Abstract
Monocytes and endothelial cells interact at sites of vascular injury during inflammatory response, thrombosis, and development of atherosclerotic lesions. Such interactions result in modulation of several biological functions of the two cell types. Because both cells, on appropriate stimulation, synthesize tissue factor (TF), we examined the effect of human umbilical vein endothelial cell (HUVEC)/monocyte coculture on the expression of TF. We found that the coincubation resulted in TF generation, which was maximal at 4 hours, increased with increasing numbers of monocytes, and required mRNA and protein synthesis. Supernatant from HUVEC/monocyte coculture induced TF activity in HUVECs, but not in monocytes, indicating that HUVEC were the cells responsible for the activity, and that soluble mediators were involved. Interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), well-known inducers of TF in HUVECs, were found in the supernatant from the coculture, and specific antibodies directed against either cytokine inhibited TF generation. The need of IL-1β and TNF-α synthesis in order to elicit TF expression was also suggested by the delay observed in TF mRNA formation and TF activity generation when monocytes were incubated with HUVECs. IL-1β and TNF-α antigen levels in the coculture supernatant, and, consequently, HUVEC TF expression, were inhibited in the presence of anti-CD18 monoclonal antibody. These findings emphasize the role of cell-cell contact and cross-talk in the procoagulant activity, which could be responsible for the thromboembolic complications observed in those vascular disorders in which monocyte infiltration is a common feature.
TISSUE FACTOR (TF) is an integral membrane glycoprotein tightly associated with phospholipids functioning as receptor and cofactor for coagulation factor VII and its active form VIIa. On binding of factor VIIa to TF, the complex acquires catalytic activity and converts factors IX and X to their active derivatives IXa and Xa, respectively, thus leading to thrombin generation and fibrin formation.1,2 TF, which under physiological conditions is not in contact with blood, can be found in the tissue adventitia surrounding blood vessels, where, after vascular injury, it becomes available for the interaction with factor VIIa, starts the coagulation process, and forms the fibrin clot that reduces or stops the loss of blood.3
Among the cells in contact with the blood stream, quiescent monocytes and endothelial cells are not endowed with TF activity, but synthesize and express TF on their membrane in response to a wide variety of stimulating agents. Physiologically relevant agents that induce TF generation in monocytes include: bacterial lipopolysaccharide (LPS),4,5 the hydrolytic fragment of the fifth component of complement,6 immune complexes,7 P-selectin,8 platelets,9 and malignant cells.10 Inducing agents for endothelial cell TF synthesis are: LPS,11,12 thrombin,13 interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α).14 The expression of TF on monocyte and endothelial cell membrane, and the consequent activation of coagulation, may be of clinical significance in a variety of pathologic conditions, such as septic shock,15,16 immune inflammatory diseases,17-19 and cancer.10,20 Moreover, expression of TF activity has been linked to the fibrin formation observed in unstable angina patients,21 and TF-producing cells have been identified in atherosclerotic plaques, thus suggesting a role for TF in the prothrombotic state associated to atherosclerotic vessels.22
Monocyte-endothelial cell interactions play a key role in the inflammatory response, thrombosis, and development of atherosclerotic lesions.23 Important initial steps for these interactions are recruitment of monocytes through soluble mediators and their consequent attachment to the vascular endothelium, mediated by a complex array of adhesion molecules.24 Such a close apposition might be predicted to result in modulation of several biological functions by both cell types.
The effect of monocyte or its products influences endothelial cell migration and proliferation, and production of several endothelial molecules. Conversely, endothelial cells, through generation of chemoattractants, adhesion molecules, and cytokines, deeply affect monocyte function.23,25 Inflammatory events, immune complex disorders, and rupture of atherosclerotic plaques lead to activation of coagulation followed by thrombus formation. In all instances, close association of monocytes to the endothelium, with consequent migration into the extracellular compartments, are commonly observed. To better understand monocyte-endothelial cell interactions, several groups have recently established and analyzed cocultures of the two cell types. Their results show that the close proximity of monocytes and endothelial cells modulates the production of molecules such as prostacyclin, plasminogen activator inhibitor, von Willebrand factor, procoagulants, mitogens, the macrophage-inflammatory protein-1α (MIP-1α), and monocyte chemoattractant protein-1 (MCP-1).26-31 In the present study we investigated whether monocyte-endothelial cell coincubation can induce the expression of TF. To test this hypothesis we used a coculture system of monocytes and human umbilical vein endothelial cells (HUVECs). Our results indicate for the first time that, in resting conditions, monocytes induce a procoagulant activity of the TF type in endothelial cells, and that cytokines are the mediators responsible for the activity. A role for CD18 integrin adhesion molecules is suggested.
MATERIALS AND METHODS
Reagents and monoclonal antibodies (MoAbs).Medium 199 (M199), newborn calf serum (NCS), penicilline, glutamine, 0.05% trypsin/0.02% EDTA, were purchased from GIBCO-BRL (Grand Island, NY). Heparin, collagenase, polymixin B, and cycloheximide were from Sigma Chemical Co (St Louis, MO). RPMI 1640 medium and fetal calf serum (FCS) were from Biochrom (Berlin, Germany). Endothelial cell growth factor (ECGF) was prepared according to Maciag et al.32 Tissue-culture dishes were from Costar Data Packing Corp (Cambridge, MA). α-Amanitin was from Calbiochem (San Diego, CA). Lymphoprep was from Nycomed (Oslo, Norway), and Percoll was from Pharmacia (Uppsala, Sweden). FVII deficient plasma was obtained from Ortho Diagnostic System (Cologno Monzese, Italy), and the Limulus assay was from Whittaker Bioproducts, Inc (Walkersville, MD). Recombinant human IL-1β and recombinant human TNF-α were from Peprotec (Rocky Hill, NJ).
The MoAbs against human TNF-α and human IL-1α were purchased from UBI (Lake Placid, NY); the MoAb against human IL-1β was from R&D System (Minneapolis, MN) and was used as purified IgG1 at 10 μg/mL. This concentration was found to be saturating. The ascite, specific for the β2 subunit of the CD11/CD18 integrin, was obtained from the hybridoma cells (clone TS1/18.1.2.11) purchased from American Type Culture Collection (ATCC; Rockville, MD). Inhibition studies with this antibody were performed with 1/500 dilution (saturating concentration). The isotype matched control MoAb anti human CD1 was also from ATCC.
Cell culture.Endothelial cells were isolated from human umbilical cord vein by digestion with 0.5% collagenase as previously described.33 For experiments, HUVECs were plated at a density of 8 × 104 cells/well in gelatin-coated 12-well plates, and grown to confluency in 199 medium, supplemented with 20% NCS, 2 mmol/L L-glutamine, 100 μg/mL streptomycin, 100 U/mL penicillin, 5 mg/mL ECGF, and 10 mg/mL heparin, in humidified atmosphere of 92.5% air/7.5% CO2 at 37°C. Cultured medium was refreshed every other day. The number of HUVECs at confluency was 2 × 105/well. HUVECs were always used at the second passage, and cells were shown to be von Willebrand factor-positive as judged by immunofluorescent staining.
Monocytes.Human monocytes were obtained from whole blood collected from healthy donors and anticoagulated with 0.1 vol of 3.8% sodium citrate/0.15 mol/L NaCl. Leukocytes were sedimented at 180g for 15 minutes at 10°C. The sedimented cells were diluted to the initial volume with citrate-saline (0.38% sodium citrate/0.15 mol/L NaCl), and sedimented again. This step was repeated to minimize platelet contamination. The cells were then layered onto Lymphoprep, and centrifuged at 700g for 20 minutes at 20°C. The ring of mononuclear cells was collected and sedimented at 620g for 7 minutes at 8°C. The pellet was resuspended in citrate-saline and the cells were washed twice. Monocytes were purified using a discontinuous Percoll density gradient; the mononuclear cell preparation was resuspended in RPMI 1640 medium supplemented with 10% heat-inactivated FCS, and was layered onto a 46% isosmotic Percoll solution. After centrifugation at 20°C for 30 min at 550g, the cells at the RPMI 1640 medium/Percoll interface were collected and sedimented at 620g. The pellet was resuspended in citrate-saline and the cells were washed two times. The final monocyte preparation was then resuspended in serum-free RPMI 1640 at the concentration required. The Percoll isolated fraction contained approximately 85% monocytes, as assessed by nonspecific esterase staining, of which >95% were viable as determined by trypan blue staining.
Coincubation studies.Confluent HUVECs were washed 3 times in serum-free RPMI 1640, and incubated in 0.5 mL of the same medium with or without monocytes for different time intervals at 37°C in 7.5% CO2 . At the end of incubation, HUVECs were extensively washed, resuspended in 300 μL RPMI 1640, and disrupted by 3 freeze-thaw cycles. The disrupted cells were stored at −20°C until procoagulant activity assay.
In selected experiments, HUVECs or monocytes were incubated for 2 hours with 10 μg/mL α-amanitin, a specific inhibitor of RNA polymerase II. After washing, the α-amanitin–treated HUVECs and the α-amanitin–treated monocytes were incubated for an additional period of 3.5 hours with monocytes and HUVECs, respectively. The cells were then washed and treated as described below for procoagulant activity assay.
When cycloheximide (10 μg/mL) or polymixin B (1 ng/mL) were used, they were added at the beginning of incubation. In some experiments, HUVECs and monocytes, alone or together, were incubated for 4 hours. At the end of incubation, the supernatants were collected, sedimented by centrifugation (5 minutes at 16,000g) to eliminate monocytes and cellular debris, added to untreated HUVECs or monocytes obtained from the same donors and cultured in parallel, and incubated for 4 hours. At the end of incubation, the cells were washed and the procoagulant activity was determined.
In experiments in which MoAbs directed against IL-1α, IL-1β, and TNF-α were used, they were incubated with HUVECs for 15 minutes at room temperature before addition of monocytes or supernatant from HUVEC/monocyte coincubation. MoAbs to CD18 and CD1 were added together with monocytes at different dilutions to the endothelial monolayer.
Cell adherence.HUVECs and monocytes were cocultured for 4 hours. At the end of incubation, the wells were rinsed 3 times to remove the nonadherent monocytes. The cells were then fixed and stained by the Diff-Quik Staining Set from Baxter Dade (Dudingen, Switzerland). The number of monocytes adhering on HUVECs was evaluated by counting in each of 20 microscopic fields per sample.
Control of endotoxin contamination.Sterile pyrogen-free working conditions were observed to avoid any contamination by endotoxin. Solutions were prepared in glassware rendered pyrogen-free by heating at 180°C for 3 hours. Reagents were dissolved in sterile pyrogen-free solvents, and, when routinely tested for endotoxin contamination by the Limulus assay, were found negative at the level of 0.1 endotoxin U/mL, corresponding to 0.01 ng/mL.
Procoagulant activity assay.Procoagulant activity was assessed by a one-stage clotting assay.8 Disrupted cells (100 μL) were mixed with 100 μL of normal human plasma at 37°C. After 30 seconds, 100 μL of 25 mmol/L CaCl2 at 37°C was added to the mixture and the time to clot formation was recorded. Results were expressed in arbitrary units (U) by comparison with a standard curve obtained using a human brain thromboplastin standard kindly donated by Dr L. Poller (Manchester, UK). This preparation was assigned a value of 1000 U for a clotting time of 20 seconds. The standard curve was linear from 1.000 to 0.01 U, corresponding to clotting times of 20 and 511 seconds, respectively. In some instances, results were also expressed in nanograms of TF by comparison with a standard curve obtained by using a recombinant human TF, lipidated lipoprotein standard (American Diagnostica, through the courtesy of Domenico Santo, Ortho Diagnostic).
IL-1β and TNF-α determination.Conditioned medium from HUVECs and monocytes, alone or together, was sedimented by centrifugation to eliminate cellular debris, and the presence of IL-1β and TNF-α was assessed by enzyme-linked immunosorbent assays (ELISA) as specified by the manufacturers (Amersham, Buckinghamshire, UK). The sensitivity of these ELISAs was 0.3 and 4.4 pg/mL for IL-1β and TNF-α, respectively.
Northern blot analysis.For northern blot analysis, HUVECs were cocultured with monocytes for different time intervals. At the end of incubation, HUVECs were extensively washed to remove monocytes. Total cellular RNA was isolated by the thiocyanate/cesium chloride method.34 Ten micrograms of each RNA sample was electrophoresed on a 1% agarose gel, and transferred to nitrocellulose (Zeta Probe Blotting Membranes, Bio-Rad, Hercules, CA) in 20× saline sodium citrate overnight, and backed at 80°C for 2 hours under vacuum. The cDNA probe for TF (pHTF8; ATCC) was labeled with 32P by the random primer method and purified by gel filtration (Sephadex G-50). Filters were prehybridized in 7% sodium dodecyl sulfate (SDS), 0.5 mmol/L sodium phosphate (pH 7.2), 1 mmol/L EDTA, 1% bovine serum albumin (BSA). Hybridization was performed in the same buffer for 16 hours at 65°C. The filters were washed twice with .5% SDS, 40 mmol/L sodium phosphate (pH 7.2), 1 mmol/L EDTA, 0.5% BSA at 65°C for 30 minutes, and twice with 1% SDS, 40 mmol/L sodium phosphate (pH 7.2), 1 mmol/L EDTA at 65°C for 30 minutes. The filters were then exposed to Kodak X-OMAT AR x-ray film (Eastman-Kodak, Rochester, NY) by using an intensifying screen at −70°C for various times. As an internal control, filters were also hybridized and autoradiographed using a 32P-labeled GAPDH.
Statistical analysis.The results are given as mean values ± SEM. Differences between groups were tested for significance using Student's t test for paired observations, unless otherwise indicated. ANOVA analysis followed by Dunnett's test was used for multiple comparisons.
RESULTS
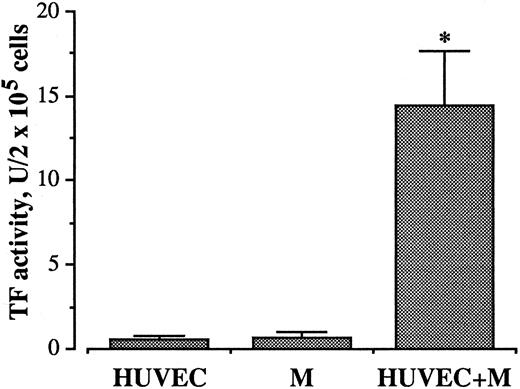
Effect of HUVEC/monocyte coincubation on TF expression.Coincubation of freshly isolated monocytes with HUVECs at a ratio of 2:1 for 4 hours at 37°C resulted in expression of procoagulant activity (Fig 1). The amount of TF protein for HUVECs, monocytes, and HUVEC/monocytes, by extrapolation with a standard curve of serial dilutions of recombinant human TF, lipidated protein standard, corresponded to 0.48, 0.57, and 10.58 ng/2 × 105 cells, respectively. The activity increased steadily by increasing the number of monocytes incubated with HUVECs.
Effect of coincubation of monocytes (M) with HUVEC on TF activity. 4 × 105 M were incubated with or without HUVEC (2 × 105/well) for 4 hours at 37°C in 7.5% CO2 . At the end of incubation the cells were washed and resuspended in 300 μL RPMI 1640. TF activity was measured in frozen and thawed samples, as described in Materials and Methods. Bars represent the mean of ten experiments ± SEM. *P < .0001 versus both HUVEC and M incubated alone.
Effect of coincubation of monocytes (M) with HUVEC on TF activity. 4 × 105 M were incubated with or without HUVEC (2 × 105/well) for 4 hours at 37°C in 7.5% CO2 . At the end of incubation the cells were washed and resuspended in 300 μL RPMI 1640. TF activity was measured in frozen and thawed samples, as described in Materials and Methods. Bars represent the mean of ten experiments ± SEM. *P < .0001 versus both HUVEC and M incubated alone.
The procoagulant activity was characterized using congenital factor-deficient plasmas. The dependence on the presence of factor VII, factor X, and prothrombin indicates this activity to be attributable to TF (not shown). Virtually, no procoagulant activity was developed when HUVECs and monocytes were cultured alone, indicating that no endotoxin contamination was affecting our experimental conditions.
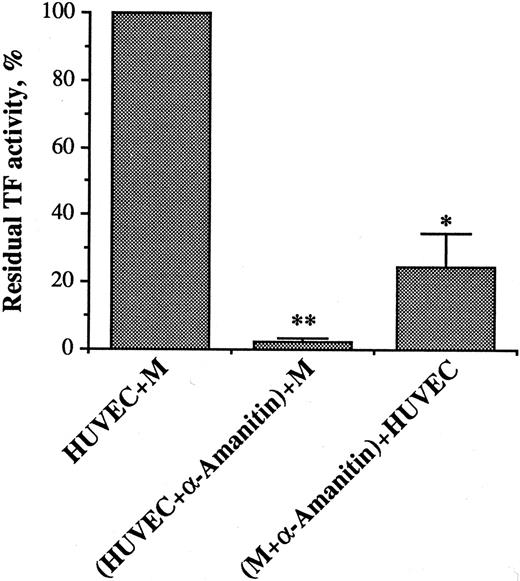
Role of protein synthesis and DNA transcription.Incubation of HUVECs with monocytes in the presence of 10 μg/mL cycloheximide virtually abolished TF activity generation, indicating the requirement for de novo protein synthesis. The role of DNA transcription for both cell types was investigated by using α-amanitin, a specific irreversible inhibitor of RNA polymerase II. In this set of experiments, HUVECs were incubated for 2 hours with 10 μg/mL α-amanitin. After washing, the α-amanitin–treated HUVECs were further incubated with monocytes that were not exposed to α-amanitin, for an additional period of 3.5 hours. Similarly, monocytes treated with α-amanitin for 2 hours were washed and further incubated for 3.5 hours with untreated HUVECs. Blockade of DNA transcription in either cell type largely prevented generation of TF activity, indicating that RNA synthesis by both HUVECs and monocytes is required for the activity (Fig 2). Neither treatment decreased cell viability, measured by trypan blue exclusion.
Effect of α-amanitin on TF activity generation during HUVEC/M coculture. α-amanitin (10 μg/mL) was incubated with HUVEC or M for 2 hours. At the end of incubation, HUVEC (2 × 105/well) and M (4 × 105/well) were washed and incubated for an additional period of 3.5 hours with untreated M and HUVEC, respectively. Cells were then processed for TF activity assay, as for Fig 1. Results are expressed in percent of TF activity. A 100% value was assigned to the activity expressed by the HUVEC/M coculture. Bars represent the mean of three experiments ± SEM. **P < .0001 and *P < .01 when compared with HUVEC + M.
Effect of α-amanitin on TF activity generation during HUVEC/M coculture. α-amanitin (10 μg/mL) was incubated with HUVEC or M for 2 hours. At the end of incubation, HUVEC (2 × 105/well) and M (4 × 105/well) were washed and incubated for an additional period of 3.5 hours with untreated M and HUVEC, respectively. Cells were then processed for TF activity assay, as for Fig 1. Results are expressed in percent of TF activity. A 100% value was assigned to the activity expressed by the HUVEC/M coculture. Bars represent the mean of three experiments ± SEM. **P < .0001 and *P < .01 when compared with HUVEC + M.
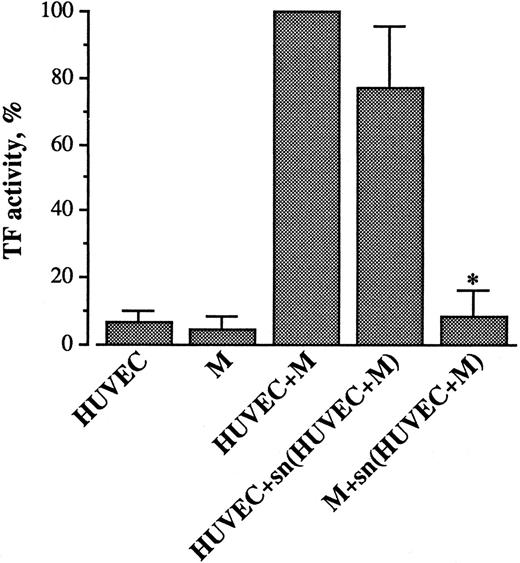
Effect of supernatant from HUVEC/monocyte coculture on TF expression.To ascertain whether direct cell-cell contact and/or secretory products were involved, HUVECs and monocytes were incubated separately or together for 4 hours. At the end of the incubation, the supernatants were collected, cleared of cells by centrifugation, and added to HUVECs or monocytes obtained from the same donors, and cultured in parallel. After 4 hours, the cells were washed and tested for TF activity. As can be seen in Fig 3, HUVECs incubated with the cell-free supernatant derived from the HUVEC/monocyte coincubation expressed a TF activity that was similar to that generated by the intact cell system. Conversely, the same supernatant could not induce TF when incubated with monocytes. Incubations of monocytes with supernatant from HUVECs and of HUVECs with supernatant from monocytes were also ineffective (not shown). These results show that the TF activity that develops during HUVEC/monocyte coincubation is solely attributable to HUVECs. Moreover, the activity is induced by soluble mediator(s) released when the two cell types are incubated together.
Effect of supernatant (sn) from HUVEC/M coincubation on TF activity in HUVECs and M. HUVEC (2 × 105/well) and M (4 × 105/well) were incubated together for 4 hours at 37°C. At the end of incubation the supernatant was collected, sedimented by centrifugation, and added to HUVEC or M obtained from the same donors and cultured in parallel. The cells were then washed and tested for TF activity, as described in Materials and Methods. Results are expressed in percent of TF activity. A 100% value was assigned to the activity expressed by the HUVEC/M coculture. Each column represents the mean of seven experiments ± SEM. *P < .01 when compared with HUVEC + M.
Effect of supernatant (sn) from HUVEC/M coincubation on TF activity in HUVECs and M. HUVEC (2 × 105/well) and M (4 × 105/well) were incubated together for 4 hours at 37°C. At the end of incubation the supernatant was collected, sedimented by centrifugation, and added to HUVEC or M obtained from the same donors and cultured in parallel. The cells were then washed and tested for TF activity, as described in Materials and Methods. Results are expressed in percent of TF activity. A 100% value was assigned to the activity expressed by the HUVEC/M coculture. Each column represents the mean of seven experiments ± SEM. *P < .01 when compared with HUVEC + M.
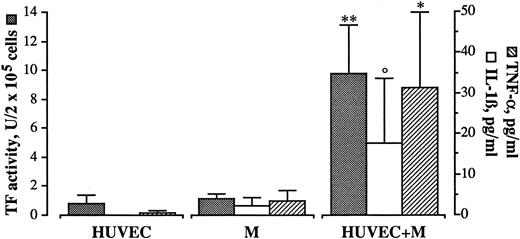
Involvement of IL-1β and TNF-α.Among the soluble mediators possibly involved, IL-1β and TNF-α, which are primarily produced by monocytes and well known inducers of endothelial cell TF,14 were good candidates. In order to investigate whether these cytokines were present in our experimental conditions, HUVECs and monocytes were incubated alone or together for 4 hours. Conditioned medium was collected, sedimented by centrifugation, and the presence of IL-1β and TNF-α assessed by ELISA, while TF activity was tested on disrupted cells. Virtually, neither IL-1β nor TNF-α could be detected in supernatants from HUVECs and monocytes cultured alone. By contrast, when HUVECs and monocytes were cultured together, antigens for IL-1β and TNF-α could be found in the conditioned medium, while HUVECs were expressing TF activity (Fig 4).
Presence of IL-1β and TNF-α during HUVEC/M coculture. HUVEC (2 × 105/well) and M (4 × 105/well) were incubated together for 4 hours at 37°C. At the end of incubation the cells were washed, the supernatant was collected, and the presence of IL-1β and TNF-α was assessed by ELISA assay, as specified by the manufacturers (Amersham), while the cells were treated for TF activity assay as described in Materials and Methods. Bars represent the mean of five experiments ± SEM. **P < .01, *P < .05, and °P < .05 (by Wilcoxon rank-sum test) versus HUVEC and M cultured alone.
Presence of IL-1β and TNF-α during HUVEC/M coculture. HUVEC (2 × 105/well) and M (4 × 105/well) were incubated together for 4 hours at 37°C. At the end of incubation the cells were washed, the supernatant was collected, and the presence of IL-1β and TNF-α was assessed by ELISA assay, as specified by the manufacturers (Amersham), while the cells were treated for TF activity assay as described in Materials and Methods. Bars represent the mean of five experiments ± SEM. **P < .01, *P < .05, and °P < .05 (by Wilcoxon rank-sum test) versus HUVEC and M cultured alone.
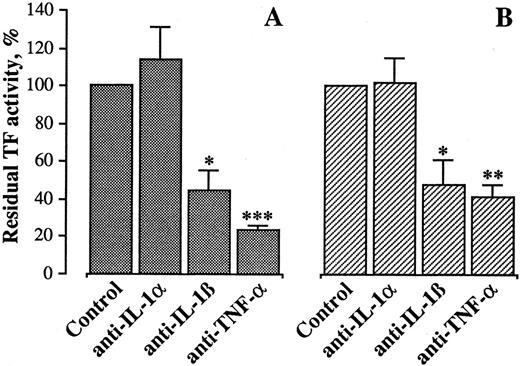
To investigate whether these cytokines were responsible for HUVEC activation, specific neutralizing antibodies to IL-1β or TNF-α were incubated with HUVECs 15 minutes before incubation with monocytes. As shown in Fig 5A, both antibodies (at a concentration of 10 μg/mL) markedly reduced the generation of TF activity, suggesting that both cytokines were involved. Similarly, when MoAbs anti–IL-1β and TNF-α were incubated with HUVEC before addition of supernatant from HUVEC/monocyte coincubation, they could inhibit TF activity generation (Fig 5B). No further inhibition was obtainable when both MoAbs were present at the same time (not shown). In both instances, a MoAb against IL-1α did not affect TF activity.
Effect of anti–IL-1α, anti–IL-1β, and anti–TNF-α MoAbs on TF activity by HUVEC cocultured with monocytes. The MoAbs, each at 10 μg/mL, were incubated with HUVEC 15 minutes before incubation with monocytes or supernatant from HUVEC/M coculture. After 4 hours, the cells were washed and processed for TF activity assay, as described in Materials and Methods. Results are expressed in percent of TF activity. A 100% value was assigned to the activity expressed by HUVEC incubated with monocytes (A) or supernatant from HUVEC/M coculture (B). Bars represent the mean of four experiments ± SEM. ***P < .001, **P < .005, and *P < .05 versus control.
Effect of anti–IL-1α, anti–IL-1β, and anti–TNF-α MoAbs on TF activity by HUVEC cocultured with monocytes. The MoAbs, each at 10 μg/mL, were incubated with HUVEC 15 minutes before incubation with monocytes or supernatant from HUVEC/M coculture. After 4 hours, the cells were washed and processed for TF activity assay, as described in Materials and Methods. Results are expressed in percent of TF activity. A 100% value was assigned to the activity expressed by HUVEC incubated with monocytes (A) or supernatant from HUVEC/M coculture (B). Bars represent the mean of four experiments ± SEM. ***P < .001, **P < .005, and *P < .05 versus control.
Taken together, these results suggest that HUVEC/monocyte coincubation results in IL-1β and TNF-α synthesis, and that both cytokines, once released, stimulate TF expression by HUVECs. To rule out the possibility that subthreshold concentrations of LPS might prime cells to become responsive to other agonists, experiments aiming at excluding this possibility were carried out. In this set of experiments, polymixin B (100 ng/mL) was incubated with HUVECs and monocytes for 4 hours at 37°C. In control experiments, this amount of polymixin B was sufficient to prevent the effect of 10 ng LPS/mL on TF expression by mononuclear cells. No differences in TF expression could be observed when HUVECs and monocytes were cocultured in the absence or in the presence of polymixin B (13.4 ± 1.5 and 12.9 ± 1.7, mean ± SEM, n = 4, respectively).
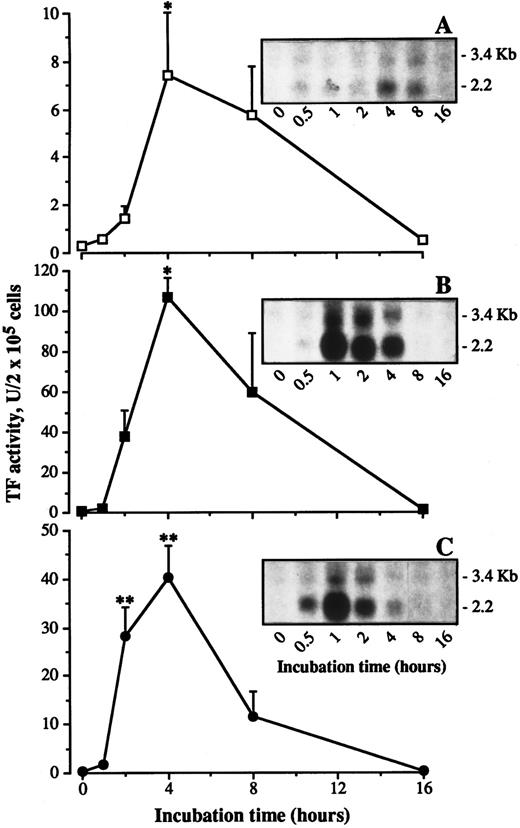
Time course of TF induction.Time course experiments were performed in which the effect of monocytes on incubation with HUVECs was compared with that of exogenously added recombinant IL-1β (rIL-1β) or recombinant TNF-α (rTNF-α). HUVECs were incubated with monocytes, rIL-1β, or rTNF-α for different time intervals. At the end of incubation, the monolayer was thoroughly washed and the cells were tested for TF activity. As previously reported,14 rIL-1β and rTNF-α stimulate HUVEC TF activity (Fig 6B and C). The stimulation occurs in a dose-dependent fashion with both cytokines (not shown). The activity is already detectable within 2 hours, reaches a maximum at 4 hours, and rapidly declines to basal values within 16 hours. When monocytes are incubated with HUVECs, a shift in time is observed: no activity is detectable after 2 hours, a maximum is attained within 4 hours, sustained through 8 hours, and baseline is reached within 16 hours (Fig 6A).
Time course in TF activity expression and TF mRNA levels during incubation of HUVEC with monocytes, IL-1β, or TNF-α. HUVEC (2 × 105/well) were incubated with monocytes (4 × 105/well) (A), IL-1β (1 ng/mL) (B), or TNF-α (1 ng/mL) (C) for the indicated time intervals. At the end of incubation the cells were treated for TF activity assay as described in Materials and Methods. In a parallel set of samples total RNA was extracted from cells, and equal amounts of RNA were analyzed by Northern blot as described in Materials and Methods. Each point represent the mean of four experiments ± SEM. **P < .01, and *P < .05 versus time zero.
Time course in TF activity expression and TF mRNA levels during incubation of HUVEC with monocytes, IL-1β, or TNF-α. HUVEC (2 × 105/well) were incubated with monocytes (4 × 105/well) (A), IL-1β (1 ng/mL) (B), or TNF-α (1 ng/mL) (C) for the indicated time intervals. At the end of incubation the cells were treated for TF activity assay as described in Materials and Methods. In a parallel set of samples total RNA was extracted from cells, and equal amounts of RNA were analyzed by Northern blot as described in Materials and Methods. Each point represent the mean of four experiments ± SEM. **P < .01, and *P < .05 versus time zero.
Northern blot analysis on TF mRNA.A similar shift in the kinetics was also observed when TF mRNA levels were studied. These experiments were conducted in parallel to those performed for determining the time course of TF activity, ie, HUVECs from the same umbilical cord and monocytes from the same donor were used on the same day. Monocytes, rIL-1β, or rTNF-α were incubated with HUVECs for different time intervals, after which the monolayer was extensively washed and the cells were treated in order to isolate mRNA, as described in Materials and Methods. Exposure of HUVECs to either rIL-1β or rTNF-α resulted in induction of a major 2.2-kb TF mRNA, consistent with the mature TF message (Fig 6, B and C, inserts). A minor TF transcript of 3.4-kb was also detected, in agreement with previous observations.35 TF mRNA, which was not detectable in unstimulated HUVECs, accumulated to maximal levels within 1 hour. A progressive decrease in TF mRNA levels followed, and by 8 hour postinduction, approximately 5% of maximal levels was detectable on the basis of band analysis by Instant Imager (Packard, Downers Grove, IL). Conversely, when monocytes were incubated with HUVECs the kinetic analysis showed that TF mRNA attained a peak at 4 hours, which was still consistent at 8 hours, and decreased to baseline value within 16 hours (Fig 6A, insert). Rehybridization with a GAPDH as internal control showed bands of similar intensity (not shown), confirming that equivalent amounts of cellular RNA had been loaded onto each lane, and that the increase in TF mRNA was specific.
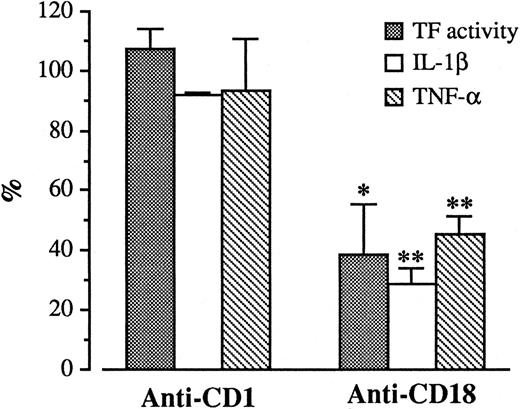
Role of adhesion molecules in TF generation.Monocyte interactions with endothelium are mediated via adhesive mechanisms. We explored the possibility that some of these mechanisms could be involved in TF expression. In our experimental conditions, monocytes adhered to HUVECs after a 4 hour incubation (120 ± 15 monocytes/mm2, by Diff Quick, as described in Materials and Methods). The number of adherent monocytes was greatly reduced in the presence of MoAb anti-CD18 (40 ± 4 monocytes/mm2 ), while MoAb anti-CD1, used as control, had no effect (140 ± 21 monocytes/mm2 ). Concomitantly, TF generation by HUVECs incubated with monocytes for 4 hours was reduced by 60.8% ± 16.3% when the anti-CD18 was present during the coculture (Fig 7). No effect could be observed with anti-CD1. Since our results show that IL-1β and TNF-α are responsible for HUVEC stimulation, the reduced induction of TF expression should correlate with a diminished synthesis of the two cytokines. We explored this hypothesis by measuring IL-1β and TNF-α antigen levels in supernatants from the same wells in which TF activity was measured. As shown in Fig 7, the anti-CD18 MoAb reduced IL-1β and TNF-α levels by 54.6% ± 6.5% and 71.3% ± 5.8%, respectively.
Effect of anti-CD18 on TF activity, IL-1β, and TNF-α levels during HUVEC/M cocultures. HUVEC (2 × 105/well) were incubated with monocytes (4 × 105/well) in the presence or in the absence of anti-CD18 or anti-CD1 for 4 hours. At the end of incubation, supernatants were collected and IL-1β and TNF-α antigen levels were measured by ELISA assay. After washing, HUVEC monolayers were tested for TF activity as described. Results are expressed in percent of TF activity, and percent of IL-1β and TNF-α antigen levels. A 100% value was assigned to the activity and antigen levels expressed by HUVEC/M coculture in the absence of anti-CD18. Bars represent the mean of three experiments ± SEM. **P < .005 and *P < .05 versus control (100%).
Effect of anti-CD18 on TF activity, IL-1β, and TNF-α levels during HUVEC/M cocultures. HUVEC (2 × 105/well) were incubated with monocytes (4 × 105/well) in the presence or in the absence of anti-CD18 or anti-CD1 for 4 hours. At the end of incubation, supernatants were collected and IL-1β and TNF-α antigen levels were measured by ELISA assay. After washing, HUVEC monolayers were tested for TF activity as described. Results are expressed in percent of TF activity, and percent of IL-1β and TNF-α antigen levels. A 100% value was assigned to the activity and antigen levels expressed by HUVEC/M coculture in the absence of anti-CD18. Bars represent the mean of three experiments ± SEM. **P < .005 and *P < .05 versus control (100%).
DISCUSSION
Monocytes adhere to endothelial cells. Although maximal adhesion is achieved with cytokine-stimulated endothelial cells,24,36 several reports have shown that monocytes spontaneously adhere to resting endothelial cells.37-40 In the present report, we show that the coincubation of purified monocytes with confluent HUVECs results in TF activity generation in the absence of exogenously added stimuli. This activity correlates with the monocyte number and requires mRNA and de novo protein synthesis.
When HUVECs or monocytes are exposed to supernatant from HUVEC/monocyte cocultures, only HUVECs are able to generate TF activity. This activity is in the same order of magnitude as that expressed by HUVEC/monocyte coculture. Thus the TF activity generated during the coculture is exclusively derived from HUVECs, and soluble mediators are involved, suggesting that exposure of monocytes to endothelial cells may create a stimulatory environment for monocytes sufficient to induce synthesis and release of molecules by these cells. For example, it has been shown that monocytes adherent to endothelial cells express monocyte-derived MIP-1α and MCP-1.30 31 Molecules synthesized and released by monocytes could then be responsible for TF synthesis and expression by HUVECs. This hypothesis is corroborated by experiments in which α-amanitin, a specific inhibitor of RNA polymerase II, was used to block RNA synthesis by HUVECs and monocytes, alternatively. The largely reduced TF activity observed under this condition clearly shows that both cell types are contributing with RNA and protein synthesis for HUVECs to express TF on their membrane.
The monocyte secretory cytokines IL-1β and TNF-α, which have the ability to shift the hemostatic balance of vascular endothelial cells toward a prothrombotic state, could likely be the monocyte-derived products responsible for induction of TF activity in HUVECs. Indeed, our ELISA studies show that the antigens of IL-1β and TNF-α are present in conditioned medium from HUVEC/monocyte coculture, and that their amount is sufficient to provoke TF synthesis by HUVECs, as assessed in dose-response experiments in which recombinant IL-1β and TNF-α were incubated with HUVECs. These findings are in agreement with previous studies, which suggested the presence of IL-1β26,28,41 and TNF-α28 during cocultures of HUVECs with monocytes or mixed leukocyte suspensions. Moreover, TF activity, both in the intact cell system and in HUVECs exposed to coculture supernatant, could be diminished by inhibitory antibodies directed against IL-1β and TNF-α, establishing a direct involvement of IL-1β and TNF-α in TF synthesis by HUVECs.
Activation of HUVECs with monocytes resulted in TF activity and mRNA levels which were delayed in their expression when compared with activation with rIL-1β and rTNF-α. This set of experiments, together with the results observed with α-amanitin, are consistent with the hypothesis that, to elicit HUVEC procoagulant response, synthesis of soluble mediators has to take place. Monocyte interactions with endothelium are dependent on the recognition by surface adhesion molecules, expressed on both monocyte and endothelial cell membrane. The leukocyte β2 integrin family (CD11/CD18) is important for the binding of monocytes to resting endothelium; antibodies directed against CD18 inhibit monocyte adherence to endothelial cells37-40 as was also found in our experimental conditions. In the presence of anti-CD18, adherence of monocytes to HUVECs was reduced by ∼60%. This inhibition in binding was accompanied by a marked reduction in TF activity generation.
Since in our experiments HUVEC TF synthesis was caused by monocyte secretory cytokines IL-1β and TNF-α, and inhibition of monocytes binding to HUVECs by anti-CD18 resulted in diminished TF activity, CD18-mediated adhesion could represent a regulatory pathway for monocyte activation and cytokine expression. Indeed, the finding that, in the presence of anti-CD18, IL-1β and TNF-α antigen levels are reduced during the cocultures, seems to sustain this hypothesis. This finding is consistent with the observation that engagement of members of the adhesion molecule family initiates signal transduction leading to expression of a number of immediate-early genes. Release of cytokines from monocytes can be triggered following engagement of surface receptors with MoAbs immobilized on plastic.42,43 However, the engagement of β2 integrins with specific antibodies as surrogate ligands seems not to provide the necessary signals for mRNA expression or protein secretion.44,45 Although receptor engagement might mimic the receptor-ligand interaction that mediates cell-cell adhesion, it is conceivable that during cell-cell interaction, more than a single adhesion molecule or signal could take place. Indeed, the anti-CD18 antibody only partially blocks monocyte adhesion and cytokine production, suggesting the involvement of other adhesion mechanisms or soluble signals as costimulatory agents in the response. A previous report showed that binding of monocytes to HUVEC could not elicit TNF-α secretion in the absence of LPS.45 However, in this case, gluteraldehyde-fixed HUVEC monolayers were used, a condition which could, at least in part, affect HUVEC/monocyte interaction. Moreover, a different sensitivity of the assays used for TNF-α detection (ELISA v L929 cytotoxicity assay) should be taken into account.
TF is widely considered to play a leading role in fibrin deposition during inflammatory and thrombotic disorders. Endothelial cells and monocytes are the only cells in the blood stream capable of expressing TF. In order to prevent disseminated intravascular coagulation, TF expression must be localized and limited at sites of endothelial cell injury or perturbation, where cell activation takes place. In this respect, a recent report has shown that adhesion of monocytes on cytokine-treated endothelial cells results in monocyte TF generation.46 The different experimental conditions could help understanding the apparent discrepancy with our results; indeed, these other investigators were using cytokine-activated endothelial cells, while, in our study, no exogenously added stimulus was used to activate HUVECs.
Wharram et al28 observed TF expression by HUVECs during HUVEC/M coculture, although LPS or/and aggregated IgG had to be present to produce a significant amount of TF. We have used an extreme precaution in our experiments to minimize LPS contamination, and no detectable amounts of this trigger could be found in our system. Moreover, polymixin B could not prevent TF activity generated during the cocultures, supporting the hypothesis that the observed TF activity was not attributable to LPS contamination. Although the different results could be explained, at least in part, by differences in calibration systems, such as the different thromboplastin standard used for calculating TF activity arbitrary units, it should be noted that these investigators were using a ratio of 1 M:8-10 HUVECs, at an M concentration of approximately 75,000/mL, approximately one tenth of that found in blood. In our experimental conditions, the ratio of 2 M:1 HUVEC used could account for the results obtained.
Also pertinent to our results, lately, it has been reported that TF antigen could be detected during coculture of HUVECs with monocytes, although most of it was claimed to be expressed on monocytes.47 The use of subconfluent HUVECs, which are still undergoing mitosis, and the detection of TF antigen instead of activity are some of the different experimental conditions that may render it difficult to compare their results with ours.
We have thus reported here the original observation that, in the absence of exogenously induced activation, HUVEC/monocyte interaction results in upregulation of TF expression in HUVECs. On the basis of these results, it is conceivable to speculate that, in pathological conditions, such as postoperative venous thrombosis, in which blood flow is reduced and circulating cells more easily interact with vascular cells, adherence of monocytes to an unperturbed endothelial layer would create a cell-cell functional cross-talk resulting in endothelial cell TF expression; this trigger would eventually lead to fibrin deposition and thrombus formation.
ACKNOWLEDGMENT
The authors thank their colleagues at the Department of Vascular Medicine and Pharmacology for critical reading of this manuscript and fruitful discussions, A. Di Castelnuovo for statistical analysis, R. Bertazzi for assistance, and the G.A. Pfeiffer Memorial Library staff for their help in the preparation of the manuscript.
Supported in part by the Italian National Research Council (CNR, Rome, Italy), Progetto Finalizzato FATMA contract No. 95.00951.41, and Convenzione CNR-Consorzio Mario Negri Sud.
Address reprint requests to Roberto Lorenzet, PhD, Consorzio Mario Negri Sud, Via Nazionale, 66030 S Maria Imbaro, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal