial growth factor (VEGF ). All of them are thought to be expressed in hematopoietic cells including stem cells. It has been proposed that VE cells and HSCs may originate from a common mesoderm-derived precursor hemangioblast. Coexpression of the same signaling elements in these distinct cell lineages may reflect their ontogeny from the common ancestry. During the development of the mouse embryo, TIE, TEK, FLK1, and FLT1 are sequentially expressed in the endothelial cells.30 Such sequential involvement of these RTKs is also possible in hematopoiesis during the ontogeny and differentiation of HSCs. In fact, FLK1-deficient embryos die earlier (E 8.5 to 9.5) than TIE or TEK-deficient mice from an early defect in the development of yolk sac HSCs and endothelial cells.5
RTKs play a critical role in pleiotropic cell functions of various cell systems including the hematopoietic system. Our findings show that HSCs would utilize several RTK signaling systems, c-Kit, FLT3/FLK2, and endothelial cell-specific RTKs during its differentiation from the most primitive self-renewing HSCs into multipotent progenitor cells without LTRA or day-12 CFU-S. c-Kit and FLT3/FLK2 play distinct roles in HSCs, but they also have overlapping functions and show functional redundancies each other.4,31 To further understand the regulatory mechanism of HSC function, it is important to clarify the distinct roles of TIE and TEK in HSCs, as well as to know the functional redundancies among these four RTKs. Rodewald and Sato32 recently reported that fetal liver cells from TIE-deficient mice could generate lymphoid and myeloid cells, and contained long-term BM reconstituting cells. Their findings may indicate the functional redundancies between TIE and other RTKs on HSCs.
Although the extracellular domains of TIE and TEK share a common overall structure, the homology at the amino acid level is relatively low, suggesting that each RTK has its own ligand. In our preliminary experiments, the agonistic antibodies against TIE or TEK, that can efficiently induce the phosphorylation of receptors, did not induce the proliferation of TIE or TEK-expressing cells. Taken together with the findings that TIE and TEK are not required for the production of angioblasts and their differentiation into endothelial cells, TIE and TEK may play distinct biological roles from those of c-Kit and FLT3/FLK2 in HSCs. Identification and assay of ligands for TIE and TEK is crucial to elucidate their specific functions in HSCs.
ACKNOWLEDGMENT
The authors thank Dr Yuji Yonemura for his technical advice and Dr Kazuo Maruyama for providing an expression vector, MKITneo.
Supported by Grants-in Aid from the Ministry of Education, Science and Culture of Japan.
Address reprint requests to Toshio Suda, MD, Department of Cell Differentiation, Institute of Molecular Embryology and Genetics (IMEG), Kumamoto University School of Medicine, 2-2-1 Honjo, Kumamoto 860, Japan.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal