Abstract
We previously showed that interleukin-3 (IL-3) alone is not sufficient, although it is essential for murine mucosal-type mast cell development and that prostaglandin E (PGE) and interferon-γ (IFN-γ) are critical for survival or differentiation of mast cell precursors. We also confirmed that IL-4 is a key inhibitor for mast cell precursors despite being a growth factor of mast cells. In the present work, mouse spleen cells were cultured with recombinant (r) IL-1β, rIL-5, rIL-6, rIL-9, granulocyte-macrophage colony-stimulating factor (GM-CSF ), stem cell factor (SCF ), tumor transforming growth factor-β (TGF-β), or tumor necrosis factor-α (TNF-α) in the presence of endogenous IL-3. After 12 days of culture, mast cell development was induced by rIL-6 and rTNF-α. rIL-1β, rIL-5, rGM-CSF, rTGF-β and even the mast cell growth factors, rIL-9 and rSCF, failed to induce mast cell development. However, unlike IL-9 and SCF, IL-6 and TNF-α did not promote the growth of mast cells already developed. Macrophage may be one of the responsive cells of IL-6 and TNF-α in the cultures, because removal of macrophages greatly reduced the mast cell development induced by the cytokines. The actions of TNF-α and IL-6 were inhibited by indomethacin, an inhibitor for prostaglandin synthesis, and by neutralizing anti–IFN-γ and anti–IL-3 antibodies. rIL-4, when added at the start of the culture, also inhibited mast cell development induced by rIL-6 and rTNF-α. Nevertheless, neutralizing anti–IL-6 and anti–TNF-α antibodies did not suppress mast cell development induced by PGE and IFN-γ. TNF-α and IL-6 enhanced IFN-γ production, but suppressed IL-4 production in the cultures. Mast cell numbers induced were inversely and directly proportional to IL-4 and IFN-γ levels, respectively. These results indicate that inflammatory mediators as triggers are important for mast cell development, although they are not the mast cell growth factors.
IN 1981, SEVERAL LABORATORIES simultaneously reported a method for induction of mast cell development from primitive precursor cells by culturing normal mouse hematopoietic cells in conditioned media derived from mitogen-activated T cells, cloned Ly-1+2− inducer T cells, or WEHI-3B cells.1-5 Since then, the method has generally been used to develop mucosal-type mast cells (MMC) in vitro.6,7 The cytokine responsible for the mast cell development in those conditioned media was later proved to be interleukin-3 (IL-3),8,9 the well-known growth factor for almost all of the cell lineages. IL-4,10,11 IL-9,12 IL-10,13 nerve growth factor,14 and stem cell factor (SCF )15 are now known to promote the action of IL-3 for mast cell proliferation and/or differentiation.
An increase in mast cell number can be seen in various inflammatory diseases and pathologic conditions, such as asthma,16 hay fever,17 scleroderma,18 rheumatoid arthritis,19 parasitic helminth infection,20 interstitial cystis,21 and at the sites of graft rejection.22 The mechanism(s) remains unclear and is difficult to be explained by mast cell growth factor alone. However, at present, much has been focused on the effects of the above-mentioned mast cell proliferation and/or differentiation factors on mast cell development. The importance of various microenvironmental factors and nonmast cell growth factors in mast cell development has been largely neglected.
We previously established a system for induction of MMC development from mouse spleen cells by a long-term liquid culture without addition of exogenous IL-3.23 Fetal calf serum (FCS) was important and could be divided into inducible and noninducible sera for mast cell development. Lipopolysaccharide (LPS) contamination in FCS was responsible for the mast cell development.24 Further experiments showed that IL-3 alone is not enough for mast cell development, although it is essential, and that certain inflammatory mediators such as prostaglandin E (PGE) and interferon-γ (IFN-γ) are also crucial, even if they are not mast cell growth factors.25,26 On the other hand, IL-4, the well-known growth factor for MMC and connective tissue-type mast cells (CTMC), is a key inhibitor for mast cell precursors.26 It is not clear whether other inflammatory factors or cytokines other than PGE and IFN-γ are able to affect mast cell development. In the present work, we provide evidence that IL-6 and tumor necrosis factor-α (TNF-α) induced mast cell development from spleen cells, but IL-1, IL-5, granulocyte-macrophage colony-stimulating factor (GM-CSF ), tumor transforming growth factor-β (TGF-β), and even the mast cell growth factors, IL-9 and SCF, failed to do so.
MATERIALS AND METHODS
Mice.Male C3H/HeN, BALB/c, and BALB/c nu/nu mice (5 to 10 weeks old) were purchased from Charles River Japan (Atsugi, Japan). Male WBB6F1 +/+ and WBB6F1 W/Wv mice (5 weeks old) were purchased from SLC Co (Shizuoka, Japan).
Reagents.The following reagents were purchased from the indicated companies: murine recombinant (r) IL-3 and GM-CSF (Intergen Co, New York, NY); murine rIL-5 and rIFN-γ (Genzyme, Cambridge, MA); rat antimurine IFN-γ neutralizing monoclonal antibody (MoAb; IgG1; Upstate Biotechnology, Lake Placid, NY); rat antimurine IL-6 neutralizing MoAB (IgG 1κ; Endogen, Cambridge, MA); rat myeloma IgG1 (Zymed Laboratories, San Francisco, CA); murine rIL-1β, rIL-4, rIL-6, rIL-9, rIL-10, rSCF, rTNF-α, and goat antimurine IL-3, IL-4, and TNF-α neutralizing antibody (IgG; R & D Systems, Minneapolis, MN); human rTGF-β1 (King Brewing Co, Central Institute, Kakogawa-shi, Japan); PGE1 (Sigma Chemical Co, St Louis, MO); LPS (Escherichia coli O26:B6; Difco, Detroit, MI); indomethacin (Wako Pure Chemical Industries, Tokyo, Japan); nonmast cell-inducible FCS (lot 1604309, Cansera International, Toronto, Ontario, Canada; lot 5C2048, JRH Biosciences, Lenexa, KS).
Mast cell development from mouse spleen cells in a liquid culture.Mast cells were developed as described previously.23-25 In brief, mouse spleen cells (6 to 10 × 106 cells/mL) were cultured for 12 days in RPMI-1640 medium (Life Technologies, Grand Island, NY) containing 10% FCS, 25 mmol/L HEPES, 200 mmol/L L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 5 × 10−5 mol/L 2-mercaptoethanol (complete culture medium). The cultures (1 mL/well) were incubated in 24-well culture plates (Iwaki Glass, Tokyo, Japan) at 37°C in a humidified atmosphere with 5% CO2. One-half of the volume of the culture medium was changed every 4 days.
To determine the effects of various stimulants or cytokines on mast cell development, they were added to the cultures with noninducible serum for mast cell development (lot 1604309) at the start of the culture or on day 0, 2, 4, 6, or 8. The stimulants and cytokines were not replenished at each feeding. Mast cell number and histamine quantity per culture were determined on day 12. To obtain MMC for colony formation assay, mast cells (>90% pure) were prepared by Histopaque-1077 (Sigma) separation of the long-term cultured cells stimulated by 1 ng/mL LPS for 16 days.
Mast cell development from mouse bone marrow cells in a liquid culture.Bone marrow cells (4 × 106 cells/mL) were cultured in complete culture medium with or without rIL-3 and other stimulants. Mast cell number and histamine quantity per culture were determined on day 12. The supernatants of days 2 and 4 in the cultures without exogenous IL-3 were recovered for endogenous IL-3 detection.
Colony formation of mast cells in a semisolid culture.Methylcellulose cultures were used for in vitro colony formation assay of mast cells. Mast cells already developed (MMC, 1 × 103) were suspended in 1 mL of complete culture medium containing 1% methylcellulose (Sigma), 20 ng/mL rIL-3, and various stimulants. The cells were then added into a culture plate of 6 wells with a diameter of 35 mm (Costar, Cambridge, MA) and were maintained at 37°C in a humidified atmosphere with 5% CO2 for an additional 12 days. Cell aggregates of 20 cells or more were scored as colonies under an inverted microscope. For histochemical identification of mast cells, smear preparations of the colonies were stained by the Wright-Giemsa method.
Separation by magnetic cell sorting (MACS) of c-kit− and c-kit+ cells.The c-kit− and c-kit+ cells in spleen cells were separated by MACS column according to the manufacturer's instructions. Briefly, spleen cells were incubated with rat antimouse CD 117 (c-kit) MoAB (IgG2b, κ; PharMingen, San Diego, CA) at 4°C for 30 minutes. After washes, the cells were further incubated with goat antirat IgG antibody conjugated with super-paramagnetic microbeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) at 4°C for 15 minutes. After washes, the cells were applied on MACS column. The negative and positive fraction was collected for culturing.
Removal of macrophages by Sephadex G-10 column.Sephadex G-10 columns (20 mL) were prepared according to the technique reported previously.27 Up to 108 spleen cells were applied to the columns and slowly eluted in 10 mL volume. The nonadherent cells were washed and used as macrophage-depleted splenic cells.
Mast cell counts.All cultures for enumeration of cells were performed in triplicate, and numbers of mast cells were expressed as mean ± standard deviation (SD). Mast cells were counted by an Alcian blue staining method specific for counting basophils and mast cells, as described previously.28
Histamine assay.A fluorometric assay was used as described previously.29 The cells recovered from long-term cultures on day 12 were washed twice with 0.9% saline, suspended in 0.1 N HCl, and boiled for 10 minutes to completely release histamine from the cells. The samples taken after the above treatments were centrifuged at 8,000g for 10 minutes, and the supernatants were collected for histamine assay. The results were expressed as mean ± SD of histamine quantity (micrograms) per culture.
Determination of IL-3, IL-4 and IFN-γ.IL-3, IL-4, and IFN-γ in supernatants, recovered from the cultures on days 2 and/or 4, were measured by kits of enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (Endogen, Boston, MA). The ELISA was conducted in duplicate and the results were expressed as mean ± SD.
Presentation of data and statistical analysis.All experiments were performed three or more times, and the data presented are from one of those experiments. Data from control and experimental groups were compared by Student's t-test. Regression analysis was performed using Statworks (Cricket Software, Philadelphia, PA). Values of P < .05 were considered statistically significant.
RESULTS
Effects of various cytokines on mast cell development.Mouse spleen cells were cultured for 12 days with complete culture medium. Various cytokines were added at the start of the cultures. Mast cell development was analyzed based on mast cell number and histamine quantity per culture. Endogenous IL-3 was detectable in supernatants of all cultures including controls without adding cytokine (data not shown). However, only rIL-6 and rTNF-α induced mast cell development. rIL-1β, rIL-5, rTGF-β, rGM-CSF, and even the mast cell growth factors, rIL-9 and rSCF, failed to do so. rIL-3 showed a weak effect on mast cell development (Table 1). To exclude a possible influence of mouse strain on the mast cell development, spleen cells from different strains of mice were stimulated by IL-6 and TNF-α. Both the cytokines induced mast cell development even from the spleen cells of mast cell-deficient W/Wv mice (Table 2). However, when c-kit+ cells were depleted by MACS, the spleen cells failed to develop mast cells, even in the presence of all factors such as IL-3, PGE, IFN-γ, IL-6, and TNF-α (data not shown). IL-6 and TNF-α failed to induce mast cell development from the spleen cells of nude mice, which lack T cells to produce endogenous IL-3. When rIL-3 was added to the cultures of nude mouse spleen cells, mast cells were developed as the spleen cells of other strains (data not shown).
Effects of Various Cytokines on Mast Cell Development
| Cytokine (per mL) . | Mast Cells/Culture (104 ± SD) . | Histamine/Culture (μg ± SD) . |
|---|---|---|
| None | 3.7 ± 1.0 | 0.0491 ± 0.0140 |
| rGM-CSF 10 ng | 0.2 ± 0.2 | 0.0232 ± 0.0017 |
| 1 ng | 0.5 ± 0.4 | 0.0321 ± 0.0027 |
| rIL-1β 20 ng | 1.9 ± 0.3 | 0.0326 ± 0.0016 |
| 10 ng | 2.7 ± 0.5 | 0.0373 ± 0.0016 |
| 1 ng | 2.0 ± 0.9 | 0.0285 ± 0.0057 |
| rIL-3 20 ng | 19.1 ± 8.5 | 0.1578 ± 0.0076 |
| 10 ng | 13.1 ± 4.4 | 0.1408 ± 0.0331 |
| 1 ng | 12.1 ± 2.9 | 0.1172 ± 0.0052 |
| rIL-5 20 U | 5.8 ± 1.5 | 0.0668 ± 0.0054 |
| 10 U | 3.7 ± 2.0 | 0.0427 ± 0.0052 |
| 5 U | 6.6 ± 2.1 | 0.0555 ± 0.0218 |
| rIL-6 20 ng | 111.6 ± 39.9 | 0.5138 ± 0.0154 |
| 10 ng | 111.2 ± 4.7 | 0.5789 ± 0.0180 |
| 1 ng | 59.1 ± 11.9 | 0.2692 ± 0.0129 |
| 0.1 ng | 6.6 ± 1.5 | 0.0203 ± 0.0038 |
| rIL-9 20 ng | 5.8 ± 0.6 | 0.0531 ± 0.0052 |
| 10 ng | 5.0 ± 1.0 | 0.0624 ± 0.0070 |
| 1 ng | 7.9 ± 2.1 | 0.0732 ± 0.0163 |
| rSCF 20 ng | 9.1 ± 4.6 | 0.0737 ± 0.0044 |
| 10 ng | 5.0 ± 1.2 | 0.0671 ± 0.0073 |
| 1 ng | 6.2 ± 1.2 | 0.0420 ± 0.0096 |
| rTGF-β1 10 ng | 4.5 ± 1.4 | 0.0196 ± 0.0018 |
| 1 ng | 1.1 ± 0.2 | 0.0178 ± 0.0043 |
| rTNF-α 20 ng | 166.2 ± 12.8 | 0.2989 ± 0.0101 |
| 10 ng | 192.9 ± 26.2 | 0.3584 ± 0.0368 |
| 1 ng | 5.0 ± 2.7 | 0.0296 ± 0.0022 |
| Cytokine (per mL) . | Mast Cells/Culture (104 ± SD) . | Histamine/Culture (μg ± SD) . |
|---|---|---|
| None | 3.7 ± 1.0 | 0.0491 ± 0.0140 |
| rGM-CSF 10 ng | 0.2 ± 0.2 | 0.0232 ± 0.0017 |
| 1 ng | 0.5 ± 0.4 | 0.0321 ± 0.0027 |
| rIL-1β 20 ng | 1.9 ± 0.3 | 0.0326 ± 0.0016 |
| 10 ng | 2.7 ± 0.5 | 0.0373 ± 0.0016 |
| 1 ng | 2.0 ± 0.9 | 0.0285 ± 0.0057 |
| rIL-3 20 ng | 19.1 ± 8.5 | 0.1578 ± 0.0076 |
| 10 ng | 13.1 ± 4.4 | 0.1408 ± 0.0331 |
| 1 ng | 12.1 ± 2.9 | 0.1172 ± 0.0052 |
| rIL-5 20 U | 5.8 ± 1.5 | 0.0668 ± 0.0054 |
| 10 U | 3.7 ± 2.0 | 0.0427 ± 0.0052 |
| 5 U | 6.6 ± 2.1 | 0.0555 ± 0.0218 |
| rIL-6 20 ng | 111.6 ± 39.9 | 0.5138 ± 0.0154 |
| 10 ng | 111.2 ± 4.7 | 0.5789 ± 0.0180 |
| 1 ng | 59.1 ± 11.9 | 0.2692 ± 0.0129 |
| 0.1 ng | 6.6 ± 1.5 | 0.0203 ± 0.0038 |
| rIL-9 20 ng | 5.8 ± 0.6 | 0.0531 ± 0.0052 |
| 10 ng | 5.0 ± 1.0 | 0.0624 ± 0.0070 |
| 1 ng | 7.9 ± 2.1 | 0.0732 ± 0.0163 |
| rSCF 20 ng | 9.1 ± 4.6 | 0.0737 ± 0.0044 |
| 10 ng | 5.0 ± 1.2 | 0.0671 ± 0.0073 |
| 1 ng | 6.2 ± 1.2 | 0.0420 ± 0.0096 |
| rTGF-β1 10 ng | 4.5 ± 1.4 | 0.0196 ± 0.0018 |
| 1 ng | 1.1 ± 0.2 | 0.0178 ± 0.0043 |
| rTNF-α 20 ng | 166.2 ± 12.8 | 0.2989 ± 0.0101 |
| 10 ng | 192.9 ± 26.2 | 0.3584 ± 0.0368 |
| 1 ng | 5.0 ± 2.7 | 0.0296 ± 0.0022 |
Mouse spleen cells were cultured with complete culture medium. Various cytokines were added at the start of the cultures. Mast cell number and histamine quantity per culture were determined on day 12.
Mast Cell Development Induced by IL-6 and TNF- α From Different Strains of Mice
| Strain . | Cytokine* (10 ng/mL) . | Mast Cells/Culture (104 ± SD) . | Histamine/Culture (μg ± SD) . |
|---|---|---|---|
| C3H/HeN | None | 2.1 ± 0.6 | 0.0227 ± 0.0036 |
| rIL-6 | 83.3 ± 8.5 | 0.4074 ± 0.0314† | |
| rTNF-α | 113.3 ± 31.6 | 0.3846 ± 0.0125† | |
| BALB/c | None | 24.1 ± 4.1 | 0.0496 ± 0.0104 |
| rIL-6 | 73.3 ± 11.4 | 0.2008 ± 0.0192† | |
| rTNF-α | 60.8 ± 9.2 | 0.1100 ± 0.0096‡ | |
| BALB/c nu/nu | None | 0 | 0 |
| rIL-6 | 0 | 0 | |
| rTNF-α | 0 | 0 | |
| WBB6F1 +/+ | None | 18.7 ± 7.1 | 0.0833 ± 0.0017 |
| rIL-6 | 65.0 ± 2.7 | 0.1887 ± 0.0179† | |
| rTNF-α | 102.5 ± 14.1 | 0.3333 ± 0.0454† | |
| WBB6F1 W/WV | None | 9.1 ± 4.2 | 0.0505 ± 0.0246 |
| rIL-6 | 53.7 ± 14.7 | 0.2329 ± 0.0239† | |
| rTNF-α | 71.6 ± 7.2 | 0.3810 ± 0.0208† |
| Strain . | Cytokine* (10 ng/mL) . | Mast Cells/Culture (104 ± SD) . | Histamine/Culture (μg ± SD) . |
|---|---|---|---|
| C3H/HeN | None | 2.1 ± 0.6 | 0.0227 ± 0.0036 |
| rIL-6 | 83.3 ± 8.5 | 0.4074 ± 0.0314† | |
| rTNF-α | 113.3 ± 31.6 | 0.3846 ± 0.0125† | |
| BALB/c | None | 24.1 ± 4.1 | 0.0496 ± 0.0104 |
| rIL-6 | 73.3 ± 11.4 | 0.2008 ± 0.0192† | |
| rTNF-α | 60.8 ± 9.2 | 0.1100 ± 0.0096‡ | |
| BALB/c nu/nu | None | 0 | 0 |
| rIL-6 | 0 | 0 | |
| rTNF-α | 0 | 0 | |
| WBB6F1 +/+ | None | 18.7 ± 7.1 | 0.0833 ± 0.0017 |
| rIL-6 | 65.0 ± 2.7 | 0.1887 ± 0.0179† | |
| rTNF-α | 102.5 ± 14.1 | 0.3333 ± 0.0454† | |
| WBB6F1 W/WV | None | 9.1 ± 4.2 | 0.0505 ± 0.0246 |
| rIL-6 | 53.7 ± 14.7 | 0.2329 ± 0.0239† | |
| rTNF-α | 71.6 ± 7.2 | 0.3810 ± 0.0208† |
Mouse spleen cells were cultured with complete culture medium. Cytokines were added at the start of the cultures. Mast cell number and histamine quantity per culture were determined on day 12.
P < .01.
P < .05 compared with data without adding cytokines.
Mast cell development from bone marrow cells.To determine whether IL-6 or TNF-α has any ability to enhance mast cell development from cell populations other than spleen cells, bone marrow cells were cultured in this system. However, in the absence of exogenous IL-3, all the stimulants, including PGE, IFN-γ, IL-6, and TNF-α, failed to induce mast cell development (Table 3). The supernatants recovered from the bone marrow cell cultures did not contain detectable levels of IL-3. This may have been because there are not sufficient T cells in bone marrow cells to produce endogenous IL-3. When exogenous IL-3 was added into the liquid cultures, mast cells were developed and synergistic effects between IL-3 and other factors were observed (Table 3).
Mast Cell Development From Bone Marrow Cells in a Liquid Culture
| Stimulant . | Mast Cells/Culture3-150 (104 ± SD) . | Histamine/Culture (μg ± SD) . | Endogenous . |
|---|---|---|---|
| (10 ng/mL) . | . | . | IL-3 in Supernatants3-151 . |
| None | 0 | 0 | Undetectable |
| PGE | 0 | 0 | Undetectable |
| rIFN-γ | 0 | 0 | Undetectable |
| rTNF-α | 0 | 0 | Undetectable |
| rIL-6 | 0 | 0 | Undetectable |
| rIL-3 | 40.8 ± 3.8 | 0.2051 ± 0.0034 | |
| rIL-3 + PGE | 163.3 ± 10.2 | 0.4059 ± 0.03133-152 | |
| rIL-3 + rINF-γ | 100.4 ± 16.2 | 0.5185 ± 0.04013-152 | |
| rIL-3 + rTNF-α | 73.3 ± 9.2 | 0.2317 ± 0.01353-152 | |
| rIL-3 ± rIL-6 | 146.2 ± 51.1 | 0.5106 ± 0.04723-152 |
| Stimulant . | Mast Cells/Culture3-150 (104 ± SD) . | Histamine/Culture (μg ± SD) . | Endogenous . |
|---|---|---|---|
| (10 ng/mL) . | . | . | IL-3 in Supernatants3-151 . |
| None | 0 | 0 | Undetectable |
| PGE | 0 | 0 | Undetectable |
| rIFN-γ | 0 | 0 | Undetectable |
| rTNF-α | 0 | 0 | Undetectable |
| rIL-6 | 0 | 0 | Undetectable |
| rIL-3 | 40.8 ± 3.8 | 0.2051 ± 0.0034 | |
| rIL-3 + PGE | 163.3 ± 10.2 | 0.4059 ± 0.03133-152 | |
| rIL-3 + rINF-γ | 100.4 ± 16.2 | 0.5185 ± 0.04013-152 | |
| rIL-3 + rTNF-α | 73.3 ± 9.2 | 0.2317 ± 0.01353-152 | |
| rIL-3 ± rIL-6 | 146.2 ± 51.1 | 0.5106 ± 0.04723-152 |
Mouse bone marrow cells were cultured with complete culture medium. Various cytokines were added at the start of the cultures. Considering the lack of endogenous IL-3 and the property of IL-3–dependent manner of MMC, rIL-3 was replenished at each feeding. Mast cell number and histamine quantity per culture were determined on day 12.
Endogenous IL-3 in the supernatants recovered from the cultures on days 2 and 4 was detected by ELISA.
P < .01 compared with data of rIL-3 alone.
Effects of IL-6, IL-9, SCF, and TNF-α on MMC growth.MMC (1 × 103 cells/mL), prepared from LPS-stimulated liquid cultures of spleen cells on day 16, were further cultured by the methylcellulose method in the presence of rIL-3. SCF increased both the number and the size of mast cell colonies. IL-9 slightly enhanced the colony formation. However, IL-6 and TNF-α had no effect on both the number and the size of mast cell colonies (Table 4). The results confirmed that IL-6 and TNF-α are not mast cell growth factors, although mast cell development was induced by them. On the contrary, IL-9 and SCF are mast cell growth factors, although they failed to induce mast cell development from spleen cells.
Effects of IL-6, IL-9, SCF, or TNF- α on Colony Formation of Purified MMC in the Presence of rIL-3
| Addition . | MCC/Culture4-150 . |
|---|---|
| rIL-3 (20 ng/mL) | 19 ± 2 |
| rIL-3 + rIL-6 (20 ng/mL) | 20 ± 3 |
| rIL-3 + rIL-9 (40 ng/mL) | 27 ± 5 |
| rIL-3 + rSCF (40 ng/mL) | 33 ± 44-151 |
| rIL-3 + rTNF-α (40 ng/mL) | 17 ± 1 |
| Addition . | MCC/Culture4-150 . |
|---|---|
| rIL-3 (20 ng/mL) | 19 ± 2 |
| rIL-3 + rIL-6 (20 ng/mL) | 20 ± 3 |
| rIL-3 + rIL-9 (40 ng/mL) | 27 ± 5 |
| rIL-3 + rSCF (40 ng/mL) | 33 ± 44-151 |
| rIL-3 + rTNF-α (40 ng/mL) | 17 ± 1 |
Abbreviation: MCC, mast cell colonies.
Mast cells (1 × 103 cells/mL), induced by a 16-day liquid culture of spleen cells with 1 ng/mL LPS and purified by Histopaque-1077 separation, were cultured by the methylcellulose method in the presence of rIL-3. MCC in triplicate cultures were determined on day 16, as described in Materials and Methods.
P < .05 compared with data of rIL-3 alone.
Time course for mast cell development induced by rIL-6 and rTNF-α.rIL-6 and rTNF-α were added on day 0, 2, 4, 6, or 8 after the start of the cultures. Mast cells were developed when they were added at the early phase (day 0, 2, or 4 of a 12-day culture, Fig 1). These results further support the conclusion that both IL-6 and TNF-α are not mast cell growth factors, since detectable mast cells appeared only after day 4 in the cultures.23
Time course for mast cell development induced by IL-6 and TNF-α. rIL-6 or rTNF-α (10 ng/mL) was added to the cultures on day 0, 2, 4, 6, or 8. Control was set up without addition of the cytokines. Mast cell number (A) and histamine quantity (B) per culture were determined on day 12.
Time course for mast cell development induced by IL-6 and TNF-α. rIL-6 or rTNF-α (10 ng/mL) was added to the cultures on day 0, 2, 4, 6, or 8. Control was set up without addition of the cytokines. Mast cell number (A) and histamine quantity (B) per culture were determined on day 12.
Effects of neutralizing anti–IL-6 and anti–TNF-α antibody on mast cell development induced by PGE1 and rIFN-γ.To study a possible role of IL-6 or TNF-α on mast cell development, anti–IL-6 or anti–TNF-α antibody was added into the cultures together with PGE1 or IFN-γ. Depletion of endogenous TNF-α or IL-6 had no effects on mast cell development induced by PGE and IFN-γ (Table 5). When endogenous TNF-α and IL-6 were depleted at the same time by adding the antibodies together, there was also no suppression on mast cell development induced by PGE or IFN-γ (data not shown). These results indicate that IL-6 and TNF-α are not essential for the proliferation and/or differentiation of mast cell precursors.
Failure to Suppress Mast Cell Development by Depleting Endogenous IL-6 and TNF-α With Neutralizing Antibody
| Stimulant (10 ng/mL) . | Antibody5-150 . | Mast Cells/Culture (104 ± SD) . | Histamine/Culture (μg ± SD) . |
|---|---|---|---|
| . | (3 μg/mL) . | . | . |
| None | −5-151 | 2.5 ± 1.0 | 0.0112 ± 0.0005 |
| rIFN-γ | − | 66.6 ± 4.2 | 0.2088 ± 0.0322 |
| Anti–IFN-γ | 1.6 ± 0.6 | 0.0116 ± 0.00055-152 | |
| Anti–IL-6 | 68.3 ± 2.3 | 0.2147 ± 0.0032 | |
| Anti–TNF-α | 60.8 ± 5.6 | 0.1931 ± 0.0118 | |
| PGE1 | − | 102.8 ± 16.2 | 0.2471 ± 0.0321 |
| Anti–IL-6 | 79.8 ± 16.9 | 0.2346 ± 0.0502 | |
| Anti–TNF-α | 88.9 ± 1.2 | 0.2693 ± 0.0157 | |
| rTNF-α | − | 102.9 ± 3.6 | 0.3156 ± 0.0193 |
| Anti–IL-6 | 109.3 ± 20.5 | 0.3977 ± 0.0567 | |
| Anti–TNF-α | 5.4 ± 2.6 | 0.0180 ± 0.00195-152 | |
| rIL-6 | − | 80.0 ± 7.1 | 0.4373 ± 0.0909 |
| Anti–IL-6 | 18.3 ± 5.0 | 0.1272 ± 0.02165-152 | |
| Anti–TNF-α | 70.4 ± 11.4 | 0.3899 ± 0.0381 |
| Stimulant (10 ng/mL) . | Antibody5-150 . | Mast Cells/Culture (104 ± SD) . | Histamine/Culture (μg ± SD) . |
|---|---|---|---|
| . | (3 μg/mL) . | . | . |
| None | −5-151 | 2.5 ± 1.0 | 0.0112 ± 0.0005 |
| rIFN-γ | − | 66.6 ± 4.2 | 0.2088 ± 0.0322 |
| Anti–IFN-γ | 1.6 ± 0.6 | 0.0116 ± 0.00055-152 | |
| Anti–IL-6 | 68.3 ± 2.3 | 0.2147 ± 0.0032 | |
| Anti–TNF-α | 60.8 ± 5.6 | 0.1931 ± 0.0118 | |
| PGE1 | − | 102.8 ± 16.2 | 0.2471 ± 0.0321 |
| Anti–IL-6 | 79.8 ± 16.9 | 0.2346 ± 0.0502 | |
| Anti–TNF-α | 88.9 ± 1.2 | 0.2693 ± 0.0157 | |
| rTNF-α | − | 102.9 ± 3.6 | 0.3156 ± 0.0193 |
| Anti–IL-6 | 109.3 ± 20.5 | 0.3977 ± 0.0567 | |
| Anti–TNF-α | 5.4 ± 2.6 | 0.0180 ± 0.00195-152 | |
| rIL-6 | − | 80.0 ± 7.1 | 0.4373 ± 0.0909 |
| Anti–IL-6 | 18.3 ± 5.0 | 0.1272 ± 0.02165-152 | |
| Anti–TNF-α | 70.4 ± 11.4 | 0.3899 ± 0.0381 |
Various stimulants and neutralizing antibody were added at the start of the cultures. Mast cell numbers and histamine quantities per culture were determined on day 12.
−, not added.
P < .01 compared with data without neutralizing antibody.
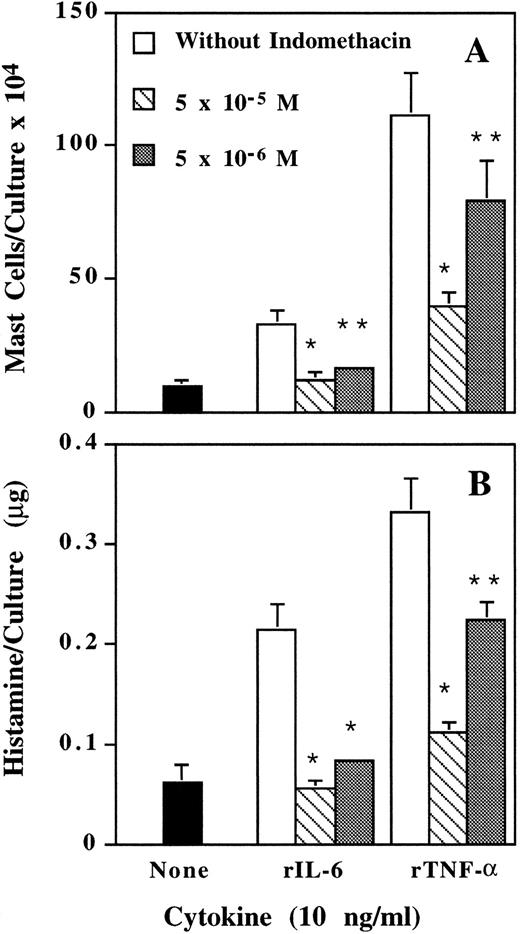
Inhibition by indomethacin of rIL-6– and rTNF-α–induced mast cell development.A possible mediator for mast cell development induced by IL-6 and TNF-α is PGE.25 When various doses of indomethacin, an inhibitor for prostaglandin synthesis, were added to the cultures together with rIL-6 or rTNF-α, indomethacin dose-dependently inhibited mast cell development (Fig 2). According to these results, PGE is, at least, involved in the mast cell development induced by IL-6 and TNF-α.
Inhibition by indomethacin of IL-6– and TNF-α–induced mast cell development. Mouse spleen cells were cultured with rIL-6 and rTNF-α in the presence or absence of various doses of indomethacin. Mast cell number (A) and histamine quantity (B) per culture were determined on day 12. * P < .01, ** P < .05 compared with data without indomethacin.
Inhibition by indomethacin of IL-6– and TNF-α–induced mast cell development. Mouse spleen cells were cultured with rIL-6 and rTNF-α in the presence or absence of various doses of indomethacin. Mast cell number (A) and histamine quantity (B) per culture were determined on day 12. * P < .01, ** P < .05 compared with data without indomethacin.
Reduction of mast cell development induced by IL-6 or TNF-α after removal of macrophages.PGE can be produced by macrophages and fibroblasts in spleen cells. To find if macrophage is a responsive cell of IL-6 or TNF-α, macrophages in the spleen cells were removed by Sephadex G-10 column. Reduction of mast cells developed in the cultures with IL-6 or TNF-α was observed (Table 6). The same tendency was also observed when macrophages were depleted by the method of plastic adhesion (data not shown). Therefore, macrophage is one of the responsive cells of IL-6 and TNF-α.
Effect of Macrophages on Mast Cell Development Induced by IL-6, TNF-α, and LPS
| Stimulant (10 ng/mL) . | Without Macrophages6-150 . | With Macrophages . | ||
|---|---|---|---|---|
| . | Mast Cells/Culture (104 ± SD) . | Histamine/Culture (μg ± SD) . | Mast Cells/Culture (104 ± SD) . | Histamine/Culture (μg ± SD) . |
| None | 1.6 ± 0.6 | 0.0182 ± 0.0027 | 11.6 ± 3.86-151 | 0.0725 ± 0.0351 |
| rIL-6 | 40.5 ± 11.4 | 0.1878 ± 0.0241 | 78.7 ± 16.76-151 | 0.5000 ± 0.06626-152 |
| rTNF-α | 59.5 ± 2.9 | 0.1249 ± 0.0263 | 85.0 ± 8.76-151 | 0.2054 ± 0.01456-151 |
| LPS | 34.1 ± 16.0 | 0.0570 ± 0.0435 | 94.1 ± 12.96-151 | 0.4378 ± 0.12116-151 |
| Stimulant (10 ng/mL) . | Without Macrophages6-150 . | With Macrophages . | ||
|---|---|---|---|---|
| . | Mast Cells/Culture (104 ± SD) . | Histamine/Culture (μg ± SD) . | Mast Cells/Culture (104 ± SD) . | Histamine/Culture (μg ± SD) . |
| None | 1.6 ± 0.6 | 0.0182 ± 0.0027 | 11.6 ± 3.86-151 | 0.0725 ± 0.0351 |
| rIL-6 | 40.5 ± 11.4 | 0.1878 ± 0.0241 | 78.7 ± 16.76-151 | 0.5000 ± 0.06626-152 |
| rTNF-α | 59.5 ± 2.9 | 0.1249 ± 0.0263 | 85.0 ± 8.76-151 | 0.2054 ± 0.01456-151 |
| LPS | 34.1 ± 16.0 | 0.0570 ± 0.0435 | 94.1 ± 12.96-151 | 0.4378 ± 0.12116-151 |
Macrophages were removed by Sephadex G-10 columns. Mast cell number and histamine quantity per culture were determined on day 12.
P < .05 compared with data of macrophage-depleted cultures.
P < .01 compared with data of macrophage-depleted cultures.
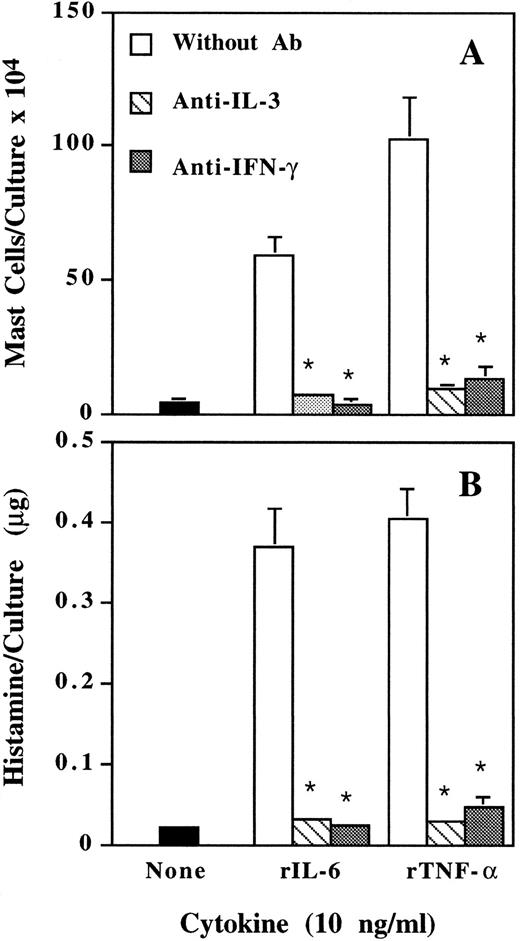
Suppression by neutralizing anti–IL-3 and anti–IFN-γ antibody of mast cell development induced by IL-6 and TNF-α.We have previously reported that, besides IL-3, IFN-γ is also an essential factor for murine mast cell development.26 When neutralizing anti–IL-3, anti–IFN-γ antibody, or nonspecific IgG was added to the cultures, both anti–IL-3 and anti–IFN-γ antibody completely suppressed mast cell development induced by IL-6 and TNF-α (Fig 3). Nonspecific IgG had no effect (data not shown). Therefore, IFN-γ may be another mediator for the actions of IL-6 and TNF-α.
Inhibition by neutralizing anti–IL-3 and anti–IFN-γ antibody of IL-6– and TNF-α–induced mast cell development. Mouse spleen cells were cultured with rIL-6 and rTNF-α in the presence or absence of neutralizing anti–IL-3 or anti–IFN-γ antibody (3 μg/mL). Mast cell number (A) and histamine quantity (B) per culture were determined on day 12. * P < .01 compared with data without antibody.
Inhibition by neutralizing anti–IL-3 and anti–IFN-γ antibody of IL-6– and TNF-α–induced mast cell development. Mouse spleen cells were cultured with rIL-6 and rTNF-α in the presence or absence of neutralizing anti–IL-3 or anti–IFN-γ antibody (3 μg/mL). Mast cell number (A) and histamine quantity (B) per culture were determined on day 12. * P < .01 compared with data without antibody.
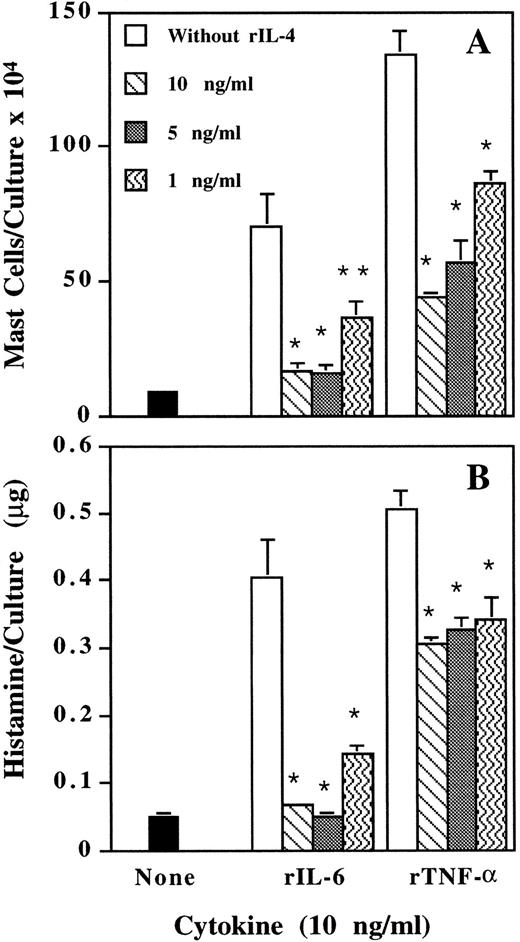
Inhibition by IL-4 of mast cell development induced by IL-6 and TNF-α.When rIL-4 was added at the start of the culture, it dose-dependently suppressed the rIL-6- and rTNF-α–induced mast cell development (Fig 4). Anti–IL-4 antibody specifically neutralized the suppressive activity of IL-4 (data not shown). Therefore, IL-4 is a downregulator for the actions of IL-6 and TNF-α on mast cell development.
Dose-dependent inhibition by IL-4 of IL-6– and TNF-α–induced mast cell development. Mouse spleen cells were cultured with rIL-6 and rTNF-α in the presence of various doses of rIL-4. Mast cell number (A) and histamine quantity (B) per culture were determined on day 12. * P < .01, ** P < .05 compared with data without adding rIL-4.
Dose-dependent inhibition by IL-4 of IL-6– and TNF-α–induced mast cell development. Mouse spleen cells were cultured with rIL-6 and rTNF-α in the presence of various doses of rIL-4. Mast cell number (A) and histamine quantity (B) per culture were determined on day 12. * P < .01, ** P < .05 compared with data without adding rIL-4.
Effects of IL-6 and TNF-α on IL-4 and IFN-γ production at the early phase of the cultures.To confirm further the actions of endogenous IFN-γ, as well as IL-4, on the mast cell development induced by IL-6 and TNF-α, IFN-γ and IL-4 levels in the supernatants, recovered on day 2 (for IFN-γ) and day 4 (for IL-4) from the cultures after stimulation with rIL-6 or rTNF-α, were detected by ELISA. Mast cell number and histamine quantity per culture were determined on day 12. TNF-α and IL-6 enhanced production of IFN-γ, but suppressed production of IL-4. Mast cell numbers induced were inversely and directly proportional to IL-4 (r = −0.95, P < .05) and IFN-γ (r = 0.97, P < .05) levels, respectively (Table 7).
Effects of IL-6 and TNF- α on IL-4 and IFN- γ Production
| Stimulant7-150 . | IL-47-151 . | IFN-γ7-151 . | Mast Cells/ . | Histamine/Culture . |
|---|---|---|---|---|
| (10 ng/mL) . | (pg/mL) . | (pg/mL) . | Culture . | (μg ± SD) . |
| . | . | . | (104 ± SD) . | . |
| Experiment 1 | ||||
| None | 174 ± 6 | 290 ± 22 | 2.9 ± 0.9 | 0.0410 ± 0.0104 |
| rIL-6 | 96 ± 77-152 | 411 ± 31‡ | 26.2 ± 2.0 | 0.2732 ± 0.0685ρ |
| Experiment 2 | ||||
| None | 199 ± 6 | 259 ± 20 | 4.2 ± 1.6 | 0.0509 ± 0.0034 |
| rTNF-α | 25 ± 2ρ | 1905 ± 131ρ | 77.5 ± 9.7 | 0.3887 ± 0.0335ρ |
| Stimulant7-150 . | IL-47-151 . | IFN-γ7-151 . | Mast Cells/ . | Histamine/Culture . |
|---|---|---|---|---|
| (10 ng/mL) . | (pg/mL) . | (pg/mL) . | Culture . | (μg ± SD) . |
| . | . | . | (104 ± SD) . | . |
| Experiment 1 | ||||
| None | 174 ± 6 | 290 ± 22 | 2.9 ± 0.9 | 0.0410 ± 0.0104 |
| rIL-6 | 96 ± 77-152 | 411 ± 31‡ | 26.2 ± 2.0 | 0.2732 ± 0.0685ρ |
| Experiment 2 | ||||
| None | 199 ± 6 | 259 ± 20 | 4.2 ± 1.6 | 0.0509 ± 0.0034 |
| rTNF-α | 25 ± 2ρ | 1905 ± 131ρ | 77.5 ± 9.7 | 0.3887 ± 0.0335ρ |
Mouse spleen cells were cultured with complete culture medium in the presence of either rIL-6 or rTNF-α. Mast cell numbers and histamine quantities were determined on day 12.
IFN-γ and IL-4 in the supernatants recovered on day 2 (for IFN-γ) and day 4 (for IL-4) from the cultures were detected by ELISA.
P < .05 compared with data without stimulants.
ρ P < .01 compared with data without stimulants.
DISCUSSION
Mast cell precursors are present in very small numbers in normal mouse spleen and cannot be identified as mast cells by morphology and histochemistry. Under certain circumstances, the precursors begin to differentiate into mast cells.7 Although many factors, such as IL-3, IL-4, IL-9, IL-10, and SCF, for mast cell proliferation and/or differentiation have been identified,8-15 30 mast cell development is difficult to explain only by mast cell growth factors.
We have previously reported that IL-3 alone is not sufficient for MMC development, although it is essential, and that PGE and IFN-γ are critical for survival or differentiation of mast cell precursors. We have also confirmed that IL-4 is a key inhibitor for mast cell precursors despite being a growth factor of MMC and CTMC.25 26 In this report, we show that IL-6 and TNF-α induced mast cell development, whereas IL-1β, IL-5, GM-CSF, TGF-β and even the mast cell growth factors, IL-9 and SCF, failed to do so.
To our knowledge, no reports have been found about the enhancing effects of IL-6 and TNF-α on murine mast cell development. Based on the data presented, mast cell development induced by IL-6 and TNF-α is mediated via an indirect process. Macrophage may be one of the responsive cells of IL-6 and TNF-α, since removal of macrophages greatly reduced the mast cell development induced by the cytokines (Table 6). IL-6 and TNF-α induce PGE and IFN-γ production, two critical stimulators for mast cell precursors, and reduce IL-4 production, a critical inhibitor for mast cell precursors, in the cultures. Ample reports are also found that show both IL-6 and TNF-α are able to increase PGE and IFN-γ production31-34 and to suppress IL-4 production.35
An increase in mast cell number can be seen in various inflammatory diseases and pathologic conditions.16-22 One thing in common should be the presence of inflammation. Inflammatory factors are clearly important, because mast cell development in vivo occurs mainly in nonhematopoietic microenvironments in which the physiological concentrations of the mast cell growth factors (except SCF ) may be limited. Our previous and present results provide the evidence that the increase of mast cell numbers is related to inflammatory mediators. Inflammatory factors, such as PGE, IFN-γ, TNF-α and IL-6, together with mast cell growth factors directly and/or indirectly trigger the differentiation and proliferation of mast cell precursors. In fact, the distribution of mast cells in vivo also suggests the importance of inflammatory mediators on mast cell development. MMC are found adjacent to respiratory and intestinal mucosal surfaces, and CTMC are spread throughout the skin, peritoneal cavity, and musculature.7 Thus, each cell type is situated at sites where different types of primary infections and inflammatory stimulation are initiated.
An interesting observation is that the IL-6–induced mast cells contained more histamine than the TNF-α–induced mast cells (Tables 1 and 3, and Figs 1-3). The presence of IL-6, however, had no effect on histamine content of mast cells already developed (data not shown). Therefore, further experiments are needed to define the mechanism.
In agreement with a previous report,30 GM-CSF did not induce mast cell development and even suppressed it. TGF-β also failed to induce mast cell development, although it is a potent chemoattractant for mast cells.36 This may have been because of the suppression by TGF-β of IFN-γ and TNF-α production,37 and the antiproliferative effects of it.38 IL-1 is able to stimulate IFN-γ and PGE production.39,40 However, IL-1 is a strong stimulator of T helper type 2 cells41 and IL-4 production.42 The IL-1–induced elevation of IL-4 in the culture may result in the failure to induce mast cell development by IL-1.
It has been reported that the precursors for mast cell lineage are positive for c-kit expression.43 When c-kit positive cells were depleted, the spleen cells failed to develop mast cells even in the presence of all essential factors including IL-3, PGE, and IFN-γ.
The importance of SCF in mast cell development is demonstrated by the lack of tissue mast cells in Sl/Sld and W/Wv mice, which are mutated at the loci encoding SCF and c-kit, respectively.44 In the culture system, however, mast cells can be easily developed in the presence of endogenous (Table 2) or exogenous IL-3,45,46 despite the dysfunctional point mutation at the intracellular tyrosine kinase domain of c-kit on mast cell precursors of W/Wv mice. This is consistent with the failure of SCF to induce mast cell development in our culture system (Table 1). The failure to induce mast cell development by IL-9 and SCF may be because they did not stimulate the production of PGE and IFN-γ and did not suppress the production of IL-4 (data not shown). Mast cell precursors were also reported to be less responsive to IL-9 and SCF than more mature mast cells.47-49 However, the above-mentioned reasons may not be sufficient to explain the differences between the results from in vivo and in vitro experiments. The complex interplay that may occur in vivo and the limitation of in vitro experiments have to be considered for the interpretation.
In summary, our present results indicate that aside from mast cell growth factors, inflammatory factors (PGE, IFN-γ, TNF-α, and IL-6) as triggers are also important for mast cell development.
Address reprint requests to Tadakatsu Shimamura, MD, Department of Microbiology and Immunology, Showa University School of Medicine, 1-5-8 Hatanodai, Shinagawa-ku, Tokyo 142, Japan.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal