Abstract
Engagement of the high-affinity IgG Fc receptor (FcγRI) activates a signal transduction pathway involving tyrosine phosphorylation of associated kinases. We compared the activation of the related protein tyrosine kinases (PTKs), Syk and ZAP-70, in FcγRI-mediated signaling. Cross-linking of the FcγRI multimeric receptor in monocytic cells results in tyrosine phosphorylation of the FcεRIγ subunit and association of Syk with this complex. We stably introduced ZAP-70 via a retroviral vector into two monocytic cell lines, U937 and THP-1, which normally do not express ZAP-70. Neither Syk nor MAP kinase activation was affected by the presence of ZAP-70. Although transduced ZAP-70 had in vitro kinase activity and associated with FcεRIγ after receptor aggregation, it was not tyrosine phosphorylated. In contrast, both ZAP-70 and Syk were phosphorylated in a T-cell line in which their respective levels of expression were similar to those detected in U937/ZAP-70 cells. Therefore, these results suggest that requirements for Syk and ZAP-70 phosphorylation are distinct in a monocytic cell context.
SIGNAL TRANSDUCTION in multiple hematopoietic cell lineages is mediated through tyrosine phosphorylation of Src family protein tyrosine kinases (PTKs) as well as the Syk and ZAP-70 PTKs.1-3 Syk and ZAP-70 are members of a class of PTKs characterized by the presence of two tandem SH2 domains and a C-terminal catalytic domain.1,4 ZAP-70 is expressed only in T cells, natural killer (NK) cells, and thymocytes, whereas Syk is expressed in thymocytes, B cells, mast cells, monocytes, macrophages, and neutrophils and at low levels in mature T cells.5 There has been some controversy regarding the degree of redundancy in signal transduction mediated by the ZAP-70 and Syk PTKs. Both ZAP-70 and Syk are activated after T-cell receptor (TCR) stimulation. However, activation of ZAP-70 has been shown to require the presence of Lck or another associated Src family PTK, whereas Syk is reported to undergo phosphorylation in an Lck-independent manner.6,7 The biologic significance of this difference is not known, because phosphorylation of Syk is augmented in the presence of an Src family PTK.5 8
Because of the controversy regarding the ability of ZAP-70 to substitute for Syk in receptor-mediated signaling, we assessed the activation of ZAP-70 and Syk after FcγRI stimulation in myelomonocytic cells. FcγRI (CD64) is a 72-kD integral membrane glycoprotein that is a member of the Fc receptor family (FcR) and binds monomeric IgG with high affinity.9,10 As described for the TCR, cross-linking FcRs results in the tyrosine phosphorylation of cell proteins; additionally, multiple immune responses are activated, including phagocytosis, antibody-dependent cellular cytotoxicity, and expression of proinflammatory genes.11
Signaling through the FcγRI and TCR receptors is hypothesized to be similar because of the high degree of homology between their respective associated subunits, FcεRIγ and TCRζ.12 Accordingly, FcεRIγ can substitute for the TCRζ homodimer in the TCR,13 and either FcεRIγ or TCRζ can associate with the FcγRIII/CD16 receptor in NK cells.14-18 The related FcεRIγ and TCRζ subunits both contain a 16-amino acid (aa) immunoreceptor tyrosine-based activation motif (ITAM) motif (YXXLX6-8YXXL)19-25 and, upon engagement of the FcγRI, FcγRIII, or TCR receptors, the two tyrosines in the ITAM are phosphorylated.26-29 These tyrosine phosphorylation events are required for subsequent downstream signaling: activation of the ras/MAP kinase pathway, increases in intracellular calcium and PLCγ1 phosphorylation, and cytokine expression.7 30-33
Stimulation of FcγRI results in the association and phosphorylation of the Src family PTKs, Lyn and Hck.34,35 Additionally, Syk is phosphorylated upon FcγRI cross-linking and is physically associated with the FcεRIγ homodimer subunit.28,29,36-42 ZAP-70 is activated in cells with a TCR containing the FcεRIγ subunit18 and it is thought that ZAP-70 associates with FcεRIγ in the context of the TCR. Therefore, we assessed whether ZAP-70 could associate with the same FcεRIγ subunit in the context of the FcγRI receptor. Myelomonocytic cells with an FcγRI receptor, transduced to express ZAP-70 as well as Syk, were used to assess the relative abilities of ZAP-70 and Syk to mediate the signaling cascade.
We now report that ZAP-70 did not undergo tyrosine phosphorylation upon FcγRI receptor aggregation even though stimulation of the receptor resulted in the association of ZAP-70 with the FcεRIγ subunit. Additionally, introduction of ZAP-70 into the U937 and THP-1 human monocytic cell lines did not affect the subset of cellular substrates phosphorylated upon FcγRI cross-linking. In contrast, both ZAP-70 and Syk were phosphorylated in a T-cell line in which the two proteins were expressed at similar levels to those observed in the ZAP-70–transduced U937 cells. These results suggest that, despite a high degree of homology, ZAP-70 and Syk are differentially activated in a myeloid cell context.
MATERIALS AND METHODS
Cells.The U937 and THP-1 monocytic cell lines as well as the Jurkat T-cell line were obtained from the American Type Culture Collection (ATCC; Rockville, MD) and cultured in RPMI 1640 with 10% fetal bovine serum (FBS; Irvine Scientific, Santa Anna, CA) and 2 mmol/L L-glutamine. The Jurkat clone 77-6.8 was generously provided by Dr K.A. Smith (Sloan-Kettering Memorial Cancer Center, New York, NY). The MB T-cell line was generated by human T-cell leukemia virus type-I (HTLV-I) transformation of peripheral T cells from a normal adult donor as previously described.43 MB cells are CD3+/CD4+ and were cultured in RPMI 1640 with 10% FBS and 240 IU/mL interleukin-2 (IL-2; a kind gift of Carolyn Paradise, Chiron Corp, Emeryville, CA). GPE (generously provided by Dr A.D. Miller, Fred Hutchinson Cancer Research Center, Seattle, WA) and PG13 (ATCC) packaging cells were grown in Dulbecco-Vogt modified Eagle's medium with high glucose (GIBCO BRL, Gaithersburg, MD) and 10% FBS. Before experiments, monocytic cells were differentiated in 400 U/mL of γ-interferon (IFN; generously provided by Genentech Corp, San Francisco, CA) for 4 days at a concentration of 5 × 105 cells/mL.29 MB cells were rested overnight in RPMI with 1% FBS in the absence of IL-2 before stimulation.
Reagents and antibodies.Polyclonal rabbit antibodies to Syk and Lck, polyclonal rabbit antisera and monoclonal antibody (MoAb) to ZAP-70, a polyclonal antisera against MAP kinase, and a polyclonal rabbit anti-FcεRIγ chain antibody were generously provided by Dr Bart Sefton (Salk Institute, La Jolla, CA), Dr Art Weiss (University of California, San Francisco, CA), Dr Jonathan Cooper (Fred Hutchinson Cancer Research Center), and Dr Jean-Pierre Kinet (National Institute of Allergy and Infectious Diseases, Bethesda, MD), respectively. Anti-FcεRIγ chain antibody (5297) was generated as described.29 FcγRI-specific MoAbs 32 (mIgG1, Fab′2 fragment) and 197 (mIgG2a, whole IgG) were obtained from Medarex, Inc (West Lebanon, NH). Polyclonal rabbit antibodies to Fyn and Lyn as well as the antiphosphotyrosine 4G10 MoAb were purchased from Upstate Biotechnology Inc (Lake Placid, NY). The biotin-linked CD3-specific antibody, Leu 4, was obtained from Becton Dickinson (San Jose, CA). The cross-linking antibody was a rabbit antimouse Fab′2 fragment (Cappel Labs, Durham, NC).
Introduction of ZAP-70 by retroviral-mediated transduction.The ZAP-70 cDNA was was excised from Bluescript KS (+) as an EcoRI fragment, blunted with klenow DNA polymerase, and cloned into the SnaBI site of the pG1XSvNa vector, a Moloney murine leukemia virus (Mo-MuLv)–based retroviral vector (generously provided by Genetic Therapy, Inc, Baltimore, MD),44 as previously described, and a high-titer PG13 amphotropic packaging line was generated.45 A single clone with a titer of 5 × 105/mL was used to transduce U937 and THP-1 cells. The LN retroviral vector, containing the bacterial neomycin phosphotransferase gene, was kindly provided as a high-titer PA317 amphotropic vector-producing fibroblast by Dr A.D. Miller. The normal ZAP-70 cDNA5 was excised from Bluescript KS (+) as an EcoRI fragment, blunted with klenow DNA polymerase, and cloned into the SnaBI site of the pG1XSvNa vector.
pG1ZAP70SvNa and LN retroviral supernatants were used to transduce U937 or THP-1 cells growing at a concentration of 1 × 105 cells/mL in media containing 3 μg/mL polybrene. At 96 hours, the transduced cells were diluted to approximately 5 × 104/mL and 1-mL aliquots were seeded into wells of a 24-well plate in 0.8 mg/mL G418 (geneticin; GIBCO BRL) for both cell types. Drug-resistant cells were observed in all wells of infected U937 and THP-1 cells, suggesting that each well represented a pool of infected cells. Six pools of G418-resistant cells from two separate transductions were assessed for ZAP-70 expression by Western blot analysis. All pools were found to express ZAP-70 and two pools from each infection were used in subsequent experiments. Similarly, U937 and THP-1 cells infected with the LN vector were obtained.
Immunoprecipitation, Western blot analysis, and kinase assays.U937 cells treated with IFN were processed essentially as reported.29 Briefly, cells were washed in cold Hanks' Buffered Saline Solution and resuspended at 1 × 107 cells/mL with 2 μg/mL of the primary MoAb (FcγRI Fab′2 , 32.2) for 15 minutes on ice. After rapid centrifugation, secondary rabbit antimouse antibody was added at 10 μg/mL for 3 minutes at 37°C, or as otherwise indicated. Jurkat cells, Jurkat clone 77-6.8, and MB cells (1 × 107/mL) were stimulated with anti-Leu4–biotin followed by streptavidin (10 μg/mL; Calbiochem, La Jolla, CA) as described.45 Cells were then lysed in an NP40 lysis buffer, immunoprecipitated with the specified antibody, and collected on protein A/G-agarose (Santa Cruz Biotechnologies, Santa Cruz, CA).46
Immunoprecipitates (from 1 × 107 cells) and whole-cell lysates (from 2 × 105 cells) were resolved on 7.5% or 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred electrophoretically to a Hybond membrane (Amersham, Arlington Heights, IL). Immunoblotting of membranes with antiphosphotyrosine MoAbs was performed as previously described.46 For immunoblotting with all other antibodies, membranes were blocked in TBS (150 mmol/L NaCl, 20 mmol/L Tris, pH 7.5) containing 5% bovine serum albumin and 0.1% Tween 20. After incubation with the primary antibody, blots were washed with TBS containing 0.1% Tween 20, incubated with horseradish peroxidase-conjugated antibody (Amersham), and visualized using the enhanced chemiluminescence (ECL) detection system (Amersham). Filters were stripped as described.46
The protein tyrosine kinase activities of ZAP-70 and Syk were determined after immunoprecipitates were washed three times in lysis buffer and twice in 150 mmol/L NaCl and 50 mmol/L Tris HCl, pH 7.5. The precipitates were incubated in 20 μL kinase buffer containing 5 μCi [γ32P] ATP (3,000 Ci/mmol/L) for 10 minutes at 30°C, resolved on a 7.5% polyacrylamide gel, and visualized by autoradiography as reported.45 For measuring kinase activity towards an exogenous substrate, 1 μg of purified GST-Lck Sh2 domain fusion protein (kindly provided by Dr C. Couture, La Jolla Institute of Allergy and Immunology, La Jolla, CA6) was added to the immunoprecipitates just before incubation with [γ32P] ATP. Samples were then treated exactly as described above, except that they were resolved on 15% polyacrylamide gels.
RESULTS
Expression of ZAP-70 in the U937 and THP-1 cell lines.Although Syk is expressed in U937 and THP-1 myeloid cell lines, expression of ZAP-70 was detected only after infection of cells with a retroviral vector, pGIZAP70SvNa, containing the ZAP-70 cDNA (Figs 1d and 2f ). As a negative control, cells were infected with a retroviral vector, LN, expressing the neomycin phosphotransferase gene alone.
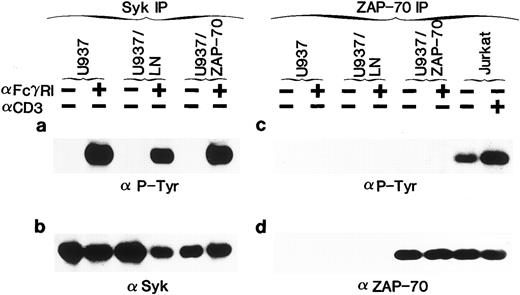
Syk, but not the introduced ZAP-70, is phosphorylated in response to FcγRI cross-linking. (a) Uninfected U937 cells or U937 cells infected with the LN (U937/LN) or ZAP-70 (U937/ZAP-70) containing retrovirus were induced with IFN for 4 days. Cells were then either incubated alone (−) or prebound with the FcγRI Fab ′2 MoAb (+) and cross-linked with rabbit α-mouse antibody. Lysates from 5 × 106 cells were immunoprecipitated with a rabbit polyclonal anti-Syk antibody, fractionated on polyacrylamide gels, analyzed by immunoblotting with the 4G10 monoclonal antiphosphotyrosine antibody, and developed with ECL to assess the phosphorylation status of Syk. (b) Blots were then stripped and reprobed with a polyclonal rabbit anti-Syk antibody. (c and d) Cell lysates were similarly immunoprecipitated with a ZAP-70–specific rabbit polyclonal antibody and probed with an antiphosphotyrosine (c) or monoclonal ZAP-70 antibody (d). For comparison, the level of expression and tyrosine phosphorylation state of ZAP-70 was assessed in Jurkat T cells that were either unstimulated (−) or stimulated with a CD3-specific antibody (+). These data are representative of results obtained in six independent experiments.
Syk, but not the introduced ZAP-70, is phosphorylated in response to FcγRI cross-linking. (a) Uninfected U937 cells or U937 cells infected with the LN (U937/LN) or ZAP-70 (U937/ZAP-70) containing retrovirus were induced with IFN for 4 days. Cells were then either incubated alone (−) or prebound with the FcγRI Fab ′2 MoAb (+) and cross-linked with rabbit α-mouse antibody. Lysates from 5 × 106 cells were immunoprecipitated with a rabbit polyclonal anti-Syk antibody, fractionated on polyacrylamide gels, analyzed by immunoblotting with the 4G10 monoclonal antiphosphotyrosine antibody, and developed with ECL to assess the phosphorylation status of Syk. (b) Blots were then stripped and reprobed with a polyclonal rabbit anti-Syk antibody. (c and d) Cell lysates were similarly immunoprecipitated with a ZAP-70–specific rabbit polyclonal antibody and probed with an antiphosphotyrosine (c) or monoclonal ZAP-70 antibody (d). For comparison, the level of expression and tyrosine phosphorylation state of ZAP-70 was assessed in Jurkat T cells that were either unstimulated (−) or stimulated with a CD3-specific antibody (+). These data are representative of results obtained in six independent experiments.
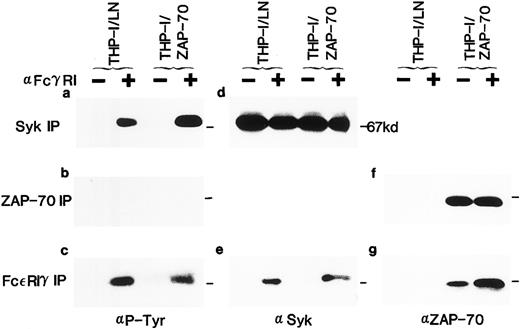
Expression of ZAP-70 and its association with the FcεRIγ subunit in THP-1 cells. Lysates from unstimulated (−) or FcγRI-stimulated (+) THP-1/LN or THP-1/ZAP-70 cells were immunoprecipitated with polyclonal rabbit anti-Syk (a and d), anti–ZAP-70 (b and f ), or anti-FcεRIγ (c, e, and g) antibodies and separated on 7.5% polyacrylamide gels. Blots were probed with either a monoclonal antiphosphotyrosine (a, b, and c), polyclonal rabbit anti-Syk (d and e), or monoclonal anti–ZAP-70 (f and g) antibody. The position of the 67-kD molecular weight marker is indicated in each panel (−). These data are representative of results obtained in five independent experiments.
Expression of ZAP-70 and its association with the FcεRIγ subunit in THP-1 cells. Lysates from unstimulated (−) or FcγRI-stimulated (+) THP-1/LN or THP-1/ZAP-70 cells were immunoprecipitated with polyclonal rabbit anti-Syk (a and d), anti–ZAP-70 (b and f ), or anti-FcεRIγ (c, e, and g) antibodies and separated on 7.5% polyacrylamide gels. Blots were probed with either a monoclonal antiphosphotyrosine (a, b, and c), polyclonal rabbit anti-Syk (d and e), or monoclonal anti–ZAP-70 (f and g) antibody. The position of the 67-kD molecular weight marker is indicated in each panel (−). These data are representative of results obtained in five independent experiments.
Because ZAP-70 is normally expressed only in T cells, NK cells, and thymocytes,5 we compared the level of ZAP-70 in the transduced pools with the endogenous production in the Jurkat T-cell line. The level of ZAP-70 production in the U937/ZAP-70, THP-1/ZAP-70, and Jurkat cells was equivalent, as assessed by immunoblotting cell lysates directly with a polyclonal α-ZAP-70 antibody (data not shown). Further experiments were performed with two different pools of U937 and THP-1 cells from two separate transductions and gave equivalent results.
Syk, but not ZAP-70, is phosphorylated upon FcγRI aggregation.Syk has previously been shown to be activated in U937 and THP-1 cells after FcγRI stimulation.28,29,36,38,39 42 To determine whether ZAP-70 as well as Syk could be phosphorylated in transduced cells, cells were stimulated with the Fab′2 portion of an MoAb to FcγRI (32.2) followed by cross-linking with rabbit antimouse Ig. Clustering of the receptor resulted in the expected tyrosine phosphorylation of Syk in U937, U937/LN, and U937/ZAP-70 cells (Fig 1a). Phosphorylated Syk was not detected before cross-linking, even though Syk was immunoprecipitated from all samples, as confirmed by immunoblotting with a polyclonal Syk antibody (Fig 1b).
The phosphorylation status of the introduced ZAP-70 was also assessed. We found that ZAP-70 was appropriately tyrosine phosphorylated in Jurkat T cells after stimulation of the TCR with an anti-CD3 antibody (Fig 1c). However, despite equivalent ZAP-70 expression in U937/ZAP-70 and Jurkat cells levels as well as comparable abilities to immunoprecipitate ZAP-70 from the two cell types (Fig 1d), FcγRI cross-linking did not result in detectable ZAP-70 phosphorylation in three independent pools of ZAP-70–transduced U937 cells (Fig 1c and data not shown). Equivalent results were also obtained in several pools of ZAP-70–transduced THP-1 cells (Fig 2b and f ). However, after long ECL exposures, very low levels of phosphorylated ZAP-70 could be detected (data not shown).
The stimulating antibody used in the experiments described above recognizes FcγRI by its Fab specificity. However, mouse monomeric IgG2A can bind FcγRI with high affinity through its Fc portion. Therefore, we assessed whether cross-linking the receptor on U937/ZAP-70 cells with a whole FcγRI IgG2A MoAb (197) would result in ZAP-70 phosphorylation. Although Syk phosphorylation was equivalent after receptor cross-linking in all transduced and wild-type U937 cells, ZAP-70 phosphorylation was not detected (data not shown).
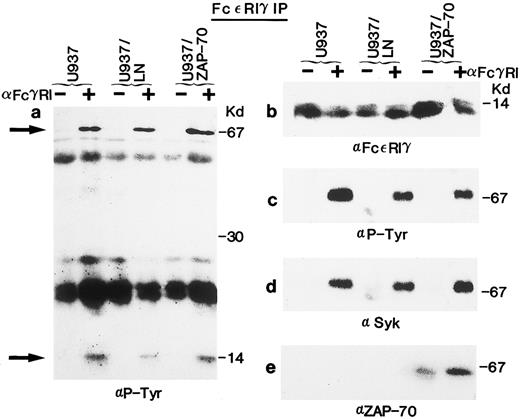
Association of Syk and ZAP-70 with the FcεRIγ subunit after receptor clustering in U937 cells. (a) Lysates from unstimulated (−) or FcγRI-stimulated (+) U937, U937/LN, or U937/ZAP-70 cells were immunoprecipitated with an anti-FcεRIγ polyclonal rabbit antibody (5297), separated on a 15% polyacrylamide gel, and analyzed by immunoblotting with an antiphosphotyrosine antibody. The positions of Syk and the FcεRIγ receptor subunit are indicated by arrows. The heavily reactive bands observed at approximately 50 kD and 25 kD are the heavy and light chains of the immunoprecipitating antibody, respectively. (b) The blot was stripped and reprobed with the anti-FcεRIγ polyclonal rabbit antibody (5297). (c, d, and e) Lysates treated as in (a) were separated on a 7.5% polyacrylamide gel and immunoblotted with either a monoclonal antiphosphotyrosine (c), polyclonal rabbit anti-Syk (d), or monoclonal anti–ZAP-70 (e) antibody. These data are representative of results obtained in six independent experiments.
Association of Syk and ZAP-70 with the FcεRIγ subunit after receptor clustering in U937 cells. (a) Lysates from unstimulated (−) or FcγRI-stimulated (+) U937, U937/LN, or U937/ZAP-70 cells were immunoprecipitated with an anti-FcεRIγ polyclonal rabbit antibody (5297), separated on a 15% polyacrylamide gel, and analyzed by immunoblotting with an antiphosphotyrosine antibody. The positions of Syk and the FcεRIγ receptor subunit are indicated by arrows. The heavily reactive bands observed at approximately 50 kD and 25 kD are the heavy and light chains of the immunoprecipitating antibody, respectively. (b) The blot was stripped and reprobed with the anti-FcεRIγ polyclonal rabbit antibody (5297). (c, d, and e) Lysates treated as in (a) were separated on a 7.5% polyacrylamide gel and immunoblotted with either a monoclonal antiphosphotyrosine (c), polyclonal rabbit anti-Syk (d), or monoclonal anti–ZAP-70 (e) antibody. These data are representative of results obtained in six independent experiments.
ZAP-70 and Syk both associate with the FcεRIγ chain.It was possible that the disparity between the phosphorylation status of the ZAP-70 and Syk PTKs after receptor aggregation was caused by differential association of the two PTKs with the FcεRIγ receptor subunit. Tyrosine phosphorylation of both ZAP-70 and Syk is dependent on their respective interactions with a phosphorylated ITAM located within the receptor subunit.11 24 Thus, it was possible that the absence of ZAP-70 tyrosine phosphorylation after FcγRI cross-linking was due to its inability to associate with the ITAM present in the FcεRIγ subunit of FcγRI.
Syk has previously been shown to associate with the tyrosine-phosphorylated FcεRIγ homodimer after receptor stimulation.28,29 36-42 We detected tyrosine-phosphorylated proteins of approximately 70 kD and a doublet of 14 kD in FcεRIγ immunoprecipitates of U937, U937/LN, and U937/ZAP-70 lysates after receptor clustering (Fig 3a). The 14-kD doublet was the FcεRIγ subunit itself, because it was detected with two polyclonal rabbit anti-FcεRIγ antibodies in both stimulated as well as nonstimulated immunoprecipitates (Fig 3b and data not shown). The protein with a molecular weight of approximately 70 kD was identified as Syk by immunoblotting the FcεRIγ immunoprecipitates with an Syk-specific antibody (Figs 2e and 3d). Syk was associated with the FcεRIγ chain after receptor clustering and this association was not affected by the presence of LN or ZAP-70 (Figs 2e and 3d).
Association of ZAP-70 with FcεRIγ was also analyzed (Figs 2g and 3e). It is interesting to note that, although Syk was only detected in FcεRIγ immunoprecipitates after clustering, low levels of ZAP-70 were associated with the FcεRIγ homodimer in unstimulated U937/ZAP-70 and THP-1/ZAP-70 cells (Figs 2g and 3e). However, the level of associated ZAP-70 increased significantly upon FcRIγ stimulation. The approximately 70-kD protein phosphorylated upon receptor aggregation could not represent both the 72-kD Syk and the 70-kD ZAP-70 because these two proteins could be clearly distinguished on the basis of their electrophoretic mobilities on a 7.5% SDS-polyacrylamide gel (data not shown). The respective associations of Syk and ZAP-70 with the FcεRIγ subunit were observed in both U937/ZAP-70 and THP-1/ZAP-70 cells after immunoprecipitations with two different polyclonal rabbit anti-FcεRIγ antibodies (see the Materials and Methods and Figs 2 and 3).
The introduced ZAP-70 protein has kinase activity that is not affected by FcγRI stimulation.To test the function of the introduced ZAP-70 protein, in vitro kinase activity was analyzed. The kinase activity of ZAP-70 in U937/ZAP-70 and Jurkat cells was comparable (Fig 4b). However, there was no increase in ZAP-70 kinase activity in the transduced U937 cells after FcγRI receptor clustering. In contrast, Syk kinase activity, which has previously been shown to be linked to receptor aggregation in various hematopoietic cells,37,38 47 was approximately twofold higher in U937, U937/LN, and U937/ZAP-70 cells after FcγRI cross-linking (Fig 4a). The amount of Syk protein immunoprecipitated in each lane was equivalent (data not shown). Moreover, the kinase activity of Syk, but not ZAP-70, towards an exogenous Lck-Sh2 domain substrate was significantly increased after receptor cross-linking (data not shown). Therefore, although the introduced ZAP-70 was functional, as assessed by its ability to be phosphorylated in an in vitro kinase assay, in contrast to Syk, its phosphorylation and kinase activity were not linked to FcγRI stimulation in U937 and THP-1 cells.
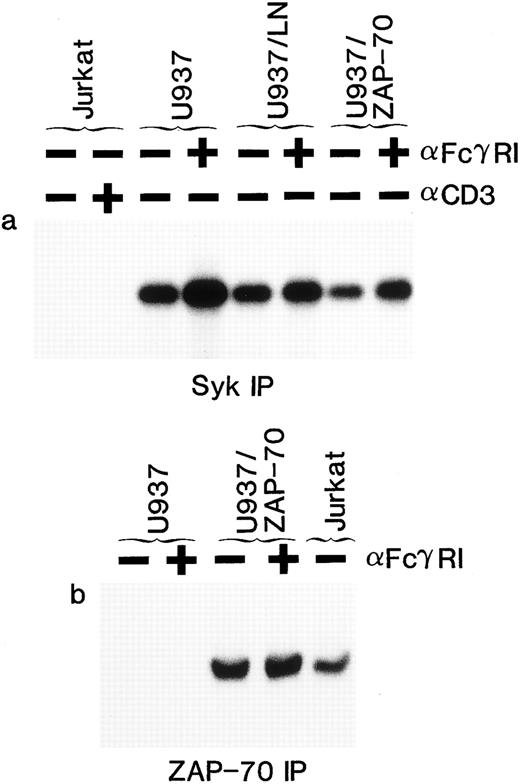
Enhancement of Syk, but not ZAP-70, kinase activity in response to FcγRI stimulation. U937, U937/LN, U937/ZAP-70, and Jurkat cells were subjected to antibody-mediated cross-linking followed by immunoprecipitation and analysis of endogenous substrate phosphorylation. Syk (a) and ZAP-70 (b) activities in immunoprecipitates from nonstimulated (−) or cross-linked (+) cells were assessed by immune complex kinase assay and autoradiography. These data are representative of results obtained in five independent experiments.
Enhancement of Syk, but not ZAP-70, kinase activity in response to FcγRI stimulation. U937, U937/LN, U937/ZAP-70, and Jurkat cells were subjected to antibody-mediated cross-linking followed by immunoprecipitation and analysis of endogenous substrate phosphorylation. Syk (a) and ZAP-70 (b) activities in immunoprecipitates from nonstimulated (−) or cross-linked (+) cells were assessed by immune complex kinase assay and autoradiography. These data are representative of results obtained in five independent experiments.
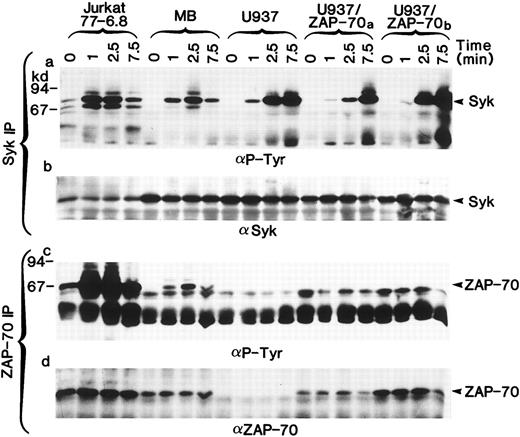
Both ZAP-70 and Syk PTKs can be phosphorylated in stimulated T cells but only Syk is phosphorylated in U937 myelomonocytic cells.The necessary use of different antibodies to detect ZAP-70 and Syk precluded a reliable comparison of their expression levels. Thus, we could not formally exclude the possibility that different concentrations of ZAP-70 and Syk accounted for the lack of ZAP-70 phosphorylation. However, by immunoblotting whole cell lysates, we were able to identify T-cell lines (Jurkat clone 77-6.8 and an HTLV-I–transformed T-cell line MB) in which the levels of ZAP-70 and Syk were approximately equal to those detected in the U937/ZAP-70 cells. In these T-cell lines, both ZAP-70 and Syk were phosphorylated after TCR stimulation (Fig 5a and c). Several molecular weight species of greater than 70 kD were also tyrosine-phosphorylated, but these other proteins were not recognized by Syk– or ZAP-70–specific antibodies (Fig 5b and d). Equivalent kinetics of ZAP-70 and Syk phosphorylation were observed in the T-cell lines with maximal phosphorylation of the two PTKs at 2.5 minutes, whereas the kinetics of Syk phosphorylation in the U937 cells differed slightly with maximal phosphorylation at 7.5 minutes. ZAP-70 phosphorylation was not detected in the transfected U937 lines, assessed up to 15 minutes after induction (Fig 5 and data not shown). It is interesting to note that even in a pool of transduced U937 cells (U937/ZAP-70b ) that had slightly higher levels of ZAP-70 as compared with the MB T-cell line, no ZAP-70 phosphorylation was detected. Thus, these data suggest that the lack of ZAP-70 phosphorylation in U937/ZAP-70 cells is not likely to be a function of ZAP-70 levels or kinetics of phosphorylation.
Syk and ZAP-70 are phosphorylated with similar kinetics in T-cell lines, whereas only Syk is phosphorylated in transduced U937/ZAP-70 cells. The Jurkat 77-6.8 T-cell line, an HTLV-I–transformed T-cell line MB, U937 cells, and two pools of ZAP-70–transduced cells (U937/ZAP-70a and U937-ZAP-70b ) were stimulated with a CD3 (for T cells) or FcγRI MoAb (for U937 cells) for the indicated times. Lysates were immunoprecipitated with either an anti-Syk (upper panel) or an anti–ZAP-70 antibody (lower panel), fractionated on a polyacrylamide gel, and immunoblotted with an antiphosphotyrosine antibody. Blots were then reprobed with either an anti-Syk (upper panel) or anti–ZAP-70 antibody (lower panel). The positions of Syk and ZAP-70 are indicated with arrows and the positions of molecular weight markers are noted. These data are representative of results obtained in six independent experiments.
Syk and ZAP-70 are phosphorylated with similar kinetics in T-cell lines, whereas only Syk is phosphorylated in transduced U937/ZAP-70 cells. The Jurkat 77-6.8 T-cell line, an HTLV-I–transformed T-cell line MB, U937 cells, and two pools of ZAP-70–transduced cells (U937/ZAP-70a and U937-ZAP-70b ) were stimulated with a CD3 (for T cells) or FcγRI MoAb (for U937 cells) for the indicated times. Lysates were immunoprecipitated with either an anti-Syk (upper panel) or an anti–ZAP-70 antibody (lower panel), fractionated on a polyacrylamide gel, and immunoblotted with an antiphosphotyrosine antibody. Blots were then reprobed with either an anti-Syk (upper panel) or anti–ZAP-70 antibody (lower panel). The positions of Syk and ZAP-70 are indicated with arrows and the positions of molecular weight markers are noted. These data are representative of results obtained in six independent experiments.
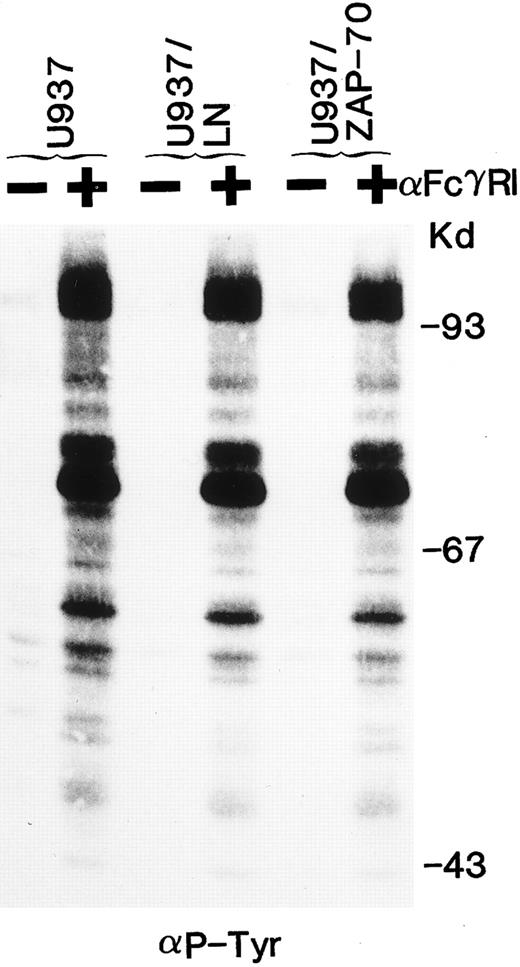
ZAP-70 expression does not alter tyrosine phosphorylation of cellular substrates and downstream signaling after FcγRI cross-linking.We then assessed whether the tyrosine phosphorylation of cellular substrates as well as downstream signaling would be affected by the presence of ZAP-70. Clustering of FcγRI increased the tyrosine phosphorylation of a large number of cellular proteins. The pattern of bands induced by FcγRI cross-linking appeared identical in U937, U937/LN, and U937/ZAP-70 cells (Fig 6). Phosphorylation of MAP kinase was examined as a measure of downstream activation. FcγRI aggregation resulted in equivalent phosphorylation of MAP kinase in U937, U937/LN, and U937/ZAP-70 cells (data not shown). These results indicate that expression of neither neomycin phosphotransferase nor ZAP-70 affected the MAP kinase pathway in U937 cells.
The global tyrosine phosphorylation pattern after FcγRI clustering is not affected by ZAP-70 expression. Uninfected U937 cells or U937 cells expressing Neo (LN) or ZAP-70 were either unstimulated (−) or cross-linked with an FcγRI MoAb (+). Lysates were fractionated by gel electrophoresis and immunoblotted with antiphosphotyrosine antibodies. These data are representative of results obtained in six independent experiments.
The global tyrosine phosphorylation pattern after FcγRI clustering is not affected by ZAP-70 expression. Uninfected U937 cells or U937 cells expressing Neo (LN) or ZAP-70 were either unstimulated (−) or cross-linked with an FcγRI MoAb (+). Lysates were fractionated by gel electrophoresis and immunoblotted with antiphosphotyrosine antibodies. These data are representative of results obtained in six independent experiments.
DISCUSSION
We find that, after FcγRI receptor aggregation, Syk, but not ZAP-70, was activated in the U937 and THP-1 myeloid cell lines. Reports assessing the relative abilities of the related ZAP-70 and Syk PTKs to mediate a response through the TCR, BCR, and FcRs have been controversial.5-7,48 However, much of the data regarding differences between ZAP-70 and Syk in receptor mediated signaling have been derived from experiments with chimeric receptors expressing ZAP-70/Syk or in nonhematopoietic cells such as COS-1 and NIH-3T3 cells transfected with various receptor subunits.6,7,26,49 50 We assessed here the activation of these two PTKs in myeloid cells that have endogenous receptors and an intrinsic ability to mediate an FcR receptor response.
A previously published study that analyzed Syk phosphorylation in U937 cells was not able to assess the activation potential of ZAP-7028 because of a lack of expression of the latter PTK. Thus, to assess the phosphorylation status of ZAP-70 in myeloid cells, we introduced an exogenous ZAP-70 gene into the U937 and THP-1 myelomonocytic cell lines by retroviral-mediated transduction. After transduction, ZAP-70 was expressed in both U937 and THP-1 cells at levels equivalent to those observed in T cells (Figs 1, 2, and 5).
The introduced ZAP-70 protein was not tyrosine-phosphorylated after its association with the FcεRIγ subunit of the FcγRI receptor (Figs 2 and 3). In contrast, ZAP-70 is phosphorylated upon interaction with the FcεRIγ subunit in the context of its association with the low-affinity IgG receptor (CD16/FcγRIII) in NK cells.9,18,51 It is unlikely that this difference is due to the fact that the introduced ZAP-70 gene was defective because the exogenously expressed ZAP-70 protein could be phosphorylated by [γ32]ATP in an in vitro kinase assay (Fig 5). Additionally, we have recently shown that transduction of ZAP-70–deficient T cells with the same ZAP-70 viral supernatant results in appropriate ZAP-70 tyrosine phosphorylation and reconstitution of signal transduction after TCR stimulation.45 Alternatively, upon potential competitive phosphorylation of ZAP-70 and Syk, ZAP-70 phosphorylation might not be detected. However, this is also unlikely, because we found that both ZAP-70 and Syk were phosphorylated in a T-cell line in which their respective levels of expression were similar to those observed in the ZAP-70–transduced U937 and THP-1 lines (Fig 5). Thus, our results show that ZAP-70 is not functionally equivalent to Syk in mediating a response through the FcγRI receptor in U937 and THP-1 monocytic cells.
ZAP-70 has recently been shown to be appropriately activated in an Syk-negative B-cell line that expresses the Src PTK and Lyn, but not Src, Lck, Fyn, Blk, Yes, or Hck.48,52 The U937 and THP-1 cells used in this study also expressed the Src family PTK Lyn (T. Jahn and N. Taylor, unpublished observations). In both B cells and myelomonocytic cells, Lyn directly associates with the respective receptor and is activated upon stimulation of the corresponding receptor.34,53 Although Lyn supports ZAP-70 activation in a B-cell line,48 we showed that this was not the case in two myeloid cell lines. Thus, the presence of a functional Src PTK does not appear to be sufficient to support ZAP-70 activation in certain cell types, such as myelomonocytic cells.
Similarly, either Lck or Fyn can support ZAP-70 activation in a T-cell leukemia line,7 whereas Lck, but neither Fyn nor Src, enhances ZAP-70 phosphorylation in activated NK cells.50 Because Lck appeared capable of mediating ZAP-70 phosphorylation in multiple cell types, we introduced Lck into U937/ZAP-70 cells by retroviral-mediated transduction. We observed that ZAP-70 phosphorylation was not enhanced after efficient expresion of either wild-type Lck or activated Lck (Lck-Y505F ) in U937/ZAP-70 cells (T. Jahn and N. Taylor, unpublished observations). It remains possible that recruitment or association of transduced Lck with the FcγRI receptor in myelomonocytic cells is impaired. In fact, in contrast with the observed phosphorylation of ZAP-70 in cultured murine thymocytes, ZAP-70 phosphorylation is not detected in freshly isolated murine thymocytes under conditions in which intrathymic interactions diminish Lck recruitment to the TCR.54 This indicates that ZAP-70 phosphorylation in thymocytes is dependent on the number of Lck molecules that are associated with the receptor rather than on the overall level of Lck expression. Therefore, the absence of ZAP-70 phosphorylation in U937 cells that constitutively express Lyn and Hck, and that were engineered to express Lck, may be caused by a relative lack of engagement of one or more of these Src PTKs by the FcγRI complex upon receptor cross-linking.
In addition to differences in their tyrosine phosphorylation status, we also showed that the abilities of ZAP-70 and Syk to be phosphorylated in an in vitro kinase assay were distinct after their respective associations with the FcεRIγ receptor subunit. We found that, after FcγRI stimulation, protein tyrosine kinase activity toward an exogenous substrate increased in Syk immunoprecipitates, but not in ZAP-70 immunoprecipitates. Our data are consistent with previous in vitro studies that have shown that Syk kinase activity is augmented after association with the single ITAM motif present in the FcεRIγ receptor subunit.41,55 Although the in vitro kinase activity of recombinant ZAP-70 increases after association with a subunit containing multiple phosphorylated ITAMs, there is no evidence that ZAP-70 kinase activity is altered after binding to a single ITAM.56 It would be of interest to assess ZAP-70 kinase activity in U937 cells engineered to express an FcγRI receptor with multiple ITAMs.
These data collectively indicate that the receptor context mediates the distinct abilities of the Syk and ZAP-70 PTKs to be activated. The ZAP-70–expressing myeloid cell lines that we have established can be used to assess the regulation of Syk– and ZAP-70–specific pathways, thereby providing further insights into signaling processes in different cell-type environments.
ACKNOWLEDGMENT
We thank Drs J. Cooper, C. Couture, J.-P. Kinet, D. Miller, T. Mustelin, B. Sefton, K. Smith, and A. Weiss for the gifts of antibodies and cells; Drs M. Sitbon, J. Nolta, and R. Parkman for critical review of the manuscript; and P. Snow for secretarial help. We appreciate the assistance and support of M. Dao. N.T. is grateful to Drs B. Sefton, A. Weiss, C. Couture, T. Mustelin, and M. Sitbon for their valuable suggestions.
N.T. was the recipient of a Howard Hughes Medical Institute Postdoctoral Physician Grant. Supported by the Fondation pour la Recherche Medicale (N.T.) and National Institutes of Health Grant No. AI25071 (K.W.).
Address reprint requests to Naomi Taylor, MD, PhD, Institut de Génétique Moléculaire de Montpellier, 1919 Route de Mende, 34033 Montpellier Cedex 1, France.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal