Abstract
The etiology of stroke in sickle cell disease is unclear, but may involve abnormal red blood cell (RBC) adhesion to the vascular endothelium and altered vasomotor tone regulation. Therefore, we examined both the adhesion of sickle (SS)-RBCs to cerebral microvessels and the effect of SS-RBCs on cerebral blood flow when the nitric oxide (NO) pathway was inhibited. The effect of SS-RBCs was studied in the rat cerebral microcirculation using either a cranial window for direct visualization of infused RBCs or laser Doppler flowmetry (LDF ) to measure RBC flow. When fluorescently labeled human RBCs were infused into rats, SS-RBCs had increased adhesion to rat cerebral microvessels compared with control AA-RBCs (P = .01). Next, washed SS-RBCs or AA-RBCs were infused into rats prepared with LDF probes after pretreatment (40 mg/kg intravenously) with the NO synthase inhibitor, N-ω-nitro-L-arginine methyl ester (L-NAME), or the control isomer, D-NAME. In 9 rats treated with systemic L-NAME and SS-RBCs, 5 of 9 experienced a significant decrease in LDF and died within 30 minutes after the RBC infusion (P = .0012). In contrast, all control groups completed the experiment with stable LDF and hemodynamics. Four rats received a localized superfusion of L-NAME (1 mmol/L) through the cranial window followed by infusion of SS-RBCs. Total cessation of flow in all observed cerebral microvessels occurred in 3 of 4 rats within 15 minutes after infusion of SS-RBCs. We conclude that the NO pathway is critical in maintaining cerebral blood flow in the presence of SS-RBCs in this rat model. In addition, the enhanced adhesion of SS-RBCs to rat brain microvessels may contribute to cerebral vaso-occlusion either directly, by disrupting blood flow, or indirectly, by disturbing the vascular endothelium.
STROKE IS A major cause of morbidity and mortality in sickle cell disease.1,2 In addition, subclinical cerebrovascular disease, documented by magnetic resonance imaging studies, is present in another 10% to 20% of patients with sickle cell disease.3,4 Both clinical and subclinical stroke in sickle cell are associated with significant cognitive deficits.4,5 Cerebrovascular damage typically occurs in either the microcirculation or the medium- to large-sized arteries.3,6,7 Pathologic studies have detected intimal hyperplasia, fibrin deposition, and thrombus formation, suggesting a role for damaged endothelium.8-10 However, the mechanism(s) of stroke in sickle cell disease is poorly understood.
Sickle red blood cells (RBCs) manifest many abnormal properties that may contribute to the pathogenesis of cerebrovascular disease. These include both decreased RBC deformability and increased adhesive properties.11,12 The increased adhesion of sickle RBCs to endothelial cells as well as to components of the subendothelial matrix has been shown in in vitro,13-18 ex vivo,19 and in vivo20,21 models. The adhesion of sickle RBCs to endothelial cells is optimal under low shear forces in vitro14; this correlates well with the observed adhesion of sickle RBCs to venules in vivo19 and known sites of cerebrovascular damage in patients with sickle cell disease.3,4 The molecular mechanisms supporting the adhesion of sickle RBCs to the vascular endothelium are complex and may involve adhesive ligands such as thrombospondin13,18 and von Willebrand factor17 and adhesive molecules on the RBC and endothelial cell surfaces such as CD36,13,22 integrins,22 and sulfated glycolipids.18 The mechanism of the sickle RBC-endothelial cell adhesion also varies depending on the source of the endothelial cell, such as microvascular versus large vein endothelium.23 The interaction of sickle RBCs with the cerebral microvascular endothelium has not been described.
In addition to the enhanced adhesion of sickle RBCs to the vascular endothelium, abnormal vasomotor tone regulation likely contributes to vaso-occlusion, including stroke, in sickle cell disease.24 Nitric oxide (NO) is an important regulator of normal vascular tone, cellular adhesion, and thrombosis.25 Exposure of cultured endothelial cells to either sickled RBCs26 or hypoxia27,28 causes a decrease in the levels of NO synthase (NOS) mRNA and protein. Therefore, both sickle RBC adhesion to the vascular endothelium and hypoxia resulting from sluggish RBC flow29 30 could influence local endothelial function, thereby contributing to the pathogenesis of vaso-occlusion in sickle cell disease.
A potential model for the pathogenesis of stroke in sickle cell disease is that sickle RBCs adhere to the cerebral vascular endothelium, causing pathologic alterations in endothelial cell function. This results in acute and chronic perturbations of vasoregulatory molecules, including decreased NO synthesis. The combination of the interaction of the abnormally adhesive sickle (SS)-RBC with the cerebral microvasculature and the disturbed vaso-motor tone regulation could then precipitate the stroke. Therefore, in this study we examined both the adhesion of SS-RBCs to cerebral vasculature as well as the effect of SS-RBC infusion on the cerebral microcirculation when the NO pathway is inhibited. The effect of SS-RBCs was investigated in the rat cerebral microcirculation using either a cranial window for direct visualization of infused RBCs or laser Doppler flowmetry for rapid, real-time measurement of RBC flow.31 We have shown that SS-RBCs have increased adhesion to the rat brain microvasculature. In addition, we have demonstrated for the first time that inhibition of NO synthesis and SS-RBC infusion predisposed to stroke, as well as death, in the majority of SS-RBC infused rats.
MATERIALS AND METHODS
Animal Preparation
Animal protocols conformed to the Guiding Principles in the Care and Use of Animals of the American Physiologic Society, were in accordance with the Guide for the Care and Use ofAnimals, and were approved by the Institutional Animal Care Committee. Adult male Sprague-Dawley rats (250 to 350 g) were anesthetized with pentobarbital (65 mg/kg, intraperitoneal) and subsequently maintained on halothane anesthesia (0.5% to 1%). Body temperature was maintained at 37°C ± 1°C using a water circulated heating pad (Model 73ATA [YSI, Yellow Springs, OH] and Model-K-2-S [American Pharmaseal, Valencia, CA]). The lungs were artificially ventilated (Model 707; Harvard Apparatus, South Natick, MA) using a 30% oxygen/70% nitrogen mixture and adjusted to maintain end-tidal pCO2 (etCO2 ) at 30 to 36 mm Hg. Catheters were placed in the femoral arteries and veins for measurement of blood pressure, collection of blood samples, and administration of RBCs and medications, respectively. Arterial blood samples were monitored to confirm physiologic pH, pO2 , pCO2 , and hemoglobin. The spleen was removed to avoid sequestration of infused human RBCs.
Cranial Window Insertion for Adhesion Experiments
To study RBC adhesion to rat cerebral microcirculation, cranial windows were used to directly visualize RBC flow and the site and duration of individual RBC adhesive events. The rat's head was placed in a stereotaxic apparatus (Model 900; David Kopf, Tujunga, CA) and the scalp and connective tissue were excised. A cranial opening of approximately 3 mm2 in diameter was created in the right parietal bone using a low speed dental drill, as previously described.32,33 The dura was opened and reflected in the center of the window and the exposed brain tissue was washed with 37°C artificial cerebrospinal fluid (CSF ). A closed, perfused, glass cranial window with an acrylic frame and 3 ports was fixed onto the skull of the rat. Intracranial pressure, measured through one of the window ports, was maintained at 5 mm Hg by adjusting the height of the reservoir attached to the outflow port. Fluorescently labeled human RBCs were infused via the femoral venous line. The cerebral microcirculation was visualized by an upright fluorescent microscope (Olympus BHS, Olympus, Lake Success, NY) equipped with a vertical epi-illuminator and modified for intravital studies.33 A 40× long working distance objective lens (Olympus ULWD40/0.5) and 2.5× video projection lens were used for these studies. The emitted light from the fluorescent RBCs was captured by an image intensifier (Videoscope VS-2525) coupled to a CCD camera (COHU 4810) and recorded on SVHS video tape (Panasonic AG-7300, Arlington Heights, IL). Arterial blood pressure, etCO2 , and intracranial pressure were continuously recorded on a Grass polygraph (Grass Instruments, Quincy, MA). After completion of the experiments, all surviving animals were killed by an overdose of pentobarbital.
Laser Doppler Flow (LDF ) Preparation for Flow Experiments
Laser-Doppler flowmetry measures bidirectional RBC flow in superficial vascular beds by detecting light frequency from a helium-neon laser that has been Doppler-shifted by the moving RBCs.31 For LDF experiments, rats were prepared as described above except that a 2- to 3-mm2 burr hole was created in the rat's skull, leaving the inner table of the skull intact as previously described.34 A drop of mineral oil was put in the burr hole to improve optical coupling between the tissue and the LDF probe (PF316, dental probe; Perimed, Stockholm, Sweden). The LDF probe, attached to a laser-Doppler flowmeter (PF3; Perimed), was lowered into the burr hole until the tip was submerged in the oil. LDF, arterial blood pressure, and etCO2 were recorded continuously on a polygraph (Model MT 9500; Astro-Med, Inc, West Warwick, RI).
RBC Preparation
Human blood was obtained from normal healthy volunteers and patients with homozygous hemoglobin SS disease after informed consent was obtained using protocols approved by the local institutional review board. Different patient samples were used for every experiment. For patients with sickle cell disease, a complete blood count and reticulocyte count were also obtained when the blood was drawn. Human RBCs (control AA-RBCs or sickle SS-RBCs) were washed three times in citrated saline (13 mmol/L NaCitrate, 33 mmol/L glucose, 124 mmol/L NaCl, pH 7.0) and resuspended in solution A (90 mmol/L NaCl, 23 mmol/L NaGluconate, 27 mmol/L NaAcetate, 5 mmol/L KCl, 2 mmol/L MgCl2 , pH 7.4) at approximately a 50% hematocrit for infusion into prepared rats. The RBCs were not subjected to hypoxic conditions. For the cranial window experiments, RBCs were fluorescently labeled to allow direct visualization of individual human RBC movement through the cerebral microcirculation. Washed RBCs, in phosphate-buffered solution A with glucose (3 mmol/L), were incubated with fluorescein isothiocyanate (FITC; Isomer I; Sigma, St Louis, MO; 0.4 mg/mL) for 3 hours (pH 7.8, room temperature) and washed twice again in solution A (pH 7.4), as previously described.35 To see if FITC labeling affected the adhesive properties of human RBCs, the adhesion of RBCs to immobilized thrombospondin, known to bind SS-RBCs, was tested in an in vitro flow chamber as previously described.18 There was no significant difference in the adhesion of washed RBCs to thrombospondin comparing RBC in vitro adhesion before and after FITC labeling (n = 2 AA-RBCs and n = 2 SS-RBCs).
Experimental Protocols
Adhesion of human RBCs to rat brain microvasculature.Cranial windows were used to directly visualize the adhesive characteristics of small volumes of infused human RBCs. Fluorescently labeled control AA-RBCs (200 to 500 μL) were infused intravenously (IV) into rats prepared with a cranial window. Cellular flow in the cortical microvessels (diameter, 4 to 100 μm) was video-recorded from two or three preselected 0.07-mm2 areas for a total of 5 to 10 minutes. After documenting the location of any remaining adherent AA-RBCs, SS-RBCs (200 to 500 μL) were infused into the rats and the same preselected fields were monitored for an additional 5 to 10 minutes. The RBC adhesive events tended to be either transient and cluster around brief time periods of less than 1 second, or more durable, with RBCs remaining adherent for time periods of greater than 1 second and frequently for the duration of the area viewing time (data not shown). Therefore, both brief adhesion events (0.1 to 1.0 second) and long adhesion events (>1.0 second) were separately quantitated (events per minute) by manual review of the video tapes.
Cerebral blood flow response to SS-RBC infusion after systemic N-ω-nitro-L-arginine methyl ester (L-NAME) or D-NAME treatment.Because laser-Doppler flowmetry is a more rapid and sensitive measure of cerebral blood flow, this method was used as an indirect measure of overall cerebral blood flow and RBC adhesion. For systemic L/D-NAME experiments, rats prepared with cranial LDF probes were stabilized for 30 to 60 minutes and either L-NAME or D-NAME (40 mg/kg) was infused IV over 10 minutes (starting at time −48 minutes). Thirty minutes after L/D-NAME treatment, washed human SS- or AA-RBCs (3 mL, approximately 10% blood volume) were infused IV over 18 minutes (time −18 to 0 minutes). Data were collected after each stabilization period and then at 1- to 5-minute intervals beginning from completion of the RBC infusion (time 0) for 30 minutes or until the rat developed stroke. For the purposes of this study, stroke was defined as cessation of observed cerebral blood flow or an LDF of less than 10 perfusion units, and death was defined as a mean arterial pressure (MAP) of less than 30 and etCO2 of less than 10. The group of rats that received L-NAME and SS-RBCs was divided into two groups: (1) rats that survived the experiment (LN-SS alive group) and (2) rats that developed stroke and died before the completion of the experiment (LN-SS stroke group). The time to stroke in the LN-SS stroke group was measured and ranged from 11 to 29 minutes after completion of the RBC infusion. To allow for comparison with the other groups and statistical analysis, the time of stroke for rats in the LN-SS stroke group was normalized to 20 minutes (median time of stroke). To better examine the events preceding the stroke, data collected for the 15 minutes immediately before stroke was also normalized in this group (normalized times of 5, 10, 15, 17, 19, and 20 minutes).
Cerebral blood flow response to SS-RBC infusion after localized superfusion of L-NAME.Because of the recognized systemic effects of L-NAME, the effect of localized NOS inhibition on cerebral circulation was also examined using the cranial window CSF perfusion system. Rats prepared with cranial windows were superfused with artificial CSF containing L-NAME (1 mmol/L) at 37°C via the cranial window ports for 60 minutes followed by IV infusion of FITC-labeled SS-RBCs (2 to 3 mL) over 5 to 10 minutes. Video recordings of six preselected 0.07-mm2 areas under the cranial window were obtained beginning from completion of the infusion of SS-RBCs (time 0) for 30 minutes or until stroke occurred. Video recordings were analyzed visually for evidence of cessation of cerebral blood flow in microvessels measuring 25 to 100 μm in diameter.
Statistical Analysis
The rates of occurrence of short and long events for the adhesion experiments were compared using the homogeneity of two Poisson processes.36 Comparisons of LDF, mean arterial pressure, and CO2 were made using a single factor within (time) and a single factor between (treatment).37 Survival distributions were compared using the log-rank test.
RESULTS
Adhesion of Human RBCs to Rat Brain Microvasculature
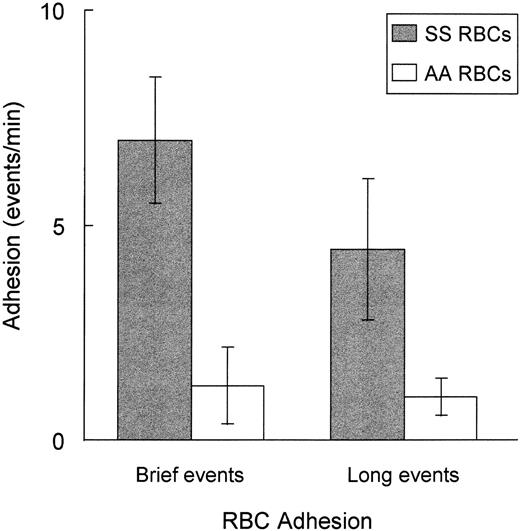
As shown in Fig 1, RBC adhesion occurred predominantly in the capillary network (approximately 65% of adhesive events) and postcapillary venules (approximately 35% of adhesive events), with less than 5% of adhesive events observed in arterioles. A similar distribution of adhesion sites was observed for both sickle and control erythrocytes. The adhesive events tended to be either transient and cluster around brief time periods of less than 1 second or more durable, remaining adherent for time periods of greater than 1 second, frequently for the duration of the area viewing time (1 to 3 minutes per field). When the number of adherent RBCs was quantitated, SS-RBCs had significantly increased adhesion for both brief adhesion events, lasting 0.1 to 1 second (P = .0063), and long adhesion events, lasting greater than 1.0 second (P = .013), compared with control AA-RBCs (Fig 2). Leukocytes can be mistaken for RBCs in some systems. To test for this effect, washed RBCs were double-labeled with FITC, which labels all cells present, and propidium iodide (50 μg/mL), to identify contaminating leukocytes and platelets in one experiment. All vascular fields were visualized with two different fluorescent filter combinations that detected either FITC or propidium iodide fluorescence. Fluorescent, adherent cells were seen only with the FITC filter and were negative for propidium iodide, verifying that adherent cells were indeed RBCs and not contaminating leukocytes or platelets (data not shown).
Adhesion of fluorescently labeled human RBCs to rat brain microvasculature. Fluorescently labeled human RBCs were injected (200 to 500 μL) into rats prepared with a cranial window as described in the Materials and Methods. Photomicrographs of video images (original magnification ×40) in two different areas of the rat brain microvasculature are shown. Area (A) focuses on capillary size vessels. Area (B) focuses on postcapillary venules. The white arrows identify fluorescent adherent human RBCs. RBC adhesion was predominately in the capillary and postcapillary venule for both sickle and control erythrocytes (SS-RBCs are shown).
Adhesion of fluorescently labeled human RBCs to rat brain microvasculature. Fluorescently labeled human RBCs were injected (200 to 500 μL) into rats prepared with a cranial window as described in the Materials and Methods. Photomicrographs of video images (original magnification ×40) in two different areas of the rat brain microvasculature are shown. Area (A) focuses on capillary size vessels. Area (B) focuses on postcapillary venules. The white arrows identify fluorescent adherent human RBCs. RBC adhesion was predominately in the capillary and postcapillary venule for both sickle and control erythrocytes (SS-RBCs are shown).
Sickle SS-RBCs have enhanced adhesion to rat brain microvasculature compared with control AA-RBCs. Fluorescently labeled control (AA)-RBCs (n = 5), followed by sickle (SS)-RBCs (n = 5), were infused into rats prepared with a cranial window as described in Fig 1. Cellular flow in the cortical microvessels (diameter 4 to 100 μm) was video-recorded from two to three preselected 0.07-mm2 areas within the cranial window for a total of 5 to 10 minutes. Brief adhesion events (0.1 to 1.0 second) and long adhesion events (<1.0 second) were separately quantitated (events per minute) by manual review of the video tapes. SS-RBCs had significantly increased adhesion compared with AA-RBCs for brief adhesion events (P = .0063) or long adhesion events (P = .013).
Sickle SS-RBCs have enhanced adhesion to rat brain microvasculature compared with control AA-RBCs. Fluorescently labeled control (AA)-RBCs (n = 5), followed by sickle (SS)-RBCs (n = 5), were infused into rats prepared with a cranial window as described in Fig 1. Cellular flow in the cortical microvessels (diameter 4 to 100 μm) was video-recorded from two to three preselected 0.07-mm2 areas within the cranial window for a total of 5 to 10 minutes. Brief adhesion events (0.1 to 1.0 second) and long adhesion events (<1.0 second) were separately quantitated (events per minute) by manual review of the video tapes. SS-RBCs had significantly increased adhesion compared with AA-RBCs for brief adhesion events (P = .0063) or long adhesion events (P = .013).
Cerebral Blood Flow Response to SS-RBC Infusion After Systemic NOS Inhibition
To study the effect of SS-RBCs on cerebral blood flow when the NO pathway was inhibited, rats were systemically treated (40 mg/kg IV) with either the NOS inhibitor, L-NAME, or the inactive control isomer, D-NAME, and then infused with approximately 10% blood volume of washed human RBCs. For this study, stroke was defined as the cessation of cerebral blood flow (LDF <10%) in the monitored cerebral microvessels. As shown in the stroke survival curve in Fig 3, all rats that were administered AA-RBCs in combination with either L-NAME (LN-AA group, n = 6) or D-NAME (DN-AA group, n = 3) survived the experimental protocol with stable LDF and therefore no evidence of stroke. Similarly, no evidence of stroke was observed in rats infused with SS-RBCs in combination with D-NAME (DN-SS group, n = 5). In contrast, 5 of 9 rats infused with SS-RBCs in combination with L-NAME (LN-SS group) developed stroke (LDF <10) within 30 minutes after completion of the RBC infusion (P = .0012).
Stroke-free survival in L/D-NAME systemically treated rats after infusion with human sickle or control RBCs. Washed human SS-RBCs or AA-RBCs (3 mL) were infused into rats systemically treated with either L-NAME or D-NAME and LDF monitored as described in the Materials and Methods and the time to stroke was measured. Stroke was defined as the time when LDF was less than 10. The probability of stroke-free survival is shown beginning from completion of the RBC infusion (time = 0 minutes) to the end of the experimental protocol (time = 30 minutes) for the four treatment groups: L-NAME +SS-RBCs (LN-SS, n = 9), L-NAME + AA-RBCs (LN-AA, n = 6), D-NAME + SS-RBCs (DN-SS, n = 5), and D-NAME + AA-RBCs (DN-AA, n = 3). Five of nine rats infused with SS-RBCs after L-NAME treatment (LN-SS) experienced a stroke within 30 minutes of the RBC infusion (*P = .0012). All rats that experienced a stroke subsequently died.
Stroke-free survival in L/D-NAME systemically treated rats after infusion with human sickle or control RBCs. Washed human SS-RBCs or AA-RBCs (3 mL) were infused into rats systemically treated with either L-NAME or D-NAME and LDF monitored as described in the Materials and Methods and the time to stroke was measured. Stroke was defined as the time when LDF was less than 10. The probability of stroke-free survival is shown beginning from completion of the RBC infusion (time = 0 minutes) to the end of the experimental protocol (time = 30 minutes) for the four treatment groups: L-NAME +SS-RBCs (LN-SS, n = 9), L-NAME + AA-RBCs (LN-AA, n = 6), D-NAME + SS-RBCs (DN-SS, n = 5), and D-NAME + AA-RBCs (DN-AA, n = 3). Five of nine rats infused with SS-RBCs after L-NAME treatment (LN-SS) experienced a stroke within 30 minutes of the RBC infusion (*P = .0012). All rats that experienced a stroke subsequently died.
To determine the relationship between the cerebral microcirculation and systemic hemodynamics after SS-RBC infusion, MAP, etCO2 , and LDF were further examined. As shown in Fig 4, MAP increased and LDF decreased in all groups treated with L-NAME (P < .01). This response is typical for the dose of L-NAME used.38 39 However, there was no difference in MAP or LDF between AA-RBC and SS-RBC groups after either D-NAME or L-NAME treatment. All 5 LN-SS animals that developed stroke also developed hypotension (Fig 4B), a decrease in etCO2 (Table 1) and died within 1 minute after the development of stroke. Because systemic vascular collapse results in a decrease in perfusion of all tissues, a decrease in metabolic rate, and consequently a decrease in the production of CO2 , the rapid and profound decrease in etCO2 was a natural consequence of severe systemic hypoperfusion. In contrast, the LN-SS animals that survived had stable MAP and etCO2 after the infusion of SS-RBCs. Interestingly, the animals in the LN-SS group that survived had a transient increase in LDF after SS-RBC infusion (Fig 4A, time −18 to 0 minutes, P < .05) compared with LN-SS stroke or control LN-AA animals. This suggests that there was a transient interaction between the SS-RBC and the cerebral circulation that resulted in a significant change in cerebral hemodynamics. In contrast, there was no change in the LDF of any of the other groups when RBCs were infused. Whereas rats in the LN-AA group experienced the same initial decrease in LDF and increase in MAP after L-NAME treatment, these animals maintained stable LDF, MAP, and etCO2 and survived to the completion of the experiment after AA-RBC infusion (Fig 4 and Table 1, LN-AA). Rats treated with the inactive isomer, D-NAME, and either SS-RBCs or AA-RBCs completed the experiment with stable LDF, MAP, and etCO2 (Fig 4 and Table 1, DN-SS and DN-AA).
LDF and MAP in rats systemically treated with L/D-NAME and infused with human RBCs. Washed human SS-RBCs or AA-RBCs (3 mL) were infused (time −18 to 0 minutes) into rats treated with either L-NAME or D-NAME (time −48 minutes) as described in Fig 3: L-NAME + AA-RBCs (LN-AA, n = 6), D-NAME + SS-RBCs (DN-SS, n = 5), and D-NAME + AA-RBCs (DN-AA, n = 3). The group of rats that received L-NAME and SS-RBCs was divided into two groups: (1) rats that survived the experiment (LN-SS alive, n = 4) and (2) rats that developed stroke and died (LN-SS stroke, n = 5). To allow for comparison with the other groups and statistical analysis, the time of stroke (LDF <10) for rats in the LN-SS stroke group was normalized to 20 minutes (median time of stroke); data collected for the 15 minutes immediately before the stroke was also normalized (normalized times of 5, 10, 15, 17, 19, and 20 minutes). The graphs represent the mean ± SE of the (A) LDF (percent change from baseline) and (B) MAP (mm Hg). †P < .01 for all L-NAME–treated groups compared with all D-NAME–treated groups. *P < .05 for LN-SS stroke group compared with LN-SS alive group. **P < .05 for LN-SS stroke group compared with all other groups. ¶P < .05 compared with time 0 within the LN-SS stroke group.
LDF and MAP in rats systemically treated with L/D-NAME and infused with human RBCs. Washed human SS-RBCs or AA-RBCs (3 mL) were infused (time −18 to 0 minutes) into rats treated with either L-NAME or D-NAME (time −48 minutes) as described in Fig 3: L-NAME + AA-RBCs (LN-AA, n = 6), D-NAME + SS-RBCs (DN-SS, n = 5), and D-NAME + AA-RBCs (DN-AA, n = 3). The group of rats that received L-NAME and SS-RBCs was divided into two groups: (1) rats that survived the experiment (LN-SS alive, n = 4) and (2) rats that developed stroke and died (LN-SS stroke, n = 5). To allow for comparison with the other groups and statistical analysis, the time of stroke (LDF <10) for rats in the LN-SS stroke group was normalized to 20 minutes (median time of stroke); data collected for the 15 minutes immediately before the stroke was also normalized (normalized times of 5, 10, 15, 17, 19, and 20 minutes). The graphs represent the mean ± SE of the (A) LDF (percent change from baseline) and (B) MAP (mm Hg). †P < .01 for all L-NAME–treated groups compared with all D-NAME–treated groups. *P < .05 for LN-SS stroke group compared with LN-SS alive group. **P < .05 for LN-SS stroke group compared with all other groups. ¶P < .05 compared with time 0 within the LN-SS stroke group.
etCO2 in Rats Treated With Systemic L/D-NAME
| Group . | −18 min . | 0 min . | +5 min . | +10 min . | +15 min . | +17 min . | +19 min . | +20 min . |
|---|---|---|---|---|---|---|---|---|
| LN-SS stroke | 34.0 (2.4) | 32.0 (4.0) | 29.0 (4.8) | 26.4 (5.5)* | 22.8 (4.1)*† | 20.8 (3.3)*† | 17.6 (4.3)*† | 7.6 (0.9)*† |
| LN-SS alive | 32.5 (1.0) | 31.5 (2.5) | 32.0 (1.6) | 32.8 (1.5) | 31.0 (1.2) | 31.0 (1.2) | 32.5 (3.0) | 32.3 (2.9) |
| LN-AA | 32.3 (0.8) | 35.0 (1.1) | 34.3 (1.5) | 33.3 (1.0) | 33.8 (1.6) | 34.3 (1.5) | 34.3 (1.5) | 34.0 (1.8) |
| DN-SS | 32.8 (1.1) | 33.0 (1.0) | 33.6 (0.9) | 33.6 (0.9) | 34.8 (1.1) | 34.8 (1.1) | 34.4 (1.7) | 34.4 (0.9) |
| DN-AA | 34.3 (2.1) | 34.7 (1.2) | 33.3 (1.2) | 34.0 (2.0) | 34.0 (2.0) | 33.3 (1.2) | 34.0 (2.0) | 33.3 (1.2) |
| Group . | −18 min . | 0 min . | +5 min . | +10 min . | +15 min . | +17 min . | +19 min . | +20 min . |
|---|---|---|---|---|---|---|---|---|
| LN-SS stroke | 34.0 (2.4) | 32.0 (4.0) | 29.0 (4.8) | 26.4 (5.5)* | 22.8 (4.1)*† | 20.8 (3.3)*† | 17.6 (4.3)*† | 7.6 (0.9)*† |
| LN-SS alive | 32.5 (1.0) | 31.5 (2.5) | 32.0 (1.6) | 32.8 (1.5) | 31.0 (1.2) | 31.0 (1.2) | 32.5 (3.0) | 32.3 (2.9) |
| LN-AA | 32.3 (0.8) | 35.0 (1.1) | 34.3 (1.5) | 33.3 (1.0) | 33.8 (1.6) | 34.3 (1.5) | 34.3 (1.5) | 34.0 (1.8) |
| DN-SS | 32.8 (1.1) | 33.0 (1.0) | 33.6 (0.9) | 33.6 (0.9) | 34.8 (1.1) | 34.8 (1.1) | 34.4 (1.7) | 34.4 (0.9) |
| DN-AA | 34.3 (2.1) | 34.7 (1.2) | 33.3 (1.2) | 34.0 (2.0) | 34.0 (2.0) | 33.3 (1.2) | 34.0 (2.0) | 33.3 (1.2) |
Shown is the mean (± SE) etCO2 (mmHg) of rats treated with systemic L-NAME or D-NAME and SS-RBCs or AA-RBCs. The LN-SS stroke group etCO2 was significantly lower than the other experimental groups beginning 10 minutes after the completion of the RBC infusion (*P < .05) and significantly different from baseline levels within the same group (time = 0) beginning 15 minutes after the completion of the RBC infusion (†P < .05).
To determine if differences within the donor SS-RBC characteristics could explain the experimental results, the reticulocyte count, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) of the SS-RBCs that were infused into rats systemically treated with L/D-NAME were compared (Table 2). There was no significant difference in these RBC parameters between any of the SS-RBC–treated groups.
Characteristics of the SS-RBCs Used in Rats Treated With Systemic L/D-NAME
| Group . | Reticulocytes (%) . | MCV (fL) . | MCH (pg) . | MCHC . |
|---|---|---|---|---|
| . | . | . | . | (g/dL) . |
| LN-SS stroke | 8.8 (3.0) | 86 (16) | 29.3 (6.2) | 33.9 (1.4) |
| LN-SS alive | 8.5 (4.8) | 83 (11) | 28.1 (3.8) | 34.0 (0.8) |
| DN-SS | 9.5 (6.9) | 86 (4) | 29.5 (1.9) | 34.3 (0.9) |
| Group . | Reticulocytes (%) . | MCV (fL) . | MCH (pg) . | MCHC . |
|---|---|---|---|---|
| . | . | . | . | (g/dL) . |
| LN-SS stroke | 8.8 (3.0) | 86 (16) | 29.3 (6.2) | 33.9 (1.4) |
| LN-SS alive | 8.5 (4.8) | 83 (11) | 28.1 (3.8) | 34.0 (0.8) |
| DN-SS | 9.5 (6.9) | 86 (4) | 29.5 (1.9) | 34.3 (0.9) |
Shown is the mean (± SD) reticulocyte count, MCV, MCH, and MCHC of the SS-RBCs that were infused into rats systemically treated with L/D-NAME. LN-SS stroke is the group of rats treated with L-NAME and SS-RBCs that developed stroke. LN-SS alive is the group of rats treated with L-NAME and SS-RBCs that survived. DN-SS is the group of rats treated with D-NAME and SS-RBCs. There was no significant difference in the reticulocyte count, MCV, MCH, or MCHC between any of these groups (P > .05, Student's t-test). SS-RBCs were from different donors for every experiment. To obtain the necessary volume of RBCs, in four experiments more than one patient's SS-RBCs were sequentially infused into a single rat (2 experiments in the LN-SS stroke group, 1 experiment in the LN-SS alive group, and 1 experiment in the DN-SS group).
Cerebral Blood Flow Response to SS-RBC Infusion After Localized Superfusion of L-NAME
To further examine the interaction of the NO pathway and RBC adhesion in the absence of the confounding systemic hemodynamic effects, the effect of localized NOS inhibition on rat cerebral blood flow was studied. L-NAME (1 mmol/L) was superfused through cranial windows followed by infusion of SS-RBCs in 4 additional rats. In 3 of these 4 rats, RBC flow initially halted in selected cerebral microvessels (Fig 5, open arrows), followed by a total cessation of cerebral blood flow in all areas visualized through the cranial window (Fig 5, solid arrows) within 15 minutes after completion of the SS-RBC infusion. The MAP and etCO2 remained stable at the time that flow first stopped in the 3 animals that developed stroke (Fig 5A, B, and D). In all 3 rats that developed stroke, the MAP subsequently decreased without recovery and 1 rat died soon after the stroke (Fig 5B). Because the endpoint for this investigation was stroke, the experiments were terminated in the other 2 rats before spontaneous death (Fig 5A and D). In 1 rat, the electroencephalogram (EEG) was monitored over the frontal area of the brain through stainless steel screws driven into the cranial bone (Fig 5D). The EEG showed a significant reduction in spontaneous brain electrical activity beginning approximately 5 minutes before the observed cessation of cerebral blood flow and further diminished after complete cessation of cerebral blood flow. This suggests that the sequence of events that occurs in the animals that died after SS-RBC infusion and L-NAME treatment is (1) SS-RBC adhesion to the microcirculation, (2) cessation of cerebral blood flow, and (3) neurological insult contributing to subsequent hypotension and death.
Cessation of cerebral blood flow (stroke) occurred in 3 of 4 rats treated with topical L-NAME (1 mmol/L) within 30 minutes after infusion of SS-RBCs. Rats were prepared with cranial windows and superfused with artificial cerebrospinal fluid containing 1 mmol/L L-NAME beginning at time −60 minutes as described in the Materials and Methods. Fluorescently labeled human SS-RBCs (2 to 3 mL) were infused into prepared rats (completed at time 0 minutes) and video images of fluorescent RBCs in 6 preselected areas within the cranial window were obtained. MAP (solid line) and etCO2 (broken line) is plotted for each animal (A, B, C, and D) that experienced stroke (solid symbols) or maintained stable cerebral blood flow (open symbols). The open arrows depict the time that blood flow first stopped in a postcapillary venule measuring greater than 25 μm in diameter. The solid arrow depicts the time the blood flow of all vessels in all observed areas ceased. Rat B died at time 11 minutes. Because the endpoint for this investigation was stroke, the experiments were terminated in rats A and D before spontaneous death. In one experiment, an EEG over the frontal cortex was also obtained and is depicted at times −60, 0, 8, and 20 minutes (D; EEG).
Cessation of cerebral blood flow (stroke) occurred in 3 of 4 rats treated with topical L-NAME (1 mmol/L) within 30 minutes after infusion of SS-RBCs. Rats were prepared with cranial windows and superfused with artificial cerebrospinal fluid containing 1 mmol/L L-NAME beginning at time −60 minutes as described in the Materials and Methods. Fluorescently labeled human SS-RBCs (2 to 3 mL) were infused into prepared rats (completed at time 0 minutes) and video images of fluorescent RBCs in 6 preselected areas within the cranial window were obtained. MAP (solid line) and etCO2 (broken line) is plotted for each animal (A, B, C, and D) that experienced stroke (solid symbols) or maintained stable cerebral blood flow (open symbols). The open arrows depict the time that blood flow first stopped in a postcapillary venule measuring greater than 25 μm in diameter. The solid arrow depicts the time the blood flow of all vessels in all observed areas ceased. Rat B died at time 11 minutes. Because the endpoint for this investigation was stroke, the experiments were terminated in rats A and D before spontaneous death. In one experiment, an EEG over the frontal cortex was also obtained and is depicted at times −60, 0, 8, and 20 minutes (D; EEG).
DISCUSSION
The major findings of this study are (1) SS-RBCs have increased adhesion to rat brain microvasculature in vivo and (2) in this rat cerebral microvascular model, the combination of NOS inhibition and sickle RBC infusion predisposed to cessation of cerebral blood flow (stroke) and death. Although all of the animals treated with both SS-RBCs and L-NAME did not die, there was a significant change in cerebrovascular hemodynamics in the animals that did not stroke, suggesting that there was a transient interaction between the SS-RBC and the cerebral circulation.
The adhesion of SS-RBCs to cerebral microvasculature has not been previously reported. In agreement with previous in vivo and in vitro studies in other vascular beds,19,21 both sickle and normal RBC adhesion was predominately observed in the postcapillary venule and the capillaries of the cerebral cortex. It is important to note that this study was not designed to examine the adhesion of RBCs to larger vessels, which also may play an important role in stroke in sickle cell disease.6 Although this study cannot rule out the possibility that RBCs stopped in the capillaries may be simply trapped behind adherent unlabeled native leukocytes, approximately 35% of the RBCs bound to the walls of the relatively larger postcapillary venules without obstruction of flow and were clearly true adhesive events. The low levels of control AA-RBC adhesion may be related to interspecies variation, because similarly labeled rat RBCs do not adhere to the cerebral microcirculation in this model (data not shown). However, the results of this investigation show that SS-RBCs have significantly increased adhesion that is beyond the baseline level of human RBC adhesion in this rat model. These results suggest a pathologic increase in RBC adhesion caused by the sickle hemoglobin disease. The adhesion of SS-RBCs was increased for both short and long events, suggesting that both transient and more tenacious adhesion is abnormal in the sickle RBC. The enhanced adhesion of SS-RBCs to rat brain microvasculature may contribute to cerebral vaso-occlusion either directly, by obstructing flow, or indirectly, by causing endothelial cell or vasomotor tone abnormalities.
The combination of NOS inhibition and infusion of sickle RBCs (LN-SS group) resulted in cessation of cerebral microvascular blood flow, or stroke, in the majority of rats tested. Because none of the control animals experienced cardio-respiratory or cerebrovascular instability, these findings cannot be explained by an isolated L-NAME effect, an isolated SS-RBC effect, or a deleterious interspecies interaction due to human RBC infusion into a live rat. In addition, the development of stroke in animals in the LN-SS group was immediately followed by death in systemically treated animals. Although the exact cause of death was not determined in this investigation, the studies in which a localized infusion of L-NAME caused stroke and subsequent hypotension in SS-RBC infused rats suggest that cerebral vaso-occlusion of critical areas of the brain, such as the brainstem, contributed to the death in these animals.
The majority of rats systemically treated with L-NAME and SS-RBCs developed hypotension and a profound decrease in etCO2 and died precipitously. The decrease in etCO2 was an expected consequence of systemic vascular collapse secondary to the decreased metabolism and production of CO2 that occurs with severe systemic hypoperfusion. However, the compromised hemodynamic status of these systemically treated rats complicates the interpretation of LDF changes. Therefore, the cranial window experiments afforded an opportunity to test a more localized effect of NOS inhibition. In agreement with the effects of systemic L-NAME infusion, the superfusion of L-NAME plus SS-RBCs in the cranial windows experiments resulted in cessation of microvascular blood flow in the majority of treated rats. Importantly, the etCO2 and MAP remained essentially unchanged despite cessation of blood flow in the observed cerebral microcirculation in the locally treated animals. In the 1 animal that developed systemic hypotension and died (Fig 5B), the etCO2 did decrease as expected due to systemic vascular collapse. In this animal, the decrease in MAP and etCO2 followed the cessation of cerebral blood flow. This suggests that profound cerebral vascular occlusion occurred as a primary event and may have contributed to the strokes and subsequent deaths observed in the systemically treated LN-SS group. In the additional experiment in which an EEG was monitored, there was a substantial decrease in amplitude in the frontal cortex EEG that began approximately 5 minutes before cessation of microvascular flow. This represents a significant functional impairment of the brain that correlated well with the development of stroke in this study.
The diversity of the responses in the group treated with both L-NAME and SS-RBCs is intriguing and may have several explanations. This diversity in response is also similar to findings in patients with sickle cell disease in which 100% have the genetic mutation (hemoglobin SS), yet only 20% to 30% develop clinical or subclinical stroke. There was no difference in the reticulocyte count or cell volumes of the SS-RBCs that were infused into the different experimental groups to account for the differences in outcomes. The significant increase in cerebral blood flow that occurred only in the LN-SS group after SS-RBC infusion in the rats that survived suggests that there was a transient interaction between the SS-RBC and the cerebral circulation that resulted in a significant change in cerebral hemodynamics. It is possible that a compensatory vasodilation of the cerebral vasculature may have been protective in these animals, consequently safeguarding them from stroke. It is possible that such transient changes could account for some of the subtle changes noted in the central nervous system of patients with sickle cell disease. Other factors that may contribute to the differences in responses within the LN-SS group include patient-to-patient variation in SS-RBC characteristics, individual differences in animal baseline hemodynamics, cerebral perfusion, leukocyte or platelet levels, or blood viscosity.
The data in this study suggest a role for the NO pathway in vaso-occlusive events, including stroke, in sickle cell disease. The role of NO as a regulator of vascular tone has been demonstrated in several disease states, including cerebral ischemia and reperfusion injury, pulmonary hypertension, and septic shock.40 In the brain, NO has been shown to be involved in the regulation of cerebral blood flow in experiments using both topically applied and IV NOS inhibitors.39 Although most evidence points to the involvement of NO in maintaining resting cerebral blood flow, its role is less clear in pathologic cerebrovascular responses.39 There is preliminary evidence that NO levels may be locally or systemically reduced in sickle cell disease. For example, exposure of isolated vascular rings to SS-RBCs decreases the vasorelaxation response to acetylcholine, suggesting that the formation of NO is inhibited by the presence of SS-RBCs.41 In preliminary work by Phelan et al,26 exposure of cultured human umbilical endothelial cells to previously sickled SS-RBCs inhibited the production of constitutive endothelial NOS mRNA and protein. In addition, leukocytes from patients with sickle cell disease have recently been reported to release higher levels of superoxide ions, a known NO scavenger, possibly contributing to lowered NO activity at sites of leukocyte activation.42 RBCs may be important in delivering nitric oxide to the microvasculature via S-nitrosylation of hemoglobin in a conformationally dependant manner.43 It is not known if sickle hemoglobin, its response to sickling conditions, or the many secondary changes in the sickle RBC membrane may adversely affect the synthesis, delivery, or regulation of nitric oxide. The combined effect of abnormal sickle hemoglobin delivery of NO and inhibition of NOS may have adverse synergistic effects.
The pathophysiology of vaso-occlusion in sickle cell disease, including stroke, is likely multifactorial. Our data provide evidence for both the increased interaction of the SS-RBC with the vascular endothelium and a critical role of NO in maintaining cerebrovascular flow in the presence of sickle RBCs in our model. The enhanced adhesion of SS-RBCs to the cerebral microvascular endothelium and subsequent perturbation of endothelial cell function may contribute to the initiation or perpetuation of clinically significant vaso-occlusion in sickle cell disease. In addition, these studies point to a therapeutic potential for treating sickle cell disease with NO or NO donors.
ACKNOWLEDGMENT
We thank Owen W. Griffith for expert advice and useful discussions. Ming C. Du is thanked for technical expertise with preparation and labeling of human RBCs. Evelyn R. Brown and Gwen Lea are thanked for assistance with obtaining blood samples.
Supported by Public Health Services Grant No. K08-HL02858 (C.A.H.) and Clinical Research Center Grant No. RR00058 from the National Institutes of Health and National Sciences Foundation Grant No. BES-9411631 (A.G.H.).
Presented previously at the 1995 meeting of the American Society of Hematology, Seattle, WA (Blood 86:142a, 1995 [abstr, suppl 1]).
Address reprint requests to Cheryl A. Hillery, MD, Blood Research Institute, The Blood Center of Southeastern Wisconsin, PO Box 2178, Milwaukee, WI 53201-2178.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal