Abstract
We have examined the pattern of human globin gene switching in transgenic mice containing three different γ and β gene constructs (HS2GγAγδβ, HS2Aγβneo, and HS2Aγenβ) and compared the results with previously described transgenics (HS2Aγβ, HS2GγAγ-117δβ, and LCRεGγAγδβ). Developmental regulation was observed in all cases with identical patterns in lines bearing the same construct. Three different patterns of switching were observed: LCRεGγAγδβ and HS2Aγβneo mice switched rapidly, HS2GγAγδβ and HS2GγAγ-117δβ at an intermediate rate, and HS2Aγβ and HS2Aγenβ mice showed delayed switching, with a plateau in late fetal-early neonatal life and readily detectable levels of γ mRNA in adults. No difference was observed in the time of switching of the HS2GγAγδβ mice compared with those with the Aγ-117 hereditary persistence of fetal hemoglobin mutation, but adult levels of γ mRNA were significantly higher (≈5%) in lines carrying the mutation than in those without (≈1%). Reversion to the rapid switch of the LCRεGγAγδβ mice was observed in three lines with the HS2Aγβ neo construct in which expression of the tk-neo gene was approximately equal to that of the globin genes. The inclusion of the Aγ enhancer in HS2Aγβ mice did not alter the pattern of switching, or reduce the relatively high levels of γ mRNA in these lines. However, unlike other HS2 mice, the combination of HS2 and the Aγ enhancer resulted in copy number-dependent expression in HS2Aγenβ lines, with intrauterine death at ≈12.5 days gestation at high copy numbers. These results demonstrate that numerous elements throughout the β globin gene cluster interact to produce the correct pattern of developmental regulation of these genes. Furthermore, extinction of γ gene expression in adult life is not completely autonomous and is incomplete when HS2 is the only LCR element present.
THE HUMAN β GLOBIN gene cluster that lies on the short arm of chromosome 11 contains five expressed genes together with an upstream regulatory locus control region (LCR), which consists of four elements (HS1 to HS4) marked by erythroid specific DNase I hypersensitive sites (HS).1 The genes are organized in the order of their expression during development, 5′LCR-ε-Gγ-Aγ-δ-β-3′, with the ε gene expressed in embryos, the duplicated γ genes transcribed in fetal life, and the δ and β genes mainly expressed in adults. Switching from γ to β gene expression occurs in the perinatal period and is unrelated to birth.
Transgenic mice have been used extensively to analyze the regulation of globin gene expression. In the absence of the LCR, expression of the human transgenes is very low or absent, being strongly influenced by the position of integration.2,3 The LCR confers erythroid specific, high level, copy number-dependent expression on the transgene,4,5 with three of its constituent elements (HS2, HS3, HS4) also capable of directing position independent expression of a linked gene at high, but not maximum, levels.3 6-13
Developmental regulation of the globin genes has also been studied in transgenic mice. Mice lack a fetal hemoglobin, having two genes, ε and βh1, which are differentially expressed in embryonic life. In the absence of the LCR, human γ and β genes, if transcribed in transgenic mice, are developmentally regulated with expression in embryonic and fetal/adult stages, respectively, indicating that sequences important for stage-specific expression lie in or around the genes themselves.14-16 In the presence of the LCR, or one of its HS elements, the γ gene is highly expressed in embryonic/fetal life, declining in adults to a greater17 or lesser18,19 degree, depending on the construct used and the number of copies present. It has been suggested that extinction of the γ gene, like that of the ε gene, is autonomous in that no other genes are necessary for its silencing.17 In contrast, when the β gene is attached to the LCR, it is expressed throughout development, only becoming correctly regulated if a γ gene is placed between it and the LCR.18,19 This suggests that competition from the γ gene is necessary to suppress β gene expression in embryos. Additional experiments have demonstrated that developmental regulation of γβ gene constructs only requires a single HS element,20-22 that individual elements may influence the developmental pattern differently,23 and that the order of the genes affects their regulation.24,25 Transfer of the complete human β globin gene complex, either as stitched together cosmid fragments26 or on yeast artificial chromosomes27 28 has now provided the normal pattern of switching when the whole LCR and all of the functional genes are in their natural organization. Expression of the ε gene is detectable in yolk sac-derived blood cells from 8.5 to 12.5 days gestation, peaking at 10.5 to 11.5 days. The human γ genes, which are expressed at higher levels than ε, are also expressed at high levels in embryonic blood from days 8.5 to 12.5, but continue to be transcribed at lower levels in the fetal liver until 16.5 days. Only occasional γ chain-containing cells are found in adult life. The pattern of human β gene expression follows that of the adult mouse β gene, βmaj , becoming detectable in the fetal liver at 11.5 days and totally replacing γ gene transcription by 16.5 days.
We21 and others20 have previously shown that, although HS2 is sufficient for γ to β gene switching, transgenic mice bearing HS2 directed γ and β gene expression show a delayed pattern of switching compared with that seen with the whole β globin cluster and one which is incomplete, with γ gene expression levels of up to 10% in adults. Although such constructs show position independence (in that all positive lines show readily detectable levels of expression), in our hands, they do not provide copy number-dependent expression levels. We now present developmental expression data on additional constructs to determine which of these aspects are specific to HS2 constructs. The results show (1) three different patterns of human globin gene switching; (2) only a small effect of an Aγ gene promoter mutation (which in humans gives rise to a markedly increased HbF level in adult life) in a large HS2 construct; (3) a change in switching pattern from delayed to rapid when a neomycin resistance gene is added to the 3′ end of an HS2Aγβ construct; (4) no effect of the Aγ enhancer on the switching pattern of HS2Aγβ mice; (5) copy number-dependent expression levels when the Aγ enhancer region is included in the HS2Aγβ gene construct; and (6) incomplete γ gene extinction in the HS2 constructs compared with those containing the whole β globin LCR.
MATERIALS AND METHODS
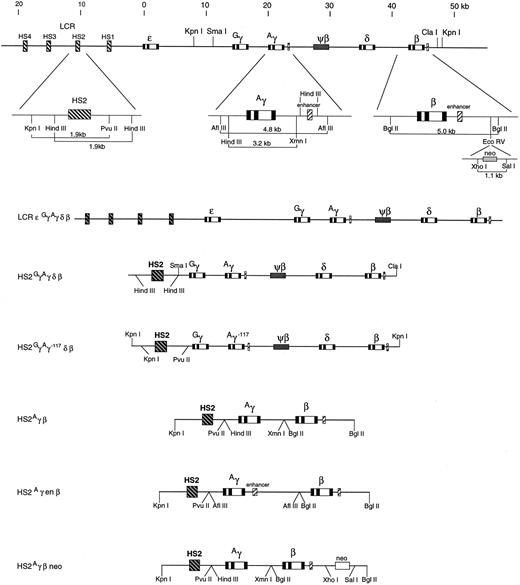
Constructs.The organization of the human β globin gene complex and the globin gene constructs used in this study appear in Fig 1. The HS2GγAγδβ construct (kindly provided by Dr P. Fraser, Erasmus University, Rotterdam, The Netherlands), consists of a 1.9-kb HindIII HS2 fragment inserted into a unique Sma I site, ≈3.5 kb upstream from the Gγ gene of cosHG28, which contains 36 kb of DNA including GγAγδ and β genes.23 This HS2 fragment differs slightly from the Kpn I-Pvu II fragment used in the other constructs, but both contain the core element together with surrounding sequences. The HS2Aγβ neo construct29 has a 1.1-kb Xho I-Sal I fragment from pMC1neo poly A (Stratagene, Cambridge, UK), containing the neomycin resistance gene under the control of a Herpes simplex virus thymidine kinase promoter. This was inserted into the EcoRV site 3′ to the β globin gene in the pN2γβ1 plasmid of Morley et al30 in the same 5′ → 3′ orientation as the globin genes. HS2Aγenβ was constructed by blunt ended ligation of a 4.8-kb Afl III fragment, containing the Aγ gene and its 3′ enhancer, into the Bgl II site of pNIIβ1530 to give pNAγenβNR. The globin gene fragment was released with Not I and gel purified before injection.
The organization of the human β globin gene complex and the globin gene constructs used in this study. At the top is the β globin gene cluster below which are expanded segments of the HS2, Aγ, and β globin genes (not to scale) showing the fragments used in the various constructs. The six constructs generated are shown below.
The organization of the human β globin gene complex and the globin gene constructs used in this study. At the top is the β globin gene cluster below which are expanded segments of the HS2, Aγ, and β globin genes (not to scale) showing the fragments used in the various constructs. The six constructs generated are shown below.
Transgenic mice.LCRεGγAγδβ transgenic mice, line 72, were kindly provided by Drs J. Strouboulis and F. Grosveld (NIMR, London, UK). HS2Aγβ and HS2GγAγ-117δβ mice were described previously21; further analysis from the earlier studies has led to revised copy number estimates in these lines.
New lines of transgenic mice were produced by microinjection of DNA into the pronuclei of fertilized C57B6 × CBA mouse eggs.31 Positive mice were identified by analysis of tail DNA and lines were produced by mating to (C57B6 × CBA) F1 mice. For expression studies, male F1 or F2 transgenic mice were mated to wild-type (C57B6 × CBA) F1 females; the morning on which a copulatory plug was observed was considered 0.5 days postcoitus.
DNA analysis.Restriction enzyme analysis by Southern blotting followed standard methods using HS2, γ and β globin,21 and neo (Xho I-Sal I fragment from pMClneo poly A) gene probes as appropriate to identify 5′ and 3′ junction fragments and tandem repeat bands. Gene copy numbers were obtained by comparison of DNA from F1 transgenic mice with a series of standard dilutions of a γ gene plasmid in normal mouse DNA, using the mouse erythropoietin gene as an internal control for DNA concentration.21
RNA analysis.RNA was prepared by the method of Chomczynski and Sacchi32 and was analyzed by RNase protection assay.21,33 Probes for the mouse and human globin genes have been described previously.21 For analysis of neo mRNA, a 417-bp Nar I-Xho I fragment from pMCIneopolyA (Stratagene), containing the transcription start site of the neo gene plus additional 5′ sequence, was ligated into pSP73 vector (Promega, Madison, WI) after cutting with Cla I and Sal I to generate pSPneoNR. After linearization with HindIII and transcription from the SP6 promoter, the 436-bp probe gives rise to a protected fragment of 162 bp.
The amount of RNA hybridized varied from 0.1 to 2.0 μg depending on source (intact embryos, embryonic blood, fetal liver, adult blood, and spleen) and was demonstrated to be in probe excess by running controls with a twofold to threefold increased amount of RNA. For accurate quantitation of RNAs present in low amounts (eg, β in early embryos, γ in adults), 10-fold more RNA was hybridized with the probe for the less abundant RNA than was hybridized with that for the more abundant RNA. The two hybridization mixtures were mixed after overnight incubation and before RNase treatment.
RESULTS AND DISCUSSION
Transgenic lines were generated with the constructs HS2Aγβneo, HS2Aγenβ, and HS2GγAγδβ. Copy numbers of the new lines were obtained from Southern blots compared with a series of standards ranging from 1 to 64 copies. The copy numbers of the new lines, together with their expression levels in adult animals are given in Table 1 and compared with the updated results for the HS2Aγβ, HS2GγAγ-117δβ, and LCRεGγAγδβ lines published previously.21 26 Full developmental profiles of γ and β mRNA levels were performed by RNase protection assay on all these lines, and the results are illustrated in Fig 2 and summarized in Fig 3.
Copy Number and Expression Data of the Various Lines Used in This Study
| Construct . | Line . | Transgene Copy No. . | % Human γ+β/Mouse α . | Expression . | γ/γ+β mRNA % in Adults . |
|---|---|---|---|---|---|
| . | . | . | Globin mRNA (in adults) . | per Copy % . | . |
| HS2 Aγβ | M2 | 6 | 39.2 ± 8.7 | 13.1 | 17.2 ± 5.3 |
| (31.5-57.5) | (5.0-19.4) | ||||
| T1 | 32 | 34.0 ± 10.8 | 2.1 | 9.5 ± 2.4 | |
| (24.7-56.8) | (6.9-14.0) | ||||
| HS2 GγAγ−117 δβ | J14 | 3 | 37.5 ± 7.5 | 25.0 | 4.7 ± 2.2 |
| (28.1-49.2) | (2.0-13.1) | ||||
| J17 | 7 | 31.3 ± 8.8 | 8.9 | 6.1 ± 2.0 | |
| (25.2-43.4) | (2.5-7.9) | ||||
| J21 | 24 | 26.6 ± 5.7 | 2.2 | 5.0 ± 2.5 | |
| (20.7-35.5) | (2.1-10.4) | ||||
| HS2 GγAγ δβ | R3 | 2 | 10.4 ± 3.1 | 10.4 | 0.6 ± 0.3 |
| (6.6-15.0) | (0.3-0.9) | ||||
| R2 | 6 | 40.5 ± 16.0 | 13.5 | 1.5 ± 0.4 | |
| (13.5-67.4) | (1.0-1.9) | ||||
| HS2 Aγβ neo | N2 | 6 | 15.4 ± 5.4 | 5.1 | 2.6 ± 1.0 |
| (10.1-22.9) | (1.1-3.4) | ||||
| N3 | 8 | 10.7 ± 3.3 | 2.7 | 2.6 ± 1.2 | |
| (8.2-17.1) | (1.1-3.4) | ||||
| N4 | 16 | 9.2 ± 2.1 | 1.1 | 3.4 ± 0.8 | |
| (7.2-14.0) | (1.4-4.2) | ||||
| HS2 Aγ en β | P5 | 1 | 11.7 ± 6.2 | 22.4 | 8.8 ± 3.6 |
| (5.0-24.2) | (5.5-17.5) | ||||
| P2 | 3 | 45.3 ± 7.3 | 30.2 | 8.7 ± 6.2 | |
| (32.1-57.4) | (1.5-20.5) | ||||
| P6 | 6 | 60.2 ± 11.5 | 20.1 | 10.4 ± 2.6 | |
| (32.4-78.6) | (7.3-14.8) | ||||
| P7 | 12 | 104.0 ± 29.5* | 17.3 | — | |
| (62.9-152.2) | |||||
| P2W | 40 | 153.5 ± 33.9* | 7.7 | — | |
| (104.0-209.5) | |||||
| LCR εGγAγδβ | 72 | 1 | 39.6 ± 12.7 | 79.2 | 0.2 ± 0.2 |
| (26.1-61.3) | (0.1-0.7) |
| Construct . | Line . | Transgene Copy No. . | % Human γ+β/Mouse α . | Expression . | γ/γ+β mRNA % in Adults . |
|---|---|---|---|---|---|
| . | . | . | Globin mRNA (in adults) . | per Copy % . | . |
| HS2 Aγβ | M2 | 6 | 39.2 ± 8.7 | 13.1 | 17.2 ± 5.3 |
| (31.5-57.5) | (5.0-19.4) | ||||
| T1 | 32 | 34.0 ± 10.8 | 2.1 | 9.5 ± 2.4 | |
| (24.7-56.8) | (6.9-14.0) | ||||
| HS2 GγAγ−117 δβ | J14 | 3 | 37.5 ± 7.5 | 25.0 | 4.7 ± 2.2 |
| (28.1-49.2) | (2.0-13.1) | ||||
| J17 | 7 | 31.3 ± 8.8 | 8.9 | 6.1 ± 2.0 | |
| (25.2-43.4) | (2.5-7.9) | ||||
| J21 | 24 | 26.6 ± 5.7 | 2.2 | 5.0 ± 2.5 | |
| (20.7-35.5) | (2.1-10.4) | ||||
| HS2 GγAγ δβ | R3 | 2 | 10.4 ± 3.1 | 10.4 | 0.6 ± 0.3 |
| (6.6-15.0) | (0.3-0.9) | ||||
| R2 | 6 | 40.5 ± 16.0 | 13.5 | 1.5 ± 0.4 | |
| (13.5-67.4) | (1.0-1.9) | ||||
| HS2 Aγβ neo | N2 | 6 | 15.4 ± 5.4 | 5.1 | 2.6 ± 1.0 |
| (10.1-22.9) | (1.1-3.4) | ||||
| N3 | 8 | 10.7 ± 3.3 | 2.7 | 2.6 ± 1.2 | |
| (8.2-17.1) | (1.1-3.4) | ||||
| N4 | 16 | 9.2 ± 2.1 | 1.1 | 3.4 ± 0.8 | |
| (7.2-14.0) | (1.4-4.2) | ||||
| HS2 Aγ en β | P5 | 1 | 11.7 ± 6.2 | 22.4 | 8.8 ± 3.6 |
| (5.0-24.2) | (5.5-17.5) | ||||
| P2 | 3 | 45.3 ± 7.3 | 30.2 | 8.7 ± 6.2 | |
| (32.1-57.4) | (1.5-20.5) | ||||
| P6 | 6 | 60.2 ± 11.5 | 20.1 | 10.4 ± 2.6 | |
| (32.4-78.6) | (7.3-14.8) | ||||
| P7 | 12 | 104.0 ± 29.5* | 17.3 | — | |
| (62.9-152.2) | |||||
| P2W | 40 | 153.5 ± 33.9* | 7.7 | — | |
| (104.0-209.5) | |||||
| LCR εGγAγδβ | 72 | 1 | 39.6 ± 12.7 | 79.2 | 0.2 ± 0.2 |
| (26.1-61.3) | (0.1-0.7) |
Expression values are given as mean and SD of 4 to 15 animals with the range given in parentheses below.
These values are from 9.5 to 11.5 day embryos.
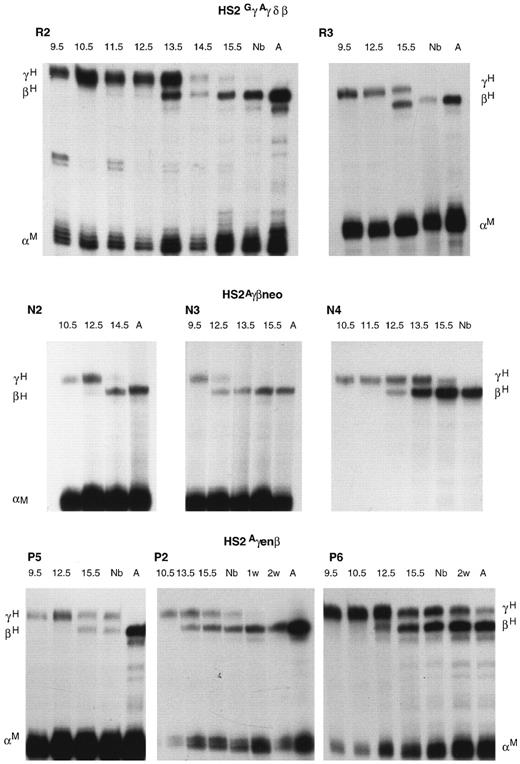
Developmental profiles of γ and β gene expression in the HS2GγAγδβ (R2, R3), HS2Aγβneo (N2, N3, N4), and HS2Aγenβ (P2, P5, P6) transgenic lines as determined by RNase protection assay. Nb, newborn, A, adult.
Developmental profiles of γ and β gene expression in the HS2GγAγδβ (R2, R3), HS2Aγβneo (N2, N3, N4), and HS2Aγenβ (P2, P5, P6) transgenic lines as determined by RNase protection assay. Nb, newborn, A, adult.
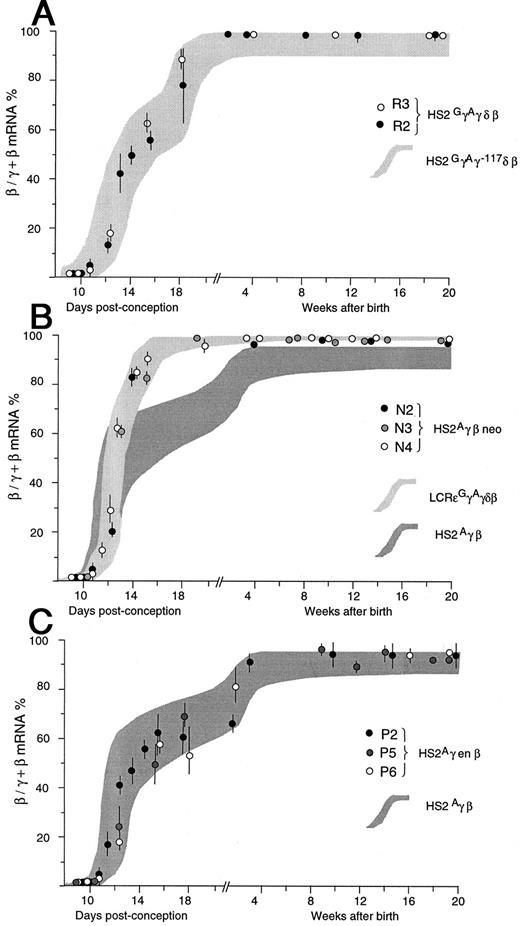
Diagrammatic representation of γ to β globin gene switching in (A) HS2GγAγδβ, (B) HS2γβneo, and (C) HS2Aγenβ transgenic lines. For comparison, the results obtained with HS2GγAγ-117δβ mice are included in (A), those for LCRεGγAγδβ and HS2Aγβ mice are shown in (B) and those for HS2Aγβ mice are illustrated in (C), as shaded areas. Each prenatal point is given as the mean value of transgenics from one to two litters (n = 3 to 12) with error bars representing 1 SD; values for adult animals are the same or are shown individually.
Diagrammatic representation of γ to β globin gene switching in (A) HS2GγAγδβ, (B) HS2γβneo, and (C) HS2Aγenβ transgenic lines. For comparison, the results obtained with HS2GγAγ-117δβ mice are included in (A), those for LCRεGγAγδβ and HS2Aγβ mice are shown in (B) and those for HS2Aγβ mice are illustrated in (C), as shaded areas. Each prenatal point is given as the mean value of transgenics from one to two litters (n = 3 to 12) with error bars representing 1 SD; values for adult animals are the same or are shown individually.
HS2GγAγδβ versus HS2GγAγ-117δβ transgenic mice.Transgenic mice (lines R2 and R3) were generated with the HS2GγAγδβ construct to determine whether the pattern obtained previously with the very similar HS2GγAγ-117δβ construct (Fig 1) was influenced by the Aγ gene promoter mutation. This mutation, G → A at position −117, occurs naturally in human populations, producing Greek hereditary persistence of fetal hemoglobin (HPFH) with HbF levels of 10% to 20% in adults.34,35 Two previous lines were generated with the HS2GγAγδβ construct, but full developmental studies were not performed.23 The pattern of globin gene switching in lines R2 and R3 are similar to the patterns of the J14, J17, and J21 lines containing the HPFH mutation (Fig 3A). Specifically, cells from both sets of mice contain approximately equal amounts of γ and β mRNA in the peripheral blood at 15.5 days gestation. In the fetal liver at this stage, the β/γ+β mRNA levels were ≈80%.
With neither construct is the γ gene completely switched off in adult life. The γ/γ+β mRNA levels of ≈1% in the HS2GγAγδβ lines are significantly lower than the ≈5% levels in the HS2GγAγ-117δβ transgenic adults (Fig 4 and Table 1). However, γ gene expression in all these lines is clearly greater than in LCRεGγAγδβ adults where it was almost undetectable (<0.2%; Fig 4).
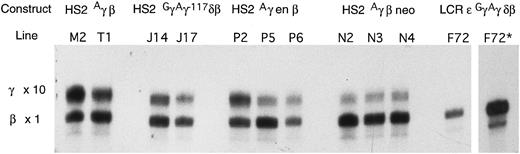
RNase protection assay to illustrate the γ/γ+β mRNA ratios in adults from the various transgenic lines. To obtain more accurate quantitation, a 10-fold greater amount of RNA was hybridized with the γ probe than with the β probe (see Materials and Methods). * The same as the preceding lane, but with a fivefold greater exposure time.
RNase protection assay to illustrate the γ/γ+β mRNA ratios in adults from the various transgenic lines. To obtain more accurate quantitation, a 10-fold greater amount of RNA was hybridized with the γ probe than with the β probe (see Materials and Methods). * The same as the preceding lane, but with a fivefold greater exposure time.
These results contrast with those of Berry et al36 who demonstrated that whereas γ gene expression was suppressed in adults carrying an LCRAγβ construct, mice with LCRAγ-117β gene showed equal levels of γ and β gene expression in adult life (later revised downwards to ≈25% γ/γ+β37). Furthermore, in mice carrying LCRAγ-117β and LCRAγ-114β (another HPFH mutation) constructs, there was a delay in the onset of β gene expression during development.36,37 In the absence of any LCR elements, the HPFH phenotype was not reproduced in GγAγ-117δβ transgenic mice.38 Our results, with a relatively small difference in adult γ mRNA levels together with the similarity in switching profiles of the HS2GγAγδβ and HS2GγAγ-117δβ mice, suggest that the −117 HPFH mutation has a much smaller effect in the setting of this large construct than in the smaller one. This discrepancy could indicate that the additional elements present in the LCR construct, but not in the HS2 constructs, are necessary to produce the HPFH phenotype in transgenic mice. Alternatively, the closer proximity of the regulatory LCR sequences to the γ gene in the LCRAγ-117β construct may favor expression of this gene, whereas in the HS2GγAγδβ constructs, a more natural arrangement is maintained.
HS2Aγβneo transgenic mice.The developmental pattern of γ and β gene expression was also studied in three lines of mice (N2, N3, and N4) transgenic for the HS2Aγβneo construct (Fig 1), originally generated for an unrelated purpose. Surprisingly, the pattern of switching in these mice (Figs 2 and 3B) did not follow the protracted switch of the previously generated HS2Aγβ mice, but showed the rapid switching pattern of the LCRεGγAγδβ mice. Expression of the γ gene was high in embryonic life, but was almost completely replaced by β gene expression by 16.5 days gestation. An identical pattern was observed in all three lines. Adult levels of γ mRNA averaged ≈3% (Fig 4 and Table 1).
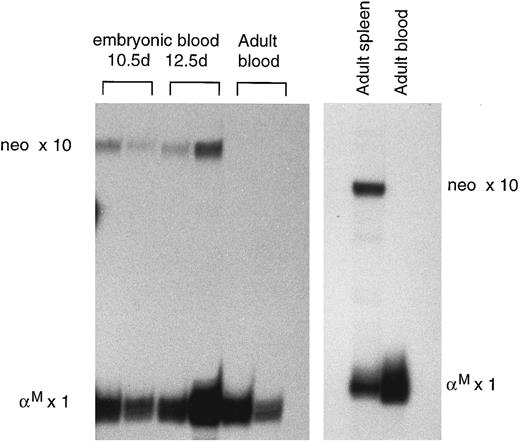
A feature of these lines was that the levels of human globin mRNA were consistently low relative to mouse mRNA compared with most of the other lines (Table 1). This was particularly marked in prenatal samples where the ratio of human γ + β mRNA over mouse α mRNA gave mean levels (± standard deviation [SD]) of 4.9% ± 1.0%, 5.4% ± 2.0%, and 4.4% ± 2.9% for N2, N3, and N4, respectively. In light of this ≈10-fold reduction in expression compared with the HS2Aγβ lines, we measured the levels of tk-neo mRNA. In the peripheral blood of normal adult transgenics, tk-neo mRNA ranged from undetectable to 0.2% of mouse α mRNA levels (Fig 5). In the peripheral blood of prenatal samples, in which there are greater numbers of immature erythroid cells, tk-neo mRNA levels were slightly higher (0.8% ± 0.5%) with the highest levels, up to 2%, found in embryonic samples. This observation, together with that showing that levels in fetal liver were twofold to fivefold higher than in corresponding blood samples, led us to suspect that the stability of the tk-neo mRNA was reduced relative to globin mRNA. To check this, we induced anemia in an adult N4 transgenic and measured the levels of tk-neo mRNA in the early erythroblasts forming in the spleen, as well as in mature peripheral blood cells. A 150-fold difference was observed, with respective ratios relative to mouse α mRNA of 3.0% and 0.02% (Fig 5). It would appear, therefore, that neo mRNA is considerably less stable than globin mRNA. Given this, together with the level of tk-neo mRNA in the spleen erythroblasts, it seems likely that the rate of transcription of the tk-neo gene in these mice is similar to that of the globin transgenes.
RNase protection assay of neomycin-resistance gene expression in mice from the N4 line. The adult blood RNA samples in the gel on the left were from untreated mice, while the adult blood and spleen samples in the gel on the right were from a mouse treated with acetylphenylhydrazine.
RNase protection assay of neomycin-resistance gene expression in mice from the N4 line. The adult blood RNA samples in the gel on the left were from untreated mice, while the adult blood and spleen samples in the gel on the right were from a mouse treated with acetylphenylhydrazine.
It has previously been shown that the LCR and its elements are capable of acting on nonglobin promoters,39,40 and it has also been shown that the LCR can apparently only activate one promoter at a time.41 Insertion of a neomycin resistance gene between most of the LCR and the globin genes leads to a marked downregulation of those genes in both mouse and human systems.42-44 This was presumed to be a result of preferential transcription of the tk-neo gene, although this was not demonstrated. We have now shown that transcription of the tk-neomycin gene occurs at a high level even when the gene is placed 3′ to the β globin gene and that this may well result in the reduced human globin RNA levels in these mice through competition between the globin and tk-neo gene promoters for access to the LCR element. It is still surprising that the thymidine kinase promoter that drives neo gene transcription should function so efficiently, because it lacks binding sites for any identified erythroid transcription factors. It is also not at all clear how tk-neo transcription would alter the developmental stage-specific selection of γ and β gene expression. As there would be no differences in any putative stage-specific transcription factors between the HS2Aγβ and HS2Aγβneo mice, one can only speculate that the altered chromatin structure brought about by having an active gene 3′ to the β globin gene in some way makes the β gene a preferential target over the γ gene for interaction with HS2 once switching is underway.
HS2Aγenβ transgenic mice.A small sequence 3′ to the Aγ gene has been demonstrated to act as an enhancer in transient transfection assays.45,46 More recently, it has been suggested that this region functions as a matrix attachment site and binds both SATB147 and HoxB2 proteins.48 Furthermore, it has previously been suggested that this region may be important for the suppression of γ gene activity in adult life.17,20-22 49
To determine more fully the role of this regulatory region, we generated six transgenic founder mice with the HS2Aγenβ construct, which is similar to the HS2Aγβ construct except that an extra 1.1 kb, including the Aγ 3′ enhancer, is present at the 3′ end of the γ gene component (Fig 1). Developmental regulation of three HS2Aγenβ lines (P2, P5, and P6) is shown in Figs 2 and 3C, and is similar to those reported earlier for the HS2Aγβ mice. Specifically, β gene expression starts at the usual time (12.5 days), but switching is slow relative to the LCRεGγAγδβ mice, with a pronounced plateau between 15.5 days gestation and 1 week after birth. Switching is not complete, with considerable variability in γ gene expression between adults (>8 weeks old) in these lines; γ/γ+β ratios ranged from 1% to 20% in heterozygous adults. This increased up to 37% in adult homozygotes of the P2 line suggesting that the increased chain imbalance, particularly in the homozygous state, may produce increased erythroid stress that results in higher γ gene expression. The γ/γ+β mRNA levels in adult mice of the three HS2Aγenβ lines were similar to those in the two HS2Aγβ lines (Fig 4 and Table 1). It does not appear, therefore, that the Aγ enhancer is sufficient to suppress expression of the γ genes in adult life.
Of the six transgenic founders generated with the HS2Aγenβ construct, one male failed to transmit the transgene (0 of 100 offspring, including 20 early embryos). When killed, this founder expressed the transgene at levels of 14.3% (relative to mouse α mRNA), with a β/β+γ mRNA ratio of ≈95%. One female founder that produced no positive newborns out of two litters, was killed at 12.5 days gestation and the embryos examined. Six normal looking embryos were negative for the transgene, whereas one dead, white embryo and one resorbing embryo were positive by DNA analysis.
One of the founders generated with the HS2Aγenβ construct, P2, transmitted the transgene to liveborn offspring, but was also found to produce a high proportion of embryos that were profoundly anemic and died in utero at 11.5 days gestation. DNA analysis showed that two separate integrants were segregating from this founder, the ones with low copy number (P2) surviving while those with a high copy number (P2W) died as embryos. A second founder with a relatively high copy number (P7) also gave rise to anemic embryos positive for the transgene that died in utero at 12.5 days gestation. Copy number analysis suggested that line P5 contained only a single copy of the transgene. Detailed restriction enzyme mapping confirmed this, with no head to tail tandem repeat band present and a single 5′ and 3′ junction fragment detected with several enzymes (data not shown).
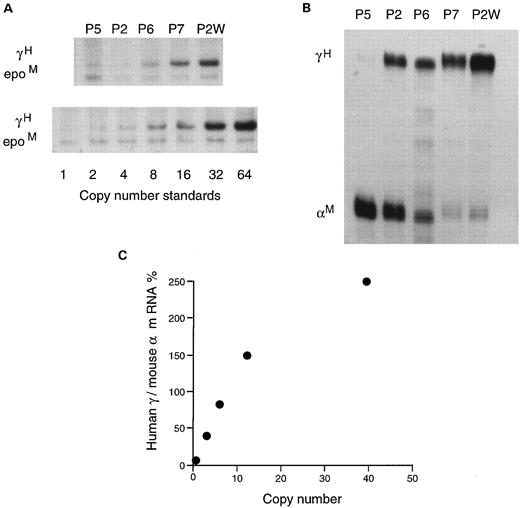
When the expression levels were compared with the copy numbers, the amount of human mRNA produced in these five lines was clearly copy number-dependent (Fig 6). Expression per copy averaged about 25% of mouse α gene expression in the lines with one to six copies. In the two higher copy number lines that resulted in intrauterine death, the absolute expression levels increased further, albeit with a slight reduction in expression per copy as has been observed previously when copy numbers are very high.
(A) Southern blot of DNA from the five HS2Aγenβ lines compared with copy number standards. (B) RNase protection assay of 10.5 days embryonic blood samples from the same lines, demonstrating the increase in the γ/αM ratio with increasing copy number. (C) Expression levels of human/mouse RNA relative to copy number in these lines.
(A) Southern blot of DNA from the five HS2Aγenβ lines compared with copy number standards. (B) RNase protection assay of 10.5 days embryonic blood samples from the same lines, demonstrating the increase in the γ/αM ratio with increasing copy number. (C) Expression levels of human/mouse RNA relative to copy number in these lines.
Transgenic mice containing the HS2Aγenβ gene construct were the only lines in which we have observed copy number-dependent expression. Although others have reported copy number dependence with HS2β gene constructs,6,50,51 this is not always observed,3,8,9,20 particularly with larger multigene constructs, and in some series there is an inverse relationship between expression per gene copy and copy number.10,21 The attainment of copy number dependence appears to be complex, with the involvement of multiple components in the LCR elements, promoter region and in the region downstream of the genes. Deletion of the NF-E2 binding site from the HS2 element in an HS2β gene construct resulted in a 20-fold drop in β globin gene expression, but maintenance of copy number dependence.50 Copy number dependent expression of a 215-bp core HS2 element attached to a β globin gene was observed, even when the GATA-1, USF, and J-BP (YY1) sites were individually mutated, but only when more than two copies were integrated in a tandem head to tail array.52 53 This suggests that cooperativity between at least two elements is necessary for copy number dependence. The fact that we observe high level expression from a single copy of the HS2Aγenβ construct suggests that the Aγ enhancer element can substitute for an LCR element to provide this function.
The importance of upstream promoter elements in copy number dependence was illustrated with μLCR-Aγ constructs that lacked the Aγ enhancer region.54 Copy number dependence was not observed when the γ gene promoter extended to position −730, but was obtained when it was truncated to −382 or −201. With a further truncation to −141, copy number dependence was lost once more. These results indicate that within the extended promoter region there are localized areas that can contribute to copy number dependence, but only in certain contexts.54 Neither HS2 nor HS3 elements could confer copy number dependence on an Aγ gene (lacking the 3′ enhancer), but both did so on Aγβ gene constructs in which the Aγ enhancer was present.22 In that study, it was argued that the β gene itself or the matrix attachment site in IVS2 of the β gene might be responsible for the copy number dependence. We suggest that on the basis of copy number dependence observed with the HS2Aγenβ transgenics, but not with the HS2Aγβ and HS2Aγβneo mice, the 3′ enhancer element is more likely to be providing this function. However, again this function appears to be context dependent. The enhancer is present in the HS2GγAγδβ and HS2GγAγ-117δβ lines, which do not show copy number dependence when their results are combined.
CONCLUSIONS
Comparison of the developmental profiles and levels of expression of the HS2GγAγδβ, HS2Aγβneo, and HS2Aγenβ transgenic lines with those reported previously for the HS2GγAγ-117δβ, HS2Aγβ, and LCRεGγAγδβ lines has demonstrated (1) a reduced effect of the Aγ-117 HPFH mutation in the context of a larger construct; (2) a major shift in the developmental pattern of γ and β gene expression as a result of the addition of the neo gene to the HS2Aγβ construct; and (3) copy number-dependent expression with the addition of the Aγ enhancer to the HS2Aγβ construct.
Three different patterns of globin gene switching were observed with these constructs. The consistent pattern of switching in different lines with the same construct indicates that it was not influenced by the site of integration, but was inherent to the transgene construct. Switching was most protracted in the HS2Aγβ and HS2Aγenβ lines in which there was a distinct plateau during late fetal and early neonatal life. Less protracted switching was observed in the HS2GγAγδβ and HS2GγAγ-117δβ lines, but the switch was still not completed until after birth, significantly later than in mice with the intact LCRεGγAγδβ complex. These differences were not due to having only the HS2 element rather than the whole LCR present, as rapid switching occurred in the HS2Aγβneo lines. These results suggest, therefore, that numerous elements throughout the β globin cluster play a role in switching. These include LCR elements, gene promoters, the number and spatial organization of the genes, as well as other regulatory elements. The combination of these factors in different constructs may determine the precise timing and pattern of switching.
The construct-dependent nature of switching has implications for the regulatory mechanisms responsible. It has been suggested that the loss of γ gene expression in fetal life is due to the interaction of repressor molecules with silencer elements in the upstream regions of the genes.17,55 However, the transcription factor content of the erythroid cells (including putative repressors) at different stages of development does not differ between lines and thus if stage-specific transcription factors were the sole determinants of switching, one would not expect the pattern to be so clearly construct-dependent. Therefore, it appears that between the different lines there must be construct specific, cis-active differences in the relative accessibility of the γ and β genes to stage-specific factors, possibly stabilized by epigenetic modifications.29
ACKNOWLEDGMENT
We thank Prof D.J. Weatherall for his continuing interest and support.
Supported by The Wellcome Trust, London, UK.
Address reprint requests to W.G. Wood, PhD, MRC Molecular Haematology Unit, Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DU, UK.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal