Abstract
Thrombopoietin (TPO) is a recently cloned cytokine that binds to its receptor, Mpl, and promotes hematopoietic expansion and maturation, primarily of the megakaryocyte lineage. The signaling pathways responsible for these events are thought to involve the Janus family of nonreceptor tyrosine kinases (JAKs) and the signal transducers and activators of transcription (STATs), which are activated by tyrosine phosphorylation. Previous investigators have studied these molecules in engineered and naturally occurring cell lines. To investigate the molecular basis for TPO signal transduction in a more physiologic target, we determined the pattern of JAK and STAT activation in purified, normal murine megakaryocytes. These results are compared with those of established cell lines that only proliferate (Ba/F3-mMPL and DA-1-TPO) or only differentiate (L8057) in response to TPO. From these findings, a model is proposed to explain the physiologic roles of JAK2, TYK2, STAT3, and STAT5 in TPO signaling. Furthermore, previous studies of the physical interaction between Mpl and the JAKs are extended, showing a difference in the association of JAK2 and TYK2 with the TPO receptor. Finally, we show that, in the cell line Ba/F3-mMPL, the closely related proteins STAT5A and STAT5B are both activated by TPO stimulation and are capable of heterodimerization. Together, these results further our understanding of the early stages of megakaryocyte and platelet development.
IN 1994 THE MPL LIGAND, thrombopoietin (TPO), was cloned by several groups and its crucial role in megakaryocyte proliferation and differentiation was elucidated (reviewed by Kaushansky1 ). The recombinant cytokine has been shown to promote lineage-specific maturation events, including increased ploidy, formation of demarcation membranes and platelet fields, and expression of specific proteins (eg, platelet factor 4, von Willebrand's factor, and glycoproteins Ib and IIb/IIIa). Furthermore, TPO accelerates platelet recovery after myelosuppressive doses of radiation or chemotherapy and is currently being tested in patients undergoing intensive chemotherapy and bone marrow transplantation. Now, with the discovery of Mpl and TPO, the signal transduction pathways activated during megakaryocytopoiesis can be investigated.
Recently, studies of cytokine receptor signaling have focused on protein tyrosine phosphorylation, which is rapidly stimulated by ligand binding. Although the cytokine receptors do not contain kinase domains, it is believed that association of two or more receptor subunits leads to the activation of nonreceptor tyrosine kinases (reviewed by Ihle2 ). We have previously shown that TPO induces tyrosine phosphorylation of numerous proteins at the same concentrations necessary for the development of megakaryocytes in cultured bone marrow cells.3 Subsequently, others have confirmed that cytokine-dependent cell lines, engineered to express Mpl, undergo dramatic increases in phosphotyrosine content in response to TPO.4-6 In addition, cell lines with megakaryocytic features7,8 and human platelets9 10 express native Mpl and also show a TPO-induced increase in tyrosine phosphorylation. Thus, it is likely that activation of tyrosine kinases is a critical early step in TPO signaling.
The Janus family of tyrosine kinases, JAK1, JAK2, JAK3, and TYK2, is likely to fulfill this role. These kinases are believed to associate with conserved membrane-proximal motifs of the receptor cytoplasmic domains, termed Box1 and Box2. Mutation or deletion of these motifs leads to the failure of cytokine-driven proliferation and loss of JAK phosphorylation. It was recently shown that alteration of the putative Box1 and Box2 sequences of v-mpl abrogates its oncogenic potential.11 Several reports indicate that TPO can activate JAK2 and TYK2 in certain cell lines and human platelets,5,9,12 whereas others show that only JAK2 is tyrosine phosphorylated.6,13 This discrepancy is important because, in general, receptors that function as homodimers (eg, growth hormone receptor, erythropoietin receptor, and granulocyte colony-stimulating factor receptor) activate only a single JAK family member.2 The precise combination of JAK family members that can interact with a specific receptor may contribute to lineage specificity.
A second important level of signaling specificity involves the STAT family of latent transcription factors (signal transducers and activators of transcription [STATs]). There are six known STATs, which under basal conditions reside in the cytoplasm and have no DNA-binding activity. After cytokine stimulation, specific STATs are rapidly tyrosine phosphorylated by Janus kinases, allowing dimerization via the intrinsic STAT-SH2 (src homology 2) domains.14,15 This event has two important consequences: (1) translocation to the nucleus and (2) DNA-binding to specific promoter regions.14,16 Phosphorylation of specific STAT family members appears to be regulated, in part, by the STAT-receptor interaction.15 Once activated, STAT proteins can homodimerize, heterodimerize, or form complexes with other transcription factors. In this manner, signaling diversity can be generated by a relatively small number of proteins.
A number of studies have examined the STATs involved in TPO signaling. There is growing evidence that STAT5 is tyrosine phosphorylated and activated in various cell lines.5-7,12,17 This same protein, originally recognized as the prolactin-stimulated mammary gland factor, has now been implicated in signaling by the erythropoietin and interleukin-3 (IL-3) receptors as well.18,19 Additional studies have identified TPO-induced tyrosine phosphorylation of STAT 36,9,12,17 and STAT1.5 20 In this report, we examine the phosphorylation and activation of each of these STAT family members in TPO-stimulated normal murine megakaryocytes.
Signal transduction studies are usually performed in cell lines that have been altered by neoplastic transformation and often engineered to express many times the physiologic levels of a specific receptor. Use of these cell lines has advantages over primary cells, which must be extensively purified and generally express only several hundred receptors per cell. Although such studies have led to great advances in understanding signaling pathways, there can be important differences in signaling intermediates when primary, physiologic cells are compared with transformed cells.21 This report represents the first study of TPO signaling in purified megakaryocytes, and significant differences were found between JAK and STAT activation in megakaryocytes and that of established cell lines. We believe that these data are likely to reflect in vivo responses to TPO and should provide a basis for further studies of the molecular mechanisms of megakaryocytopoiesis.
MATERIALS AND METHODS
Antibodies.Polyclonal rabbit antisera against JAK1, JAK2, JAK3, STAT1, STAT2, and monoclonal antibody against phosphotyrosine (4G10) were purchased from Upstate Biotechnology Inc (Lake Placid, NY) and were used for immunoprecipitations, Western blotting, and supershift analysis (STAT1 only) according to manufacturer's recommendations. Monoclonal antibodies against STAT4 and STAT6 were purchased from Transduction Laboratories (Lexington, KY) and were used for Western blotting and immunoprecipitation. Polyclonal antisera against TYK2, STAT1, and STAT3 were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA) and used for immunoprecipitation, Western blotting, and supershift analysis (STAT3 only). Polyclonal rabbit antisera raised against the carboxyl termini of STAT5A22 was a generous gift of James N. Ihle (St Jude Children's Research Hospital, Memphis, TN). Polyclonal rabbit antisera specific for STAT5B6 was a generous gift of Chris Saris (Amgen Corp, Thousand Oaks, CA). Both STAT5 antisera were used for Western blotting, immunoprecipitation, and supershift analysis. Polyclonal antisera against c-Mpl, as previously described,3 was developed and generously provided by Charles Hart (Zymogenetics, Inc, Seattle, WA).
Cell lines and growth conditions.Ba/F3-mMPL cells were developed by Zymogenetics, Inc, as previously described.23 They were grown in RPMI 1640 (BioWhittaker, Walkersville, MD) plus 10% heat-treated fetal calf serum (HyClone, Logan, UT), 2 mmol/L L-glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin, and 0.25 mg/mL amphotericin B (BioWhittaker), supplemented with murine IL-3 (mIL-3; 0.2% vol/vol conditioned culture medium from baby hamster kidney cells engineered to constitutively secrete mIL-3) and 500 μg/mL synthetic neomycin (Geneticin; Life Technologies Inc, Gaithersburg, MD). DA-1–TPO cells were selected from parental DA-1 cells on the basis of TPO-dependent proliferation.24 DA-1–TPO cells were grown in the same media except that recombinant murine TPO (0.2% vol/vol conditioned culture medium) was substituted for mIL-3. L8057 cells were a generous gift from Hideki Sasaki (Yokohama City University, Yokohama, Japan). They were grown in Iscove's modified Dulbecco's medium (IMDM; BioWhittaker) plus 10% fetal calf serum, antibiotics, and L-glutamine as described above. No exogenous cytokines were necessary for growth. HepG2 (human hepatoma) cells were obtained from the American Type Culture Collection (Rockville, MD) and grown in Dulbecco's modified Eagle's medium (DMEM; BioWhittaker) plus 10% fetal calf serum, antibiotics, and L-glutamine as described above. 2FTGH human sarcoma cells were a generous gift of George Stark (Cleveland, OH). They were grown in the same media as that used for HepG2.
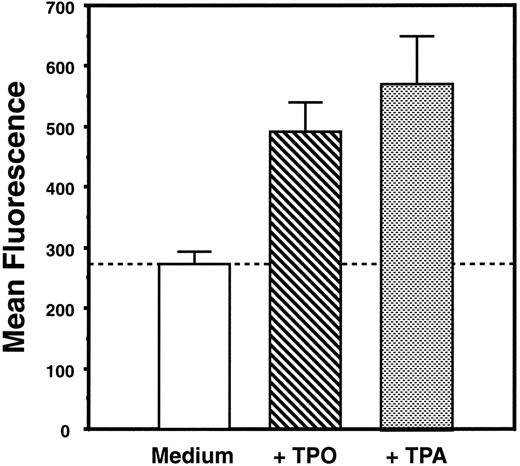
Acetylcholinesterase assay.L8057 cells were grown in the presence of recombinant murine TPO (4% vol/vol conditioned medium) or 50 nmol/L 12-O-tetradecanoylphorbol 13-acetate (TPA; Sigma Chemical Co, St Louis, MO) for 3 days. Cells were then washed once in IMDM and twice in serum-free media (Ultraculture; BioWhittaker) and aliquoted into Nunc V-bottom well plates at 2 × 106 cells/well. Acetylcholinesterase content was measured as previously described25 and is expressed as the mean total fluorescence of triplicate measurements.
Purification of murine megakaryocytes.BDF-1 or Balb-C mice (Jackson Labs, Bar Harbor, ME) received daily subcutaneous injections with pure, recombinant murine TPO for 5 days (10 μg/kg/d; Zymogenetics). The mice were then killed and marrow cells were flushed from the femurs and tibias into serum-free IMDM supplemented with 1% Nutridoma (Boehringer Mannheim, Indianapolis, IN) and penicillin, streptomycin, amphotericin B, and L-glutamine. Single-cell suspension was achieved by vigorous pipeting, and marrow cells were resuspended at 1 × 106 cells/mL and incubated (37°C, 5% CO2 ) in the same serum-free media plus recombinant TPO (3% vol/vol conditioned medium) for 72 to 96 hours. Mature megakaryocytes were harvested by low-speed centrifugation (400 RPM for 10 minutes) and then overlayered on a discontinuous bovine serum albumin (BSA; Sigma) density gradient (0%/1.5%/3.0% BSA in phosphate-buffered saline [PBS]). Megakaryocytes made up greater than 90% of the cells that settled to the bottom within 30 minutes at 1× gravity as determined by visual inspection of cells in culture or after Wright's stain. In some cases, fractions that contained mixed populations of megakaryocytes and other hematopoietic cells were subjected to a second round of density separation. The final preparation of megakaryocytes was collected by low-speed centrifugation and resuspended in IMDM + 0.5% BSA for 10 hours of serum and cytokine deprivation.
Stimulation of cells.Cell lines were incubated at 37°C in serum-free, cytokine-free medium for 16 hours; megakaryocytes were starved for 10 hours. Recombinant murine TPO (in the form of conditioned culture medium) was added at final volume of 2% vol/vol (several times the concentration required for maximal proliferation) and allowed to incubate for 1 to 60 minutes. Adherent HepG2 cells were stimulated with 50 ng/mL human IL-6 (Genzyme Corp, Cambridge, MA) for 15 minutes. 2FTGH cells were stimulated with 10 U/mL interferon-α-2A (Roche Laboratories, Nutley, NJ) for 15 minutes. The reaction was stopped by adding ice-cold PBS and washing the cells twice at 4°C. Protein lysates were prepared by solubilization in Triton X-100, as previously described.3 For Fig 7A, nuclear and cytoplasmic proteins were prepared detergent-free by freezing and thawing the cells in hypertonic saline solution (20 mmol/L HEPES, pH 7.9, 20 mmol/L NaF, 1 mmol/L sodium vanadate, 1 mmol/L sodium pyrophosphate, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L dithiothreitol, 1 mmol/L phenylmethylsulfonyl fluoride, 1 μg/mL leupeptin, 1 μg/mL aprotinin, 420 mmol/L sodium chloride, 20% glycerol), as described by Tweardy et al.26
EMSA. (A) Megakaryocyte extracts [5 to 10 μg, detergent-free, before (−) or after (+) 15 minutes of TPO stimulation] were analyzed for the ability to bind the hSIE probe (shifted band = S). As indicated below the autoradiogram, STAT1- and STAT3-specific antisera as well as an irrelevant antiserum (IRREL) were tested for the ability to induce a supershift (SS). (B) Lysates derived from Ba/F3-mMPL cells and megakaryocytes (5 to 10 μg, detergent-containing) were evaluated for TPO-induced binding to the PRE probe (S). Antibodies specific for STATs 3, 5B, and 5A as well as irrelevant antiserum (IRREL) were evaluated for supershift activity (SS). In each of these experiments, the first two lanes had no added antiserum.
EMSA. (A) Megakaryocyte extracts [5 to 10 μg, detergent-free, before (−) or after (+) 15 minutes of TPO stimulation] were analyzed for the ability to bind the hSIE probe (shifted band = S). As indicated below the autoradiogram, STAT1- and STAT3-specific antisera as well as an irrelevant antiserum (IRREL) were tested for the ability to induce a supershift (SS). (B) Lysates derived from Ba/F3-mMPL cells and megakaryocytes (5 to 10 μg, detergent-containing) were evaluated for TPO-induced binding to the PRE probe (S). Antibodies specific for STATs 3, 5B, and 5A as well as irrelevant antiserum (IRREL) were evaluated for supershift activity (SS). In each of these experiments, the first two lanes had no added antiserum.
Western blots.The protein concentration was determined using the Protein/DC Assay (BioRad, Hercules, CA) to assure equal loading between lanes. Protein was denatured by boiling for 5 minutes in loading buffer containing a final concentration of 62.5 mmol/L Tris/base, pH 6.8, 1% β-mercaptoethanol, 1% sodium dodecyl sulfate, 10% glycerol and was separated on a 7.5% acrylamide gel.27 Protein was electrophorectically transferred to nitrocellulose (Schleicher & Schuell, Keane, NH) and blocked in Tris-buffered saline with 0.05% TWEEN 20 (TBST) and 3% BSA for at least 1 hour. Primary antibodies were added to the blocking solution at a final concentration of 1:1,000 (MPL and STAT5B antisera), 1:3,000 (STAT5A antiserum), or as recommended by the manufacturer. After four washes with TBST (10 minutes each), a detecting antibody was added (either goat antimouse or goat antirabbit coupled to horseradish peroxidase [BioRad]) at a final concentration of 1:5,000 in blocking solution for 1 hour. The nitrocellulose was washed four more times with TBST (10 minutes each) and then incubated with chemiluminescence reagents and exposed to film as directed (Amersham, Arlington Heights, IL). In some cases, the blots were stripped of antibody by washing at 50°C for 20 minutes in 62.5 mmol/L Tris (pH 6.8), 2% sodium dodecyl sulfate, and 100 mmol/L β-mercaptoethanol; were reblocked; and then were reprobed.
Immunoprecipitation.Lysates were diluted 1:5 with additional lysis buffer. STAT4 and STAT6 antibodies immunoprecipitated more efficiently when the lysates were first denatured by boiling 5 minutes in the presence of 1% sodium dodecyl sulfate; all other reactions were performed on native protein. Antisera was added according to manufacturer's guidelines (or 3 μL crude antisera/500 μg total protein when not specified) and incubated at 4°C for 2 hours. Immune complexes were collected by adding 50 μL of protein A-sepharose beads (Upstate Biotechnology Inc) and incubated on a rocker at 4°C for 1 hour. Beads were washed three times in lysis buffer and then boiled in sample buffer before Western blot analysis.
Electrophoretic mobility shift assay (EMSA).Two double-stranded DNA sequences, known to recognize STAT proteins, were used, ie, human sis-inducible element (hSIE; GATCCATTTCCCGTAAATCGATC)26 and the prolactin response element (PRE; GATCAGATTTCTAGGAATTCAAATCGATC).26 Probes were radiolabeled with α32P-dCTP by hybridizing with a short 5′ terminal primer and filling in the second strand using the large fragment of DNA polymerase (Life Technologies, Inc) per the manufacturer's specifications. The labeled probe was precipitated after adding oyster glycogen as carrier (Boehringer Mannheim). Cell lysates (5 to 10 μg), which were made in the presence or absence of cytokine stimulation, were incubated with the radioactive probe (0.8 pmol/L) for 20 minutes in a final concentration of 15 mmol/L HEPES, pH 7.9; 125 mmol/L NaCl; 1 mmol/L dithiothreitol; 0.15 mmol/L EDTA; 8% wt/vol glycerol; 4 μg poly dIdC (Pharmacia, Piscataway, NJ). Detergent-free protein lysates were required for hSIE/STAT3 binding. Excess unlabeled probe was used as well as an unrelated, unlabeled double-stranded oligonucleotide (both 16 pmol/L) to show that the protein/DNA interaction was specific (not shown). Supershift analysis was performed by incubating the whole cell lysates with 1 μg of STAT3 antibody (Santa Cruz Biotechnology, Inc) or 2 μg of STAT1 antibody (Upstate Biotechnology Inc) before the addition of radiolabeled probe. For STAT5B and STAT5A supershift studies, cell lysates were generated in 1% Triton X-100 and 2 μL of each antisera was added after incubation with the labeled probe. The reactions were analyzed by electrophoresis on 4% acrylamide gels made in 0.25× TBE buffer. After 60 minutes of pre-electrophoresis, the samples were loaded and run in 0.5× TBE buffer at 80 V for 2 to 3 hours. The gels were dried and exposed to film.
RESULTS
Characterization of cell lines.Three cell lines were used in our investigations to illustrate distinct aspects of TPO biology. Ba/F3-mMPL, derived from the prolymphoid Ba/F3 cell line, was engineered to express murine Mpl on its surface.23 DA-1–TPO is a subclone of a lymphoblastic leukemia cell line, which spontaneously expresses functional Mpl receptor on the cell surface.24 Like Ba/F3-mMPL cells, these are cytokine-dependent cells that proliferate in TPO but show no evidence of megakaryocytic differentiation. Specifically, changes in morphology, acetylcholinesterase expression, and cell surface markers were sought but not detected (D. Sabath, manuscript in preparation, and Sabath et al24 ). In contrast, L8057 is a murine megakaryoblastic leukemia cell line that grows in a cytokine-independent fashion and has been shown to undergo megakaryocytic differentiation in response to the phorbol ester, TPA.28 We have shown that growth of these cells in supplemental TPO results in the expression of platelet-specific proteins29 and increased acetylcholinesterase expression (Fig 1), consistent with partial megakaryocytic differentiation. Exogenous TPO had no effect on the proliferative rate of L8057 cells.29
TPO induces increased acetylcholinesterase content of L8057 cells. L8057 cells were grown in IMDM with 10% fetal calf serum alone (medium) or with supplemental TPO (4% conditioned medium) or with 50 nmol/L TPA. Acetylcholinesterase content was determined by fluorimetric assay; the graph indicates the mean of triplicate determinations and standard error of mean. These results are representative of five separate experiments.
TPO induces increased acetylcholinesterase content of L8057 cells. L8057 cells were grown in IMDM with 10% fetal calf serum alone (medium) or with supplemental TPO (4% conditioned medium) or with 50 nmol/L TPA. Acetylcholinesterase content was determined by fluorimetric assay; the graph indicates the mean of triplicate determinations and standard error of mean. These results are representative of five separate experiments.
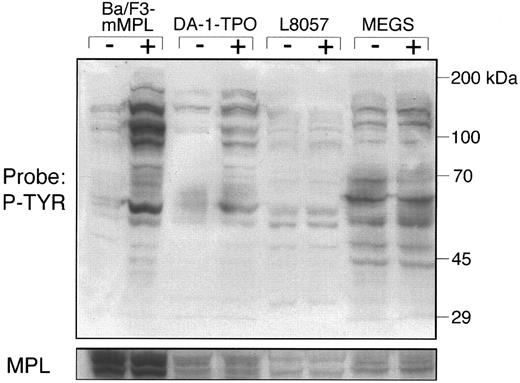
TPO-induced tyrosine phosphorylation of megakaryocytes and cell lines. To determine the effect of TPO on cellular tyrosine phosphorylation, lysates were generated from purified murine megakaryocytes and each of the three cell lines either before or after TPO stimulation for 15 minutes. Through timecourse experiments, we have determined that 15 minutes represents the point of maximal phosphorylation for each of the cell lines (data not shown). Cellular lysates (50 μg) were analyzed by Western blot using a monoclonal antiphosphotyrosine antibody, 4G10 (Fig 2). The cell lines that proliferate in response to TPO (Ba/F3-mMPL and DA-1–TPO) displayed multiple bands with increased phosphotyrosine content. Lysates from Ba/F3-mMPL cells showed evidence of greater TPO-stimulated tyrosine phosphorylation than DA-1–TPO, which may be a function of increased receptor number on the cell surface of these engineered cells (Fig 2). In contrast, the murine megakaryocyte and L8057 lysates showed very little TPO-induced tyrosine phosphorylation. This finding cannot be explained by a lack of c-Mpl expression, which was present in approximately equal amounts to DA-1–TPO cells when the blot was stripped and reprobed (Fig 2). Although not apparent by this method, we reasoned that changes in phosphotyrosine content of specific signaling molecules might be evident if enough megakaryocytes could be purified to perform immunoprecipitations.
Tyrosine phosphorylation of total cellular lysates. Protein was generated from Ba/F3-mMPL, DA-1–TPO, L8057 cells, and megakaryocytes before (−) and after (+) TPO-stimulation for 15 minutes, as described in the Materials and Methods. Fifty micrograms of each sample was evaluated by Western blot and probed with a phosphotyrosine-specific antibody (P-TYR). The apparent decreased intensity of a 70-kD band in the last lane was an artifact due to incomplete transfer and was not seen in four similar experiments. The blot was stripped and reprobed with an MPL-specific antiserum (lower strip).
Tyrosine phosphorylation of total cellular lysates. Protein was generated from Ba/F3-mMPL, DA-1–TPO, L8057 cells, and megakaryocytes before (−) and after (+) TPO-stimulation for 15 minutes, as described in the Materials and Methods. Fifty micrograms of each sample was evaluated by Western blot and probed with a phosphotyrosine-specific antibody (P-TYR). The apparent decreased intensity of a 70-kD band in the last lane was an artifact due to incomplete transfer and was not seen in four similar experiments. The blot was stripped and reprobed with an MPL-specific antiserum (lower strip).
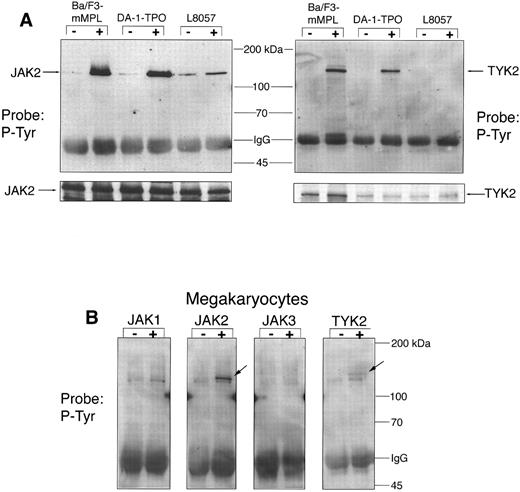
Tyrosine phosphorylation of JAKs in response to TPO.We have previously reported that TPO leads to rapid phosphorylation of JAK2 in Ba/F3-mMPL cells, an event that has been shown to cause kinase activation.3 Several investigators have also identified TYK2 as a substrate for TPO-induced phosphorylation.5,9 12 To more fully explore the role of Janus kinases in TPO signaling, we studied all four JAK family members in the three cell lines described above and in purified murine megakaryocytes. JAK1-, JAK2-, JAK3-, and TYK2-specific antisera were used to immunoprecipitate lysates from each of the cell lines or from megakaryocytes both before and after TPO stimulation. When analyzed by Western blot, JAK2 was found to be tyrosine phosphorylated after exposure to TPO in all cell lines as well as megakaryocytes (Fig 3A and B). L8057 cells had a significant amount of phosphorylated JAK2 before stimulation and a less dramatic increase in JAK2 phosphotyrosine content after TPO than the other cell lines, despite equal amounts of JAK2 detected by immunoblotting (Fig 3A). TYK2 phosphorylation was clearly evident for the proliferative cell lines, Ba/F3-mMPL and DA-1–TPO (Fig 3A). However, tyrosine phosphorylation of TYK2 was much less evident in the L8057 and megakaryocyte extracts (Fig 3A and B). This is not due to an absence of TYK2 in the lysates, as can be appreciated by reprobing the blots with the TYK2 antibody (shown for L8057, Fig 3A). In all cell types tested, there was no detectable JAK1 or JAK3 tyrosine phosphorylation (data shown only for megakaryocytes, Fig 3B).
JAK phosphorylation. Specific JAK proteins were immunoprecipitated and analyzed by Western blot for changes in phosphotyrosine content before (−) and after (+) TPO stimulation for 15 minutes. (A) In the three cell lines tested, 300 μg of total protein was used for each immunoprecipitation. The blots were stripped and reprobed to show equal precipitation and loading of JAK2 and TYK2 (see strip below). (B) For purified megakaryocytes, 75 μg of total protein was used for each immunoprecipitation. Tyrosine phosphorylated JAK2 and TYK2 are indicated by arrows. A slightly smaller band, most prominent in the JAK1 lane, was nonspecifically precipitated and was found not to be a JAK protein when the blots were reprobed with JAK-specific IgG. In two similar experiments, this artifactual band was not seen.
JAK phosphorylation. Specific JAK proteins were immunoprecipitated and analyzed by Western blot for changes in phosphotyrosine content before (−) and after (+) TPO stimulation for 15 minutes. (A) In the three cell lines tested, 300 μg of total protein was used for each immunoprecipitation. The blots were stripped and reprobed to show equal precipitation and loading of JAK2 and TYK2 (see strip below). (B) For purified megakaryocytes, 75 μg of total protein was used for each immunoprecipitation. Tyrosine phosphorylated JAK2 and TYK2 are indicated by arrows. A slightly smaller band, most prominent in the JAK1 lane, was nonspecifically precipitated and was found not to be a JAK protein when the blots were reprobed with JAK-specific IgG. In two similar experiments, this artifactual band was not seen.
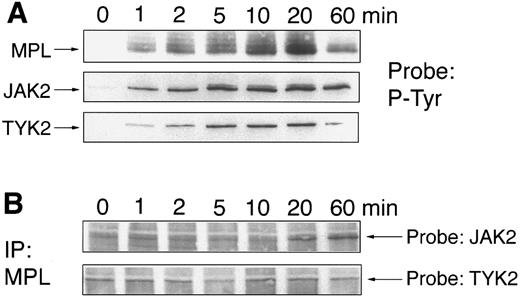
Coimmunoprecipitation of JAK2 and TYK2 with Mpl.We have previously reported that JAK2 physically associates with Mpl after 10 to 20 minutes, which is much later than the initial increase in tyrosine kinase activity. We have now repeated these experiments using a more sensitive detection method and also examined the kinetics of TYK2 association with Mpl. Ba/F3-mMPL cells were stimulated with TPO for 0 to 60 minutes, lysates were generated, and immunoprecipitations were performed with the JAK2, TYK2, and Mpl antisera immediately. Tyrosine phosphorylation of JAK2, TYK2, and c-Mpl was detectable within 1 minute, peaked at 10 to 20 minutes, and decreased by 60 minutes (Fig 4A). In addition, we looked at the ability of Mpl antiserum to coprecipitate receptor-associated JAK2 or TYK2 (Fig 4B). We found that TYK2 was precipitated by Mpl antiserum at a low and constant level before stimulation and during the entire 60 minutes of TPO exposure. Similarly, a low but detectable amount of JAK2 was coprecipitated before stimulation and through 10 minutes of TPO exposure. However, as previously reported,3 the amount of Mpl-associated JAK2 increased markedly at 20 and 60 minutes. The blots were stripped and reprobed to show equal loading of Mpl (not shown).
Time course of JAK phosphorylation and JAK/c-Mpl coimmunoprecipitation. Ba/F3-mMPL cells were stimulated with TPO (2% conditioned medium) for 0 to 60 minutes, and cellular lysates were obtained. Immunoprecipitations were performed with the indicated antisera and were analyzed by Western blot. (A) Phosphotyrosine immunoblots (P-Tyr) show the kinetics of MPL, JAK2, and TYK2 phosphorylation in response to TPO. (B) Samples were immunoprecipitated with MPL-specific antiserum and probed to detect coimmunoprecipitation of JAK2 and TYK2 (arrows indicate the position of each protein). The blots were stripped and reprobed to confirm equal immunoprecipitation and loading (not shown).
Time course of JAK phosphorylation and JAK/c-Mpl coimmunoprecipitation. Ba/F3-mMPL cells were stimulated with TPO (2% conditioned medium) for 0 to 60 minutes, and cellular lysates were obtained. Immunoprecipitations were performed with the indicated antisera and were analyzed by Western blot. (A) Phosphotyrosine immunoblots (P-Tyr) show the kinetics of MPL, JAK2, and TYK2 phosphorylation in response to TPO. (B) Samples were immunoprecipitated with MPL-specific antiserum and probed to detect coimmunoprecipitation of JAK2 and TYK2 (arrows indicate the position of each protein). The blots were stripped and reprobed to confirm equal immunoprecipitation and loading (not shown).
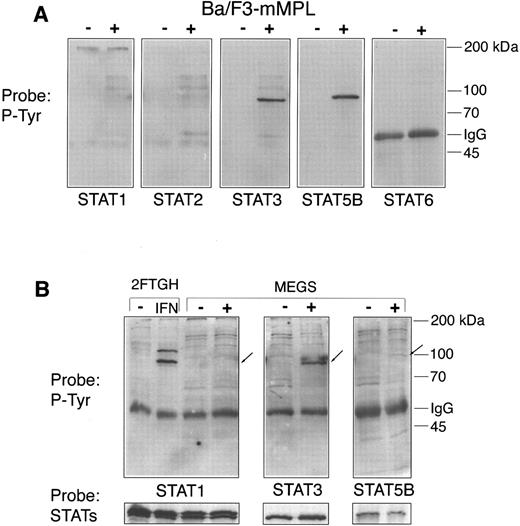
STATs in cell lines and megakaryocytes.Because the STAT proteins are early and important substrates of activated JAKs, we next studied which of these transcription factors are involved in TPO signaling. Five of the known STAT proteins were immunoprecipitated from Ba/F3-mMPL cells both before and after TPO stimulation. STAT4 was excluded from this analysis because numerous immunoblots failed to show STAT4 protein in Ba/F3 and megakaryocyte lysates (data not shown). Western blot analysis was used to detect TPO-induced changes in phosphotyrosine content (Fig 5A). We observed that only STATs 3 and 5 were phosphorylated under these conditions. The blots were then stripped and reprobed to assure adequate immunoprecipitation of each protein (not shown).
STAT phosphorylation. Lysates were generated from Ba/F3-mMPL cells and purified megakaryocytes before (−) and after (+) TPO stimulation for 15 minutes. Specific STAT proteins were immunoprecipitated from (A) 300 μg of total protein derived from Ba/F3-mMPL cells or (B) 75 μg of megakaryocyte extract. These samples were analyzed by Western blot for changes in phosphotyrosine content (P-Tyr). For megakaryocytes, the exact positions of the immunoprecipitated proteins are indicated by arrows. Equivalent loading in the paired lanes was shown by reprobing with the same antibody used for immunoprecipitation (lower strip).
STAT phosphorylation. Lysates were generated from Ba/F3-mMPL cells and purified megakaryocytes before (−) and after (+) TPO stimulation for 15 minutes. Specific STAT proteins were immunoprecipitated from (A) 300 μg of total protein derived from Ba/F3-mMPL cells or (B) 75 μg of megakaryocyte extract. These samples were analyzed by Western blot for changes in phosphotyrosine content (P-Tyr). For megakaryocytes, the exact positions of the immunoprecipitated proteins are indicated by arrows. Equivalent loading in the paired lanes was shown by reprobing with the same antibody used for immunoprecipitation (lower strip).
STATs 1, 3, and 5 have each been implicated in TPO signaling in various cell lines or human platelets5-7,9,12,17,20; however, tyrosine phosphorylation of these early transcription factors had not been studied in the most relevant physiologic setting, purified megakaryocytes. We performed immunoprecipitations of megakaryocyte extracts to assess the affect of TPO on these signaling molecules (Fig 5B). STAT3 was strongly phosphorylated in response to TPO; in contrast, STAT5B was barely detectable using a phosphotyrosine antibody but was clearly present when the blot was reprobed with the STAT5B antibody (Fig 5B, lower panel). STAT1 phosphorylation was not detectable in mature megakaryocytes despite easily detectable levels of STAT1 protein. 2FTGH cells stimulated with interferon-α were used as a positive control for STAT1 tyrosine phosphorylation. The most striking difference between TPO-stimulated megakaryocytes and Ba/F3-mMPL cells is the dramatically lower level of STAT5B tyrosine phosphorylation in megakaryocytes.
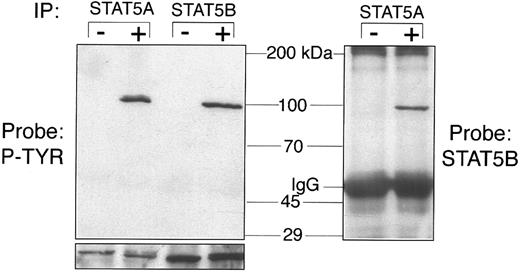
STAT5A and STAT5B can heterodimerize.The closely related STAT5A and STAT5B proteins differ only in the carboxyl terminus. It is not clear whether these transcription factors serve distinct purposes in vivo, whether they can be differentially activated by a single cytokine, or whether they can heterodimerize (STAT5 does not heterodimerize with other STAT proteins). These events were studied in Ba/F3-mMPL cells before and after TPO stimulation. As seen in Fig 6, both STAT5A and STAT5B were clearly tyrosine phosphorylated in response to TPO. Furthermore, we showed that immunoprecipitation of STAT5A causes coprecipitation of STAT5B only after TPO stimulation, indicating that the phosphorylated form of these transcription factors are capable of heterodimerization as was recently shown in erythropoietin signaling.22
STAT5A and STAT5B heterodimerization. Ba/F3-mMPL cellular extracts were generated before (−) and after (+) TPO stimulation for 15 minutes. Immunoprecipitation was performed with STAT5A- and STAT5B-specific antisera and analyzed by Western blot to detect changes in phosphotyrosine (P-TYR) content (first 4 lanes). Equivalent protein in the −/+ lanes is shown by probing a parallel blot with the same antibody used for immunoprecipitation (lower strip). In the pair of lanes to the right of the molecular weight markers, STAT5A antiserum was used for immunoprecipitation and STAT5B antiserum was used for detection.
STAT5A and STAT5B heterodimerization. Ba/F3-mMPL cellular extracts were generated before (−) and after (+) TPO stimulation for 15 minutes. Immunoprecipitation was performed with STAT5A- and STAT5B-specific antisera and analyzed by Western blot to detect changes in phosphotyrosine (P-TYR) content (first 4 lanes). Equivalent protein in the −/+ lanes is shown by probing a parallel blot with the same antibody used for immunoprecipitation (lower strip). In the pair of lanes to the right of the molecular weight markers, STAT5A antiserum was used for immunoprecipitation and STAT5B antiserum was used for detection.
EMSA.Tyrosine phosphorylation of STAT proteins is a prerequisite for transcriptional activation, which can be assessed in vitro by the ability to bind specific double-stranded DNA sequences. EMSA analysis was next performed to confirm that tyrosine phosphorylation of STAT proteins correlated with DNA-binding activity. Cellular extracts were generated from Ba/F3-mMPL cells and purified megakaryocytes either before or after 15 minutes of TPO stimulation. To study STAT1 and STAT3 activation, radiolabeled hSIE was used as a DNA target sequence, and detergent-free lysates were generated because of our observation that Triton X-100 prevents STAT3 binding (data not shown). EMSA showed that megakaryocyte extracts contain TPO-induced DNA binding activity, which was supershifted by STAT3- but not STAT1-specific antiserum (Fig 7A). Similar results were obtained using extracts from Ba/F3-mMPL cells (data not shown). Both the STAT1 and STAT3 antibodies were capable of inducing supershift under these conditions using extracts from HepG2 cells stimulated with IL-6 (data not shown).
To test STAT5 activation, the radiolabeled PRE was used as a DNA target sequence, and lysates were generated in the presence of detergent (which interferes with STAT3 binding but not STAT5). TPO-induced PRE-binding activity was readily detectable in Ba/F3-mMPL extracts (Fig 7B). This band was supershifted by both STAT5A- and STAT5B-specific antisera; however, neither STAT3-specific IgG nor an irrelevant antiserum induced a supershift. In contrast, megakaryocyte extracts contain significantly less TPO-induced PRE-binding activity (Fig 7B); furthermore, none of the antisera (STAT3, STAT5A, STAT5B, or irrelevant) were capable of supershifting this band. At present, the identity of the protein(s) that binds PRE in megakaryocytes is unknown. It is possible that this activity represents a novel STAT family member.
DISCUSSION
We have studied early signaling events in purified murine megakaryocytes and have found significant differences when these results are compared with those of established cell lines. Upon TPO-stimulation, both JAK2 and TYK2 kinases are obviously tyrosine phosphorylated in cell lines that have a mitogenic response. In contrast, polyploid megakaryocytes as well as cells that differentiate in the presence of TPO (L8057) show substantially less TYK2 phosphorylation. Furthermore, both STAT3 and STAT5 are activated by TPO stimulation in the TPO-proliferative cell lines, whereas there is extremely little STAT5 tyrosine phosphorylation or activation in mature megakaryocytes. Finally, differences in receptor association have been shown between JAK2 and TYK2, suggesting that these kinases may play distinct roles during TPO signaling, and STAT5A and STAT5B were shown to be capable of heterodimerization during TPO-induced proliferation.
Tyrosine phosphorylation of JAK2 and TYK2.Conflicting data have been reported regarding JAK activation by TPO. Several publications claim that JAK2 is the only family member affected,6,13 whereas others claim that both JAK2 and TYK2 are tyrosine phosphorylated.5,9,12 Our results indicate that either of these conclusions can be correct, depending on the specific cells studied. In cell lines that undergo TPO-dependent proliferation, both JAK2 and TYK2 were tyrosine phosphorylated after TPO exposure. In contrast, cells that primarily respond to TPO by differentiation (polyploid megakaryocytes and L8057) displayed much less TYK2 phosphorylation, although JAK2 phosphorylation was still readily apparent. Based on these findings, we hypothesize that JAK2 and TYK2 may both play a role in TPO-induced proliferation but that TYK2 may not contribute to the final stages of megakaryocyte differentiation. In support of this model, Mu et al6 failed to detect TYK2 phosphorylation in a 32D/Mpl cell line, which differentiates and has decreased proliferation in response to TPO. Furthermore, TYK2 tyrosine phosphorylation in TPO-stimulated human platelets appeared to be substantially less than that of JAK2.9 The precise function of each of these kinases is an active area of ongoing investigation.
Coprecipitation of JAKs and Mpl.Upon ligand binding, two or more receptor subunits are brought into close contact, allowing associated kinases to interact. It is believed that cross-phosphorylation of JAK molecules on separate subunits is the primary mechanism for kinase activation.2 At present, Mpl is thought to form a homodimer in response to TPO, which is relevant because homodimeric receptors generally activate only one JAK family member. In contrast, receptors that use two or more distinct signaling subunits have been found to activate multiple JAKs.2 On a molecular level, we propose three potential mechanisms for the activation of both JAK2 and TYK2: (1) both proteins associate with Mpl either at a shared site or distinct sites; (2) the complete TPO receptor may be heterodimeric and include an additional, as yet unidentified subunit, allowing one kinase to associate with each subunit; and (3) only one of the JAKs may physically associate with c-Mpl and, upon activation, could rapidly phosphorylate the second kinase, even though the latter does not directly interact with the receptor. We have shown that the kinetics of JAK2 and TYK2 tyrosine phosphorylation are very similar (Fig 4A) and that both proteins appear to be associated with Mpl at a low but detectable level before TPO stimulation (Fig 4B). These findings appear to support the first model described above.
The most profound difference between JAK2 and TYK2 in Ba/F3-mMPL cells is the striking increase in JAK2, but not TYK2, that was coprecipitated by Mpl-specific antiserum at 20 and 60 minutes of TPO exposure. This finding suggests that the JAK2-Mpl interaction was increasing at a time when overall tyrosine phosphorylation was already decreasing. It is possible that this relatively late event is necessary for turning off the signaling cascade. We hope that identifying distinguishing characteristics of JAK2 and TYK2 in the TPO signaling pathway may ultimately provide clues as to the structure of the signaling complex.
STAT activation by TPO.The STAT transcription factors are believed to play a critical role in the early aspects of cytokine signaling. These proteins were originally identified as differentiation factors because of their ability to upregulate transcription of lineage-specific genes.2,16 There is growing evidence that STATs may also play a role in cytokine-induced proliferation.30,31 Studies of TPO signaling have suggested that various combinations of STATs 1, 3, and 5 are activated in a number of cell lines and human platelets.5-7,9,12,17,18 20 Our results indicate that STAT3, STAT5A, and STAT5B were activated by TPO stimulation in Ba/F3-mMPL cells. In contrast, only STAT3 was substantially activated in murine megakaryocytes.
Two reports suggest that STAT1 is involved in TPO signaling. The first, by Gurney et al,20 showed that STAT1 could be purified on the basis of increased DNA-binding activity from TPO-stimulated lysates of CMK cells. This result may be explained by a low basal activation of STAT1 in this cell line, which could then bind to the gamma activation sequence used for purification. The second report by Sattler et al5 shows STAT1 phosphorylation in various cell lines but does not show evidence for increased STAT1 DNA binding activity. In addition, an antibody used to detect the phosphorylated form of STAT1 (phosphoSTAT1) is known to crossreact with STAT5.5 Moreover, there are also reports that specifically sought but failed to detect STAT1 activation in MO7e cells17 and human platelets.9 Together with these considerations, our inability to detect tyrosine phosphorylation or DNA binding of STAT1 in transfected cells and normal megakaryocytes leads us to conclude that this protein is unlikely to play a significant role in TPO-induced megakaryocytopoiesis. This finding also helps illustrate differences between TPO signaling and that of other cytokines that can influence megakaryocyte development (ie, IL-6 and IL-11 both induce STAT1 activation).32
STAT3 was originally identified as the acute phase response factor (APRF ), which is activated by IL-6.33 It has since been shown that STAT3 is an important factor in signaling by all receptors that use the gp130 signaling subunit as well as the granulocyte colony-stimulating factor and epidermal growth factor receptors.34 Our group has recently shown that TPO does not signal through the gp130 subunit.35 Furthermore, Baumann et al36 found that the Mpl cytoplasmic domain was not capable of upregulating transcription of acute phase proteins via the IL-6 response element. These differences between gp130 and Mpl signaling, both of which involve STAT3, can potentially be reconciled, because gp130-containing receptors also activate STAT1, in contrast to Mpl. These results are consistent with the hypothesis that transcription of acute phase proteins may be induced by STAT1/STAT3 heterodimers or STAT1 homodimers, rather than STAT3 alone.
STAT5, which was first classified as a mammary gland factor, has now been identified as a transcription factor involved in erythropoietin and IL-3 signaling as well as that of prolactin.18,19 The role of STAT5 in these signaling pathways has been extensively studied but remains controversial. Several recent reports suggest that STAT5 is primarily involved in proliferation (or the inhibition of differentiation).30,31 On the other hand, an engineered form of the erythropoietin receptor that does not activate STAT5 has been reported to support normal proliferation.22 Our investigations indicated that STAT5 phosphorylation and activation was most pronounced in cells that have a mitogenic response to TPO (ie, Ba/F3-mMPL and DA-1–TPO) and was virtually absent in purified megakaryocytes. It should be emphasized that the megakaryocytes used for our experiments were highly differentiated, with a mean ploidy of 32 to 64 N, as determined by propidium iodide stain and fluorescent cell sorting (unpublished observations). We hypothesize that STAT5 is involved in TPO signaling during proliferation but not in megakaryocytes undergoing terminal differentiation. Consistent with this model, 32D/Mpl cells, which partially differentiate when grown in TPO but proliferate in IL-3, were reported to have substantially less STAT5 phosphorylation after TPO stimulation than that seen with IL-3.6 To test the hypothesis that STAT5 activation plays a role in the expansion of immature megakaryocyte precursors, a different cell population will need to be purified and tested, experiments for which are presently underway.
From our data as well as other published results, we can begin to construct an comprehensive model of STAT activation during megakaryocytopoiesis. STAT5 may be essential for early expansion and/or early maturation of megakaryocyte progenitors, whereas STAT3 might play a greater role during terminal differentiation. In support of this hypothesis, IL-3 (which activates STAT5 but not STAT319 ) induces large numbers of early megakaryocytes, which seldom exceed 4N ploidy and do not acquire ultrastructural elements of mature megakaryocytes such as platelet fields and demarcation membranes.37 IL-6 and IL-11, which activate STATs 1 and 3 (but not 5), induce little or no megakaryocyte proliferation independently but are relatively potent inducers of terminal differentiation.25,38,39 When IL-3 and IL-11 are combined, resulting in STAT 3 and 5 activation, megakaryocytopoiesis more closely resembles that seen with TPO based on acetylcholinesterase production1 and morphologic/ultrastructural development.40 More definitive confirmation of the specific roles played by each of these transcription factors during megakaryocyte development is likely to be forthcoming with the development of STAT3 and STAT5 knockout mice.
Extensive bone marrow infiltration by Hodgkin's lymphoma. On trephine biopsy, there is replacement of normal hematopoietic cells by Reed-Sternberg and mononuclear Hodgkin's cells, surrounded by small lymphocytes and a fibroblastic reaction. Bone marrow aspirate shows infiltration by mononuclear Hodgkin's cells. (Courtesy of P.T. Murphy and C.S. Chapman, Department of Haematology, Leicester Royal Infirmary, Leicester, UK.)
Extensive bone marrow infiltration by Hodgkin's lymphoma. On trephine biopsy, there is replacement of normal hematopoietic cells by Reed-Sternberg and mononuclear Hodgkin's cells, surrounded by small lymphocytes and a fibroblastic reaction. Bone marrow aspirate shows infiltration by mononuclear Hodgkin's cells. (Courtesy of P.T. Murphy and C.S. Chapman, Department of Haematology, Leicester Royal Infirmary, Leicester, UK.)
ACKNOWLEDGMENT
The authors thank the following investigators who provided critical reagents used in this study: H. Sasaki (L8057 cells), G. Stark (2FTGH cells), D. Foster (Ba/F3-mMPL cells and purified, recombinant TPO), C. Hart (c-Mpl antisera), J. Ihle (STAT5A antisera), and C. Saris (STAT5B antisera). We also thank C. Carow for analysis of megakaryocyte ploidy.
Supported by the National Institutes of Health Grant No. R01DK49855. J.G.D. received postdoctoral support from the National Institutes of Health, a molecular medicine training grant (University of Washington), and the ASH Fellow Scholar Award.
Address reprint requests to Jonathan G. Drachman, MD, University of Washington Medical Center, Division of Hematology, Box 357710, Seattle, WA 98195.

![Fig. 7. EMSA. (A) Megakaryocyte extracts [5 to 10 μg, detergent-free, before (−) or after (+) 15 minutes of TPO stimulation] were analyzed for the ability to bind the hSIE probe (shifted band = S). As indicated below the autoradiogram, STAT1- and STAT3-specific antisera as well as an irrelevant antiserum (IRREL) were tested for the ability to induce a supershift (SS). (B) Lysates derived from Ba/F3-mMPL cells and megakaryocytes (5 to 10 μg, detergent-containing) were evaluated for TPO-induced binding to the PRE probe (S). Antibodies specific for STATs 3, 5B, and 5A as well as irrelevant antiserum (IRREL) were evaluated for supershift activity (SS). In each of these experiments, the first two lanes had no added antiserum.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/2/10.1182_blood.v89.2.483/4/m_bl_0032f7.jpeg?Expires=1767697442&Signature=wuWcgruf9gfdbNksifPDFcnnblLaKn1wmYy-5R3jDseT7xsfbNZHU2rts0O3q51wuUl3ETCXB4IrtGkw~VK04nv7DUyaZKbSAIRgofXyW~R-ovW0QqhhgZ1ovVkxHfBfux3J4RDSAnfHqtIEJgqEjwB7nRJafyIWvj6xkn-UZhbfJFPsganFkv8pKG-v0yy7XIPmjLgEz~eU~OvmhdKlEwaP9P52Wqv6d~i5UrV4fMuiVK7Klgi3MV2SNI6obuuTtw0KR5mrYnKcq7rlTAs5vUNLtiE02hiCTGpqEOAbUst--LojuWNbuAoalm3EJQx7TSzmlfOqNs~4eDeAlv6Exw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal