Abstract
Treatment with a combination of cytokines and chemotherapy can effectively stimulate the release of hematopoietic stem cells (HSC) into the peripheral blood (PB), which can then be harvested for transplantation. The cell cycle status of the harvested HSC from mobilized PB (MPB) is of interest because of the impact that cell cycling may have on optimizing the conditions for ex vivo expansion, retrovirus-mediated gene transfer, and the engraftment of transplanted tissues. Therefore, we characterized the cell cycling status of mobilized HSC from mice and humans. The murine HSC, which express the phenotype c-kit+ Thy-1.1lo Lin−/lo Sca-1+, were purified from PB, bone marrow (BM), and spleen after the mice were treated with the mobilizing regimen of granulocyte colony-stimulating factor (G-CSF ) or a combination of cyclophosphamide (CTX) and G-CSF. Human HSC (CD34+ Thy-1+ Lin−) and progenitor cells (CD34+ Thy-1− Lin−) were isolated from the BM of untreated healthy volunteers and from MPB of healthy volunteers and patients treated with G-CSF or a combination of CTX and GM-CSF. Cell cycle status was determined by quantitating the amount of DNA in the purified cells after staining with the dye Hoechst 33342. Fluorescence-activated cell sorting analysis of the progenitor cells from the murine and human samples showed an unexpected finding, ie, virtually none of the cells from the MPB was cycling. The G0/G1 status of HSC from MPB was surprising, because a significant proportion of HSC from BM are actively proliferating and, after mobilization, the HSC in the spleen and BM were also actively cycling.
THE COLLECTION AND purification of hematopoietic stem cells (HSC) from peripheral blood (PB) after treatment with stem cell mobilizing agents has important clinical applications. In autologous transplantations, these purified HSC may provide tumor-free grafts for cancer patients to regenerate a complete hematopoietic system. Over the past 10 years, autologous PB stem cells harvested by apheresis have become a major source of hematopoietic grafts. PB stem cell transplantation has replaced autologous bone marrow (BM) transplantation as the treatment of choice after chemotherapy.1,2 Recent studies have shown successful allogeneic transplantation of mobilized PB (MPB) HSC.3-5 In allogeneic transplantation, these purified HSC may provide a better graft to abrogate the potential for graft versus host disease.
PB stem cell transplants can accelerate hematopoietic recovery, typically 7 to 10 days faster than autologous BM transplants.6 The accelerated hematopoietic recovery after transplanting MPB cells could be due to an increase of CD34+ progenitors in MPB collected by apheresis.1,7 8 Alternatively, HSC and progenitors may be activated when they are released into the blood circulation by cytokines or the combination of chemotherapy and cytokine treatment. These activated mobilized hematopoietic progenitor cells could be more proliferative and thus contribute to a more rapid hematopoietic recovery when engrafted into patients.
Human and murine HSC can be purified from normal BM and MPB based on phenotype (reviewed in Watt and Visser9 and Morrison et al10 ). In mice, HSC can be defined phenotypically as a population of cells with the expression profile of Thy-1.1lo Lin−/lo Sca-1+ or c-kit+ Thy-1.1lo Lin−/lo Sca-1+ (KTLS).11-14 We have previously reported that, in humans, the CD34+ Thy-1+ Lin− cells from BM and mobilized PB (MPB) are highly enriched for candidate HSC.15-18
In this study, we characterized the cell cycle status of highly purified populations of stem cells from both mouse and man after treating with mobilizing regimens of cytokine alone (ie, granulocyte colony-stimulating factor [G-CSF ]) or a combination of high-dose chemotherapy followed by cytokine treatment (ie, CTX/G-CSF or CTX/granulocyte-macrophage colony-stimulating factor [GM-CSF ]). The benzimide dye, Hoechst 33342, was used to analyze the cell cycle status by flow cytometry. Hoechst 33342 actively intercalates into the AT-rich regions of DNA and can be used to quantitate the amount of DNA in viable cells and show its cell cycle status.
Approximately 7% to 20% of Thy-1.1lo Lin−/lo Sca-1+ cells from murine BM are in the S/G2/M phases of the cell cycle,11,13,19 whereas the long-term reconstituting HSC,13 the Rh123lo subset,19 and small-sized HSC subsets20 are relatively more quiescent. Based on the choice of mobilization treatments, we hypothesized that mobilized HSC are in an activated state and would therefore have a large fraction of cells that were proliferating. Indeed, we observed a significant increase in the number of KTLS in spleen after G-CSF treatment. However, when we analyzed the cell cycle status of mobilized stem cells from these mice, we were surprised to find that, after mobilization, the stem cells that were isolated from the PB were exclusively in the G0/G1 phases of cell cycle, whereas the stem cells from the BM and spleen were actively cycling. We also analyzed the cell cycle status of mobilized HSC from humans and found that the trend was consistent for both mice and humans. A kinetic analysis of entry of MPB HSC into S/G2/M cycle was performed in vitro to define further whether these cells are G0 or G1 cells. MPB CD34+ Thy-1+ Lin− HSC displayed delayed cell cycle progression into S phase compared with BM CD34+ Thy-1+ Lin− HSC or the G0/G1 CD34+ Thy-1− Lin− progenitors from BM and MPB. Understanding the cell cycle characteristics of HSC that have been mobilized with different treatment protocols has important implications for the in vitro self-renewal potential of these cells, their use for transplantation, ex vivo expansion, and their manipulation as targets for gene therapy.
MATERIALS AND METHODS
Mouse strains.The C57BL/Ka-Thy-1.1 (Thy-1.1, Ly-5.2) and C57BL/Ka-Ly-5.1 (Thy-1.2, Ly-5.1 mouse strains)21 were bred and maintained in the animal care facility at SyStemix (Palo Alto, CA). Mice were treated with a mobilization regimen at 10 to 13 weeks of age. In a set of experiments to determine the number of stem cells in cycle from untreated animals, BM was obtained from 4- to 9-week-old mice.
Mobilization of murine stem cells.Human recombinant G-CSF (Filgrastim; Amgen, Thousand Oaks, CA) and CTX (Cytoxan; Bristol Myers Squibb, Princeton, NJ) were used to mobilize murine stem cells. The dose of G-CSF and CTX administration and the length of treatment were determined based on the literature22-25 and optimized for this study.26 Mice were anesthetized with a mixture of ketamine hydrochoride (50 mg/kg) and zylazine hydrochloride (25 mg/kg) and implanted with osmotic minipumps (Alza, Palo Alto, CA). Osmotic pumps continuously administered G-CSF (250 μg/kg/d) or phosphate-buffered saline (PBS) with 0.2% bovine serum albumin (BSA; Sigma, St Louis, MO) as a control. In a separate experiment, mice were injected intraperitoneally with CTX (200 mg/kg) 1 day before implanting the osmotic pumps. The mice were then infused with G-CSF for 5 or 7 days. In one experiment, mice were splenectomized 7 weeks before G-CSF (250 μg/kg/d) administration.
Purification of c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells.After continuous G-CSF infusion, mice were killed to obtain blood samples, long bones (2 femurs and 2 tibias per mouse), and spleens for stem cell isolation. Blood samples were collected into a Vacutainer (Becton Dickinson, Rutherford, NJ) containing acetate citrate dextrose solution to prevent clotting. Red blood cells were lysed using ammonium chloride buffer (SyStemix, Palo Alto, CA). BM cell suspensions were obtained by flushing the long bones with PBS containing 2% fetal calf serum (FCS; Hyclone Laboratory, Logan, UT). Spleens were teased with the plunger of a 10-mL syringe and the suspension was filtered through a Cell Strainer (Becton Dickinson Labware, Franklin Lakes, NJ).
c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells were stained and isolated from these tissues as described previously.11,13 Briefly, the antibodies used to remove cells with lineage markers included RA3-6B2 for the B-lineage marker B220; RM.-5 (CD2), GK. 1.5 (CD4), 53-7.3 (CD5), and 53.6.72 (CD8) for T-cell markers; RB6-8C5 (GR-1) and M1/70.15.11.5 (Mac-1) for myelomonocytic markers; and TER-119 for erythrocytes. Antibodies specific for the lineage markers were obtained from Pharmingen (San Diego, CA) and were detected with phycoerythrin-conjugated polyclonal antirat antibody (Caltag, South San Francisco, CA) or phycoerythrin-conjugated 145-2C11 (CD3) monoclonal antibody (MoAb). The cells were incubated with biotinylated Sca-1 MoAb. Sca-1+ cells were positively selected using the MACS magnetic bead system (Miltenyi Biotec, Auburn, CA). The positive selected cells were further stained with fluorescein-conjugated 19XE5 (Thy-1.1) and allophycocyanin-conjugated 2B8 (c-kit) and steptavidin-Texas Red (BioMeda, Foster City, CA). The cells were incubated for 20 minutes on ice for each step. After the final wash, cells were resuspended in PBS/2% FCS buffer containing 2 μg/mL propidium iodide to discriminate between viable and nonviable cells.27 The labeled cells were analyzed and sorted with a three-laser FACS (Becton Dickinson Immunocytometry Systems, San Jose, CA). Dead cells were identified by their propidium iodide staining characteristic and excluded from analysis. After sorting, the purity of c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells was reanalyzed by flow cytometry.
Hoechst 33342 staining for analysis of cell cycle.The sorted c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells were resuspended in PBS containing 2% FCS (≤106 cells/mL) and incubated with Hoechst 33342 (10 μmol/L; Molecular Probes, Eugene, OR) at 37°C for 1 hour. The stained cells were analyzed on the FACStar Plus with the UV laser tuned to 360 nm excitation at a light regulated output of 50 mW. Linear blue fluorescence emission of the Hoechst/DNA was measured using a 424/40 bandpass filter. In some cases, verapamil (50 to 100 μmol/L; Sigma) was added to the mixture to ensure that the Hoechst 33342 would not be effluxed.13 28 We analyzed cells that were treated with or without verapamil and found that the efflux of the Hoechst dye was minimal regardless of whether verapamil was present.
Purification of human HSC.BM was aspirated from the posterior iliac crest of healthy adult volunteers after obtaining informed consent according to criteria established by the Institutional Review Board at the Scripps Clinic (San Diego, CA), Stanford Medical Center (Stanford, CA), and the University of Nevada Medical Center (Reno, NV). Low-density (<1.077 g/mL) mononuclear BM cells were isolated by using ficoll-hypaque (Pharmacia, Piscataway, NJ). In some cases, CD34+ BM cells were positively selected using paramagnetic beads (CD34 selection kit; Miltenyi, Sunnyvale, CA) before sorting with the FACStar Plus (Becton Dickinson Immunocytometry Systems).
Apheresed PB samples were collected from adult multiple myeloma patients treated at the University of Arkansas Medical Center (Little Rock, AR) after obtaining informed consent. These patients were treated with CTX (6 g/m2) followed by the administration of GM-CSF (0.25 mg/m2). Apheresed PB was also collected from healthy volunteers that were treated with G-CSF (10 to 15 μg/kg) at either the Stanford Medical Center, the Fred Hutchinson Cancer Research Center (Seattle, WA), or the MD Anderson Center (Houston, TX).
CD34 antigen was detected by staining cells with anti-CD34 (Tuk3; mouse IgG3 from Dr A. Ziegler, University of Berlin, Berlin, Germany) and sulforhodamine-conjugated Fab′2 Tuk-3 reagents prepared at SyStemix. Thy-1 expression was detected by staining cells with phycoerythrin (PE)-conjugated MoAb or Fab′2 fragments specific for Thy-1 (GM201; mouse IgG1 from Dr W. Retting, Ludwig Cancer Research Institute, New York, NY). Fluorescein isothiocyanate (FITC)-conjugated antibodies were used to remove cells that expressed lineage markers, allowing for the isolation of the lineage-negative (Lin−) population: anti-CD19 for B lineage, anti-CD2 for T lineage, anti-CD14, anti-CD15 and CD16 for myelomonocytic lineages (Becton Dickinson Immunocytometry Systems), and antiglycophorin A (Immunotech, Westbrook, ME) for erythroid lineage. The cells were incubated for 20 minutes on ice for each step. After the final wash, cells were resuspended in Hanks' Balanced Salt Solution or PBS/2% FCS containing 2 μg/mL propidium iodide. The labeled cells were analyzed and sorted with the three-laser FACStar Plus or the dual-laser FACS Vantage (Becton Dickinson Immunocytometry Systems). Dead cells that positively stained with propidium iodide were excluded from analysis.
In vitro culture of human BM and MPB CD34+ Thy-1+ Lin− and CD34+ Thy-1− Lin− populations.For in vitro culture analysis of the progression of BM and MPB HSC and primitive progenitors into cell cycle, the CD34+ Thy-1+ Lin− and CD34+ Thy-1− Lin− subsets from BM and MPB were purified as described above. Sorted CD34+ Thy-1+ Lin− cells were cultured on a pre-established monolayer of a mouse stromal cell line (SyS-1) in 96-well flat-bottom plates as described previously.15 17 The cultures contained human interleukin-3 (IL-3; 10 ng/mL), human IL-6 (10 ng/mL), human steel factor (SLF; 50 ng/mL), human GM-CSF (10 ng/mL), and human FLK2 ligand (50 ng/mL). The medium consisted of 50% Iscove's modified Dulbeco's medium (IMDM; JRH Bioscience, Lenexa, KS), 50% RPMI with 10% FCS, 2-mercaptoethanol (40 μmol/L), 1 mmol/L sodium pyruvate, 50 U/mL penicillin-streptomycin, and 2 mmol/L glutamine (JRH Bioscience). Time points were taken at 0, 12, 24, 36, and 48 hours of culture. At each time point, cells were incubated at 37°C for 1 hour in the presence of Hoechst 33342 (10 mmol/L) and verapamil (25 mmol/L). Cells were stained with FITC-conjugated CD34 (Becton Dickinson Immunocytometry Systems) and PE-conjugated Thy-1 (SyStemix) for cell cycle analysis.
RESULTS
Distribution of c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells in the different tissues after G-CSF mobilization.Using this murine model for HSC mobilization, we were able to assess both distribution and numbers of KTLS cells throughout the mice. Table 1 shows the distribution and numbers of the KTLS cells in different tissues of nonsplenectomized or splenectomized mice after treatment with G-CSF or PBS/BSA control. Others have previously reported redistribution of colony-forming units-spleen (CFU-S), colony-forming cells (CFC), or long-term engrafting cells and progenitors after cytokine administrations.22-25 29 However, none of these reports looked in a quantitative manner at a phenotypically enriched population of HSC in BM, PB, and spleen. Although there appear to be very low levels (≤0.01%) of KTLS cells in the control spleen, after mobilization, the frequency of KTLS cells increased dramatically in the spleen to 0.25% and 0.35%. The numbers of KTLS in mobilized spleen (∼4 to 6 × 105 cells/spleen) increased concomitantly. In normal and PBS/BSA-treated control mice, KTLS cells could not be detected in the PB. After mobilization, KTLS cells, normally a rare subset of the population, were detectable at levels ranging from 0.01% to 0.04% and 0.07% of PB from splenectomized mice.
Distribution of c-kit+ Thy-1.1lo Lin−/lo Sca-1+ Cells in the Different Tissues After G-CSF Mobilization
| . | Tissue source . | No. Cells/Tissue* . | % KTLS . | No. KTLS/Tissue . |
|---|---|---|---|---|
| Experiment I | Control PB/mL | 4.2 × 106 | <0.01 | — |
| Control BM | 4.8 × 107 | 0.07 | 3.4 × 104 | |
| Control SPL | 1.5 × 108 | <0.01 | — | |
| G-CSF PB/mL | 3.5 × 107 | 0.04 | 1.5 × 104/mL | |
| G-CSF BM | 3.7 × 107 | 0.04 | 1.6 × 104 | |
| G-CSF SPL | 2.3 × 108 | 0.25 | 5.8 × 105 | |
| Experiment II | Control PB/mL | 8.6 × 106 | <0.01 | — |
| Control BM | 3.6 × 107 | 0.08 | 3.0 × 104 | |
| Control SPL | 8.4 × 107 | <0.01 | — | |
| G-CSF PB/mL | 2.1 × 107 | 0.02 | 5.0 × 103/mL | |
| G-CSF BM | 3.4 × 107 | 0.07 | 2.4 × 104 | |
| G-CSF SPL | 1.1 × 108 | 0.37 | 4.2 × 105 | |
| Experiment III | Control PB/mL | 6.4 × 106 | <0.01 | — |
| Control BM | 3.6 × 107 | 0.08 | 3.0 × 104 | |
| Control SPL | 3.1 × 107 | <0.01 | — | |
| Splenectomized | G-CSF PB/mL | 7.2 × 107 | 0.01 | 9.3 × 103/mL |
| Splenectomized | G-CSF BM | 3.7 × 107 | 0.07 | 2.7 × 104 |
| . | Tissue source . | No. Cells/Tissue* . | % KTLS . | No. KTLS/Tissue . |
|---|---|---|---|---|
| Experiment I | Control PB/mL | 4.2 × 106 | <0.01 | — |
| Control BM | 4.8 × 107 | 0.07 | 3.4 × 104 | |
| Control SPL | 1.5 × 108 | <0.01 | — | |
| G-CSF PB/mL | 3.5 × 107 | 0.04 | 1.5 × 104/mL | |
| G-CSF BM | 3.7 × 107 | 0.04 | 1.6 × 104 | |
| G-CSF SPL | 2.3 × 108 | 0.25 | 5.8 × 105 | |
| Experiment II | Control PB/mL | 8.6 × 106 | <0.01 | — |
| Control BM | 3.6 × 107 | 0.08 | 3.0 × 104 | |
| Control SPL | 8.4 × 107 | <0.01 | — | |
| G-CSF PB/mL | 2.1 × 107 | 0.02 | 5.0 × 103/mL | |
| G-CSF BM | 3.4 × 107 | 0.07 | 2.4 × 104 | |
| G-CSF SPL | 1.1 × 108 | 0.37 | 4.2 × 105 | |
| Experiment III | Control PB/mL | 6.4 × 106 | <0.01 | — |
| Control BM | 3.6 × 107 | 0.08 | 3.0 × 104 | |
| Control SPL | 3.1 × 107 | <0.01 | — | |
| Splenectomized | G-CSF PB/mL | 7.2 × 107 | 0.01 | 9.3 × 103/mL |
| Splenectomized | G-CSF BM | 3.7 × 107 | 0.07 | 2.7 × 104 |
Mice were treated with 250 μg/kg/d of G-CSF or PBS/BSA as a control for 7 days. Cells obtained from PB, BM from four long bones representing 15% to 20% of total BM, and spleen (SPL) were purified according to the phenotypic markers c-kit+ Thy-1.1lo Lin−/lo Sca-1+ and the percentage of KTLS cells isolated from each tissue is shown.
Numbers of cells per milliliter of PB.
Cell cycle analysis of purified murine c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells after mobilization with G-CSF.We purified c-kit+ Thy-1.1lo Lin−/lo Sca-1+ (KTLS) cells from mice and stained the cells with Hoechst 33342 dye to determine the cell cycle status. We have found that, in normal mice, 4 to 9 weeks of age, 7% (standard deviation = 2.2; 6 independent experiments) of KTLS cells from BM are in S/G2/M (data not shown). When 10-week-old control mice were mock-treated with a 7-day infusion of BSA, the analysis of the cell cycle status of KTLS cells isolated from BM shows that only 2% to 4% of the purified cells are cycling in S/G2/M (Fig 1). After stem cells were mobilized by treating age-matched mice with a continuous infusion of G-CSF for 7 days, a higher percentage of the KTLS cells from the BM were observed cycling. Figure 1 shows that 24% to 26% of KTLS cells isolated from the BM and 5% to 7% from the spleen were in S/G2/M. In each experiment, about 17 mL of blood was collected from 20 G-CSF–treated mice. Only 1,000 to 2,000 KTLS cells were sorted from MPB. After Hoechst dye staining, even fewer KTLS cells (278 and 366) were available for cell cycle status analysis. Because of this technical limitation, we estimated that the percentage of cells in the S/G2/M phases based on very low numbers of cells; only 6 of 278 and 10 of 366 cells were cycling. Thus, we can only estimate that less than 3% of KTLS cells in the MPB were in the S/G2/M phase of the cell cycle (Fig 1).
Cell cycle analysis of purified mouse c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells after mobilization with G-CSF. Mice were infused for 7 days with of G-CSF (250 μg/kg/d). c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells were purified and stained with Hoechst 33342 for analysis of DNA content. The data from two replicatel experiments are shown. The FACS plots display Hoechst 33342 staining versus forward scatter of c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells isolated from (a and e) BM of PBS/BSA mock-treated mice or from G-CSF–treated (b and f ) BM, (c and g) spleen, and (d and h) MPB. The percentage of cells in G0/G1 or S/G2/M is indicated on left or right side of each FACS plot, respectively.
Cell cycle analysis of purified mouse c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells after mobilization with G-CSF. Mice were infused for 7 days with of G-CSF (250 μg/kg/d). c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells were purified and stained with Hoechst 33342 for analysis of DNA content. The data from two replicatel experiments are shown. The FACS plots display Hoechst 33342 staining versus forward scatter of c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells isolated from (a and e) BM of PBS/BSA mock-treated mice or from G-CSF–treated (b and f ) BM, (c and g) spleen, and (d and h) MPB. The percentage of cells in G0/G1 or S/G2/M is indicated on left or right side of each FACS plot, respectively.
Analysis of G-CSF–mobilized stem cells from splenectomized mice.Stem cells were mobilized with G-CSF in splenectomized mice to determine the influence that a spleen has on the cell cycle status of KTLS cells. In mice, the spleen can become a primary hematopoietic site after lethal irradiation followed by transplantation of hematopoietic cells, as shown by spleen colony formation.30,31 To mimic the human system, some investigators splenectomize mice before mobilization.22 29 From the splenectomized mice, KTLS cells were isolated from PB and BM and were analyzed for DNA content after staining with Hoechst 33342. FACS analysis showed that, similar to nonsplenectomized mice, virtually all of the KTLS cells isolated from the MPB were resting in G0/G1. As shown in Fig 2, this differs from the cell cycle status of the KTLS isolated from the BM. One-third of the purified KTLS subset from BM are in S/G2/M phases of cell cycle, indicating that there is active proliferation in progress.
Cell cycle analysis of purified c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells from splenectomized mice after G-CSF mobilization. Mice were splenectomized and, after 7 weeks, infused continuously for 7 days with G-CSF (250 μg/kg/d). c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells were purified and the cells were stained with Hoechst 33342 for analysis of DNA content. The FACS plots show Hoechst 33342 staining versus forward scatter of c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells isolated from the (a) BM or (b) MPB of G-CSF–treated mice. The percentage of cells in G0/G1 or S/G2/M is indicated on the left or right side of each FACS plot, respectively.
Cell cycle analysis of purified c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells from splenectomized mice after G-CSF mobilization. Mice were splenectomized and, after 7 weeks, infused continuously for 7 days with G-CSF (250 μg/kg/d). c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells were purified and the cells were stained with Hoechst 33342 for analysis of DNA content. The FACS plots show Hoechst 33342 staining versus forward scatter of c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells isolated from the (a) BM or (b) MPB of G-CSF–treated mice. The percentage of cells in G0/G1 or S/G2/M is indicated on the left or right side of each FACS plot, respectively.
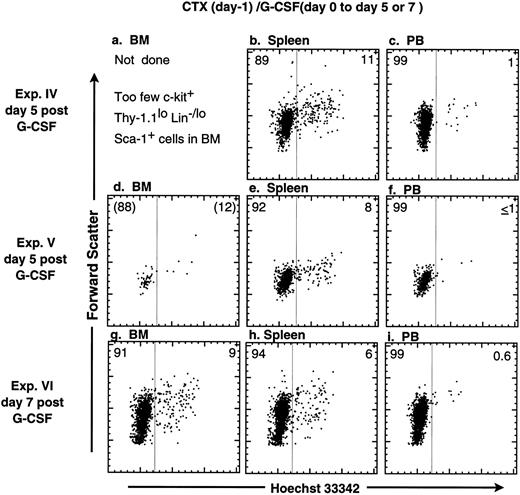
Cell cycle status of KTLS cells after mobilization with both CTX and G-CSF.The combination of a high-dose CTX and cytokines such as G-CSF is an effective mobilization treatment for patients with neoplastic disease that allows for the harvest of a large quantity of hematopoietic progenitors. Therefore, we wanted to know how treating mice with a combination of chemotherapeutic agents, such as CTX, and the cytokine G-CSF affects the cell cycle status of KTLS cells that are isolated from different tissues. After mice were treated with a mobilization regime of CTX and G-CSF, KTLS cells were purified from the BM, spleen, and MPB. The purified cells were stained with Hoechst 33342 to determine the cell cycle status. The cell cycle profile of the KTLS cells was similar in each case to the cycling profile obtained when stem cells were mobilized after a regime of G-CSF alone. As shown in Fig 3, the analysis of KTLS cells isolated from the MPB showed that greater than 99% of the purified subset were in G0/G1. KTLS cells isolated from the BM and spleen had 6% to 11% in S/G2/M (Fig 3).
Cell cycle analysis of purified mouse c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells after mobilization with combination of CTX and G-CSF. Mice were treated with CTX (200 mg/kg) and, 24 hours later, infused continuously with G-CSF (190 μg/kg/d) for 5 (Exp. IV and V) or 7 days (Exp. VI). c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells were purified and the cells were stained with Hoechst 33342 for analysis of DNA content. The FACS plots from three separate experiments show Hoechst 33342 staining versus forward scatter of c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells isolated from (d and g) BM, (b, e, and h) spleen, and (c, f, and g) MPB. There were not enough KTLS cells isolated from BM during Exp. IV for analysis. The percentage of cells in G0/G1 or S/G2/M is indicated on the left or right side of each FACS plot, respectively.
Cell cycle analysis of purified mouse c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells after mobilization with combination of CTX and G-CSF. Mice were treated with CTX (200 mg/kg) and, 24 hours later, infused continuously with G-CSF (190 μg/kg/d) for 5 (Exp. IV and V) or 7 days (Exp. VI). c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells were purified and the cells were stained with Hoechst 33342 for analysis of DNA content. The FACS plots from three separate experiments show Hoechst 33342 staining versus forward scatter of c-kit+ Thy-1.1lo Lin−/lo Sca-1+ cells isolated from (d and g) BM, (b, e, and h) spleen, and (c, f, and g) MPB. There were not enough KTLS cells isolated from BM during Exp. IV for analysis. The percentage of cells in G0/G1 or S/G2/M is indicated on the left or right side of each FACS plot, respectively.
Cell cycle analysis of human stem cells mobilized with G-CSF or a combination of CTX and GM-CSF.The mouse system showed that KTLS in circulating blood after mobilization are not in the S/G2/M phases of the cell cycle. We wanted to investigate whether the cell cycle status of human stem cells after mobilization would have the same cycling pattern as the murine stem cells, ie, whether MPB stem cells were almost exclusively in the G0/G1 phases of the cell cycle. In a preliminary study, we evaluated the cell cycle status of CD34+ populations from BM. We found that about 11% of CD34+ Lin− and 22% of CD34+ Linlo/+ BM cells were in the S/G2/M phases (data not shown). Next, we examined the CD34+ subset obtained from three different sources: BM of normal volunteers, MPB from patients treated with G-CSF, and MPB from patients treated with a combination of CTX and GM-CSF. Figure 4 shows the FACS analysis of the purified CD34+ subset comparing the Hoechst staining with the expression of Thy-1 (note that these samples were not stained for lineage markers). The majority of CD34+ cells that are in the S/G2/M phase do not express the Thy-1 antigen. Very few of the CD34+ Thy-1+ BM cells appeared to be in cycle. The CD34+ cells from MPB have a smaller proportion of cells in S/G2/M when compared with the CD34+ subset from BM. Strikingly, virtually none of the cells in the CD34+ Thy-1+ subsets from either of the MPB samples is in the S/G2/M phases.
Cell cycle analysis of human CD34+ progenitors from BM and MPB after mobilization with G-CSF or CTX/G-CSF. The CD34+ subset of cells was obtained stained with Hoechst 33342 and antibodies specific for Thy-1. The FACS plots of the CD34+ gated populations show Hoechst 33342 staining versus Thy-1 expression from cells isolated from (a) BM from an untreated donor, (b) MPB from a donor treated with G-CSF, and (c) MPB from a patient treated with a combination of CTX/GM-CSF. The percentage of CD34+ cells is shown in each quadrant of the FACS plot.
Cell cycle analysis of human CD34+ progenitors from BM and MPB after mobilization with G-CSF or CTX/G-CSF. The CD34+ subset of cells was obtained stained with Hoechst 33342 and antibodies specific for Thy-1. The FACS plots of the CD34+ gated populations show Hoechst 33342 staining versus Thy-1 expression from cells isolated from (a) BM from an untreated donor, (b) MPB from a donor treated with G-CSF, and (c) MPB from a patient treated with a combination of CTX/GM-CSF. The percentage of CD34+ cells is shown in each quadrant of the FACS plot.
To characterize the more primitive lineage-negative population of CD34+ cells, the CD34+ Thy-1+ Lin−, and the CD34+ Thy-1− Lin−, subsets from BM and MPB were sorted then analyzed for their cell cycle status (Table 2). In the CD34+ Lin− fraction from BM, 6.4% and 6.3% of Thy-1+ and Thy-1− subsets, respectively, are in S/G2/M phases of cell cycle. In contrast, virtually all of the more primitive populations (ie, CD34+Thy1+) that were isolated from G-CSF–treated MPB are G0/G1 (P < .0001). A similar G0/G1 cell cycle status was observed in the CD34+ Thy-1+ subset isolated from MPB from patients treated with the combination of CTX and GM-CSF. The lack of cells in S/G2/M phases within the CD34+ Thy-1+ population was observed in fresh samples as well as those shipped overnight, indicating that this observation is not a consequence of holding cells for long periods. These data confirm the trend that was observed in mice and seen in human MPB stem cells after mobilization (Fig 4).
Cell Cycle Status of Human CD34+ Thy-1+ Lin− Cells
| Tissue . | Population . | % S/G2/M . | n . |
|---|---|---|---|
| BM | CD34+ Thy-1+ Lin− | 6.4 ± 1.4 | 5 |
| CD34+ Thy-1− Lin− | 6.3 ± 1.3 | 5 | |
| MPB (G-CSF) | CD34+ Thy-1+ Lin− | <0.2 | 5 |
| CD34+ Thy-1− Lin− | <0.5 | 5 | |
| MPB (CTX/GM) | CD34+ Thy-1+ Lin− | <0.1 | 2 |
| CD34+ Thy-1+ | 0.33 | 1 | |
| CD34+ Thy-1− | 0.7 | 1 |
| Tissue . | Population . | % S/G2/M . | n . |
|---|---|---|---|
| BM | CD34+ Thy-1+ Lin− | 6.4 ± 1.4 | 5 |
| CD34+ Thy-1− Lin− | 6.3 ± 1.3 | 5 | |
| MPB (G-CSF) | CD34+ Thy-1+ Lin− | <0.2 | 5 |
| CD34+ Thy-1− Lin− | <0.5 | 5 | |
| MPB (CTX/GM) | CD34+ Thy-1+ Lin− | <0.1 | 2 |
| CD34+ Thy-1+ | 0.33 | 1 | |
| CD34+ Thy-1− | 0.7 | 1 |
The defined CD34+ populations were obtained from these sources: BM of untreated healthy donor; G-CSF–mobilized MPB from a healthy donor; and CTX/GM-CSF–mobilized MPB from cancer patients. CD34+ Thy-1+ Lin− and CD34+ Thy-1− Lin− cells from these sample were sorted, stained with the Hoechst 33342, and analyzed for their DNA content. One sample from CTX/GM MPB, the CD34+ enriched population, was stained with Hoechst 33342 and MoAbs against CD34 and Thy-1 as shown in Fig 4c. The percentage of CD34+ Thy-1+ Lin− cells in S/G2/M from MPB was significantly less than that from BM (P < .0005).
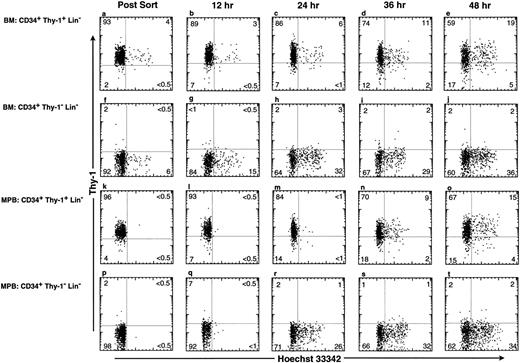
Delayed entry of MPB G0/G1 CD34+ Thy-1+ Lin− cells into S phase upon in vitro culture.To ascertain whether MPB stem cells are predominantly G0 or G1 cells, we compared the cell cycle progression of BM and MPB CD34+ Lin− subsets. The CD34+ Thy-1+ Lin− and the CD34+ Thy-1−Lin− populations from BM and MPB were isolated and then cultured on a mouse stromal layer SyS-1 in the presence of IL-3, IL-6, SLF, GM-CSF, and FLK2L. Under these culture conditions, the CD34+ Thy-1+ Lin− BM subset continuously produced cells entering S phase and the percentage of S/G2/M cells increase from 4% at t = 0 to 24% at t = 48 hours after culture (Fig 5a through e). Compared with the CD34+ Thy-1+ Lin− BM cells, the CD34+ Thy-1− Lin− BM progenitors showed both a faster kinetics of entry and a higher percentage of cells into S phase. Greater than 30% of cells were in the S/G2/M phases after 24 hours of culture (Fig 5f through j). In contrast, the CD34+ Thy-1+ Lin− MPB cells showed no detectable levels of S phase cells at 24 hours. By 36 to 48 hours after culture, 11% and 19% of the CD34+ Thy-1+ Lin− MPB cells were in the S/G2/M phases (Fig 5k through o). Interestingly, the MPB CD34+ Thy-1− Lin− cells were in G0/G1 at 12 hours and then progressed rapidly into the S phase of cell cycle by 24 hours (Fig 5q through t). These data suggest that the MPB CD34+ Thy-1+ Lin− HSC appears to be more quiescent than either BM CD34+ Thy-1+ Lin− HSC or BM and MPB CD34+ Thy-1− Lin− progenitors, as determined by the time required for these cells to become actively cycling. However, once MPB CD34+ Thy-1+ Lin− HSC enter active cell cycle, their proliferative potential is similar to that of the BM CD34+ Thy-1+ Lin− HSC population.
Kinetic appearance of actively cycling cells from human BM and MPB CD34+ Thy-1+ Lin− and CD34+ Thy-1− Lin− subsets during in vitro culture. BM CD34+Thy-1+Lin− (a through e), BM CD34+Thy-1−Lin− (f through j), MPB CD34+Thy-1+Lin− (k through o), and MPB CD34+Thy-1−Lin− (p through t) cells were isolated and were cultured on a murine BM stromal layer SyS-1 in the presence of IL-3, IL-6, SLF, GM, and FLK2L. At each time point, these cells were stained with Hoechst 33342 for analysis of DNA content and with MoAbs specific for CD34 and Thy-1. The FACS plots show the expression of Thy-1 versus Hoechst 33342 staining in viable cells. Cells were analyzed at t = 0 (a, f, k, and p), 12 (b, g, l, and q), 24 (c, h, m, and r), 36 (d, l, n, and s), and 48 (e, j, o, and t) hours. The percentage of cells in each quadrant of the plot is shown. At 48 hours, approximately 94%, 74%, 97%, and 94% of cells in the culture initiated from BM CD34+Thy-1+Lin− (e), BM CD34+Thy-1−Lin− (j), MPB CD34+Thy-1+Lin− (o), and MPB CD34+Thy-1−Lin− (t), respectively, were CD34hi.
Kinetic appearance of actively cycling cells from human BM and MPB CD34+ Thy-1+ Lin− and CD34+ Thy-1− Lin− subsets during in vitro culture. BM CD34+Thy-1+Lin− (a through e), BM CD34+Thy-1−Lin− (f through j), MPB CD34+Thy-1+Lin− (k through o), and MPB CD34+Thy-1−Lin− (p through t) cells were isolated and were cultured on a murine BM stromal layer SyS-1 in the presence of IL-3, IL-6, SLF, GM, and FLK2L. At each time point, these cells were stained with Hoechst 33342 for analysis of DNA content and with MoAbs specific for CD34 and Thy-1. The FACS plots show the expression of Thy-1 versus Hoechst 33342 staining in viable cells. Cells were analyzed at t = 0 (a, f, k, and p), 12 (b, g, l, and q), 24 (c, h, m, and r), 36 (d, l, n, and s), and 48 (e, j, o, and t) hours. The percentage of cells in each quadrant of the plot is shown. At 48 hours, approximately 94%, 74%, 97%, and 94% of cells in the culture initiated from BM CD34+Thy-1+Lin− (e), BM CD34+Thy-1−Lin− (j), MPB CD34+Thy-1+Lin− (o), and MPB CD34+Thy-1−Lin− (t), respectively, were CD34hi.
DISCUSSION
In this study, we have characterized the cell cycle status of HSC from mice and humans after mobilization treatments. Based on the assumption that a mobilization treatment causes extensive proliferation of primitive progenitors, we anticipated that the HSC harvested from MPB would be actively cycling. In the mouse model, HSC appear to be actively cycling in the BM and spleen, the primary sites for hematopoiesis. Therefore, it was unexpected when we found that virtually all HSC that could be detected in MPB are in the G0/G1 phase of the cell cycle.
Our results are in good agreement with three recent reports that show a decrease in the cycling activity of progenitor cells from MPB. Donahue et al32 treated nonhuman primates with the combination of G-CSF and SCF and analyzed the CD34+ Thy-1+ progenitor population. Their results showed that 9.6% of CD34+ Thy-1+ cells isolated from PB are in S/G2/M, whereas 36.3% of the CD34+ Thy-1+ BM cells are in S/G2/M.32 Roberts and Metcalf33 performed tritiated thymidine suicide assays to determine the percentage of hematopoietic progenitors in S-phase. They showed that, in G-CSF–treated mice, 47% and 52% of CFC from mobilized BM and spleen, respectively, are in S phase, whereas only 7% of PB progenitor CFC are in S phase at the time of isolation.33 A recent correspondence by Siegert and Serke34 also showed the absence of S/G2/M phases among cells expressing CD34+ in PB isolated from patients mobilized by GM-CSF. Our data further confirm and extend these observations by analyzing extensively purified phenotypically defined HSC from different tissues and the dye Hoechst 33342 to analyze cell cycle status. Staining with the Hoechst dye will not differentiate between cells that are in the G0 and the G1 stage of the cell cycle. If the MPB CD34+ Thy-1+ Lin− cells are predominately G1, we should have observed a distinct number of actively cycling cells (ie, in S/G2/M phases) by 12 to 24 hours under the culture conditions. However, our data show that the MPB CD34+ Thy-1+ Lin− cells are delayed in their entry to S-phase until between 36 and 48 hours. These data indicate that MPB CD34+ Thy-1+ Lin− HSC cells are more quiescent than BM CD34+ Thy-1+ Lin− HSC or MPB and BM CD34+ Thy-1− Lin− progenitor populations. To distinguish between the G0 and G1 phases, it may be necessary to simultaneously use different metabolic markers such as levels of mitochondrial function or RNA.
It is not clear why we observed primarily G0/G1 HSC in MPB. Are the G0/G1 cells selectively released from the BM into the MPB after the stromal environment is altered? Is there a signal that triggers the dormancy of mobilized stem cells? Or are the noncycling stem cells less likely to be released from the BM? Whatever the mechanism of HSC mobilization, it will be important to understand the kinetics of proliferation for HSC in vitro, especially if HSC from MPB are to be used as targets for gene therapy. In vitro retroviral transduction protocols may best be optimized according to the timing of entry of the isolated MPB HSC into the S-phase of cell cycle before they lose primitive progenitor activity.
ACKNOWLEDGMENT
We thank Dr A. Schlageter for preparing and critically reviewing the manuscript. We also thank the SyStemix's flow cytometry, cell processing, and comparative medicine and animal care departments for providing us excellent core facility supports. We express special thanks to R. DiGiusto, B. Ford, L. Osborne, and J. Combs for technical support and inspiration to the study. We are grateful to Drs I. Weissman, M. Bonyhadi, and B. Hill for stimulating discussions and reviewing the manuscript.
D.H. and A.M.F. contributed equally to the manuscript.
Address reprint requests to Nobuko Uchida, PhD, Systemix Inc, 3155 Porter Dr, Palo Alto, CA 94304.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal