Abstract
The heat stable antigen (HSA, or murine CD24) is a glycosyl phosphatidylinositol-linked surface glycoprotein expressed on immature cells of most, if not all, major hematopoietic lineages, as well as in developing neural and epithelial cells. It has been widely used to stage the maturation of B and T lymphocytes because it is strongly induced and then repressed again during their maturation. Terminally differentiated lymphocytes, as well as most myeloid lineages, are negative for HSA. Erythrocytes are an exception in that they maintain high levels of HSA expression. HSA on naive B cells has been shown to mediate cell-cell adhesion, while HSA on antigen-presenting cells has been shown to mediate a costimulatory signal important for activating T lymphocytes during an immune response. Here, we characterize mice that lack a functional HSA gene, constructed by homologous recombination in embryonic stem cells. While T-cell and myeloid development appears normal, these mice show a leaky block in B-cell development with a reduction in late pre-B and immature B-cell populations in the bone marrow. Nevertheless, peripheral B-cell numbers are normal and no impairment of immune function could be detected in these mice in a variety of immunization and infection models. We also observed that erythrocytes are altered in HSA-deficient mice. They show a higher tendency to aggregate and are more susceptible to hypotonic lysis in vitro. In vivo, the mean half-life of HSA-deficient erythrocytes was reduced. When infected with the malarial parasite Plasmodium chabaudi chabaudi, the levels of parasite-bearing erythrocytes in HSA-deficient mice were also significantly elevated, but the mice were able to clear the infection with kinetics similar to wild-type mice and were immune to a second challenge. Thus, apart from alterations in erythrocytes and a mild block in B-cell development, the regulated expression of HSA appears to be dispensable for the maturation and functioning of those cell lineages that normally express it.
THE MATURATION of hematopoietic cells is associated with the regulated expression of numerous genes. Some of these genes encode cell surface receptors that mediate maturation-stage–specific signals into and out of the cell by binding to a variety of substances, such as soluble growth or maturation factors like interleukins or to adhesion receptors either on other cells or in the extracellular matrix. Many of these interactions are crucial for the ordered maturation of hematopoietic lineages. The heat stable antigen (HSA, mouse CD24) is one of these stage-specific genes. It is heavily glycosylated and anchored in the plasma membrane by glycosyl phosphatidylinositol.1-3 HSA is strongly expressed on most immature hematopoietic lineages, but usually absent from cells that have reached their final differentiation stage. Indeed, HSA has proven to be a very useful marker for characterizing the maturation stage of both B and T lymphocyte lineages,4-11 being maximally expressed at the time when the cells rearrange their antigen receptors. HSA is also expressed on myeloid precursors,3-9 as well as in developing neurons,12-14 regenerating muscle,15 dendritic cells,16,17 and epithelial cells.13
We have been interested in studying the role that the HSA might play in the maturation and function of B and T lymphocytes. Using either antibodies directed against HSA or by manipulating its expression on cells, HSA has been implicated in cellular adhesion18,19 and lymphocyte activation.20-24 However, the importance of HSA relative to other adhesion or signaling receptors is not known. We have previously reported that in chimeric mice bearing a mixture of hematopoietic progenitor cells, some normal and some deficient for HSA expression, B and probably T-lineage cells can mature without expressing HSA.25 Here, we describe the production and characterization of mice that completely lack HSA expression. We examined the maturation of hematopoietic cells in these mice, particularly lymphopoiesis, and describe a leaky block in the production of B lymphocytes. We have characterized the immune response in HSA-/- mice when challenged with a variety of antigens. We also present results suggesting that erythrocytes are altered in HSA-deficient mice.
MATERIALS AND METHODS
Construction of HSA targeted mice.The ES cell line N1, derived from C57BL/6 mice,26 was cultured, electroporated with the targeting vector pX62HBneo18TK, and homologous recombinants were identified by polymerase chain reaction (PCR) screening as previously described.25 Of 216 ES cell transfectants screened, 11 were PCR positive and eight of these were confirmed by Southern blotting to have one targeted allele and one wild-type allele for the CD24a gene. Targeted ES cell clones were microinjected into blastocysts derived from fertilized BALB/c females and transferred to pseudopregnant foster mothers. Chimeric offspring were paired with C57BL/6 mice and the resulting mice, heterozygous for the targeted deletion, were paired with each other to generate HSA-deficient mice.
For analysis of mRNA expression, total RNA was extracted from mouse organs, separated on denaturing agarose gels and hybridized with a mouse HSA cDNA probe by standard procedures as previously described.27 The analysis of the CD24a locus in genomic DNA by Southern blotting and hybridization has also been described.25
Many of the experiments described here were performed with HSA-/- mice derived from two different ES clones, and no difference in the results was observed. Mice were kept in restricted-access breeding facilities and were found to be negative in routine screens for common murine infectious agents.
Flow cytometry.The expression of cell surface antigens was examined on a FACScan cytometer (Becton Dickinson, San Jose, CA) using Lysis II or Cell Quest software (Becton Dickinson). Single-cell suspensions of lymphocytic organs and cellular labeling with antibody conjugates was done as previously described.25 Antibodies used were: anti-HSA, M1/69,28 fluorescein isothiocyanate (FITC); J11d,8 FITC; 79,18 biotin; or 20C9,20 FITC; anti-B220, RA3-6B2,29 biotin, phycoerythrin (PE) or FITC; anti-μa, RS3.1,30 biotin or FITC; anti-μb, AF6-78.25,31 biotin or FITC; anti-mCD4, GK1.5,32 biotin or FITC; anti-mCD8, 53-6.72,33 biotin; anti-Thy1.2, 30-H12,33 biotin or FITC; anti-BP-1 antigen, 6C3,34 biotin; anti-CD43, S7,35 biotin; anti-Ly5.1, A104.2.1,36 FITC or biotin; anti-Ly5.2, A20.1.737 FITC or biotin; anti Mac1, M1/70,28 FITC and biotin; and Gr1, RB6-8C5 (R.L. Coffman, DNAX Corp, Palo Alto, CA) biotin. Biotinylated antibodies were visualized with streptavidin coupled either to FITC, PE, or Red 670 (GIBCO-BRL, Gaithersburg, MD). Fc-receptors were blocked with 40 μg/mL anti-FcγRII antibody 2.4G2.37
Immunizations and infections.Serum antibody titers in immunized mice were determined using an enzyme-linked immunosorbent assay (ELISA) procedure described earlier.25 To measure a T-cell–dependent antigen response, five HSA-deficient and five C57BL/6 wild-type mice were immunized with 20 μg dinitrophenol (DNP)-ovalbumin (coupling ratio 20:1 DNP:ovalbumin) absorbed on alum and supplemented with a 1:200 dilution of heat-killed Bordetella pertussis. To generate the immunogen, 400 μg DNP-ovalbumin was mixed with 160 μL alum (Alu-Gel-S, Serva 12261) in a total volume of 320 μL. After incubation at room temperature for 1 hour, the insoluble alum with the adsorbed DNP-ovalbumin was washed three times with phosphate-buffered saline (PBS) and resuspended in 2 mL PBS. Then, 10 μL killed B pertussis (Behring Ch.B.393396) was added and 100 μL was injected per mouse, subcutaneous (SC) at the base of the neck. After 17 days, isotype-specific anti-DNP serum titers were determined by ELISA, and the mice were boosted with 20 μg DNP-ovalbumin intraperitoneally (IP). Serum anti-DNP antibodies were determined again after 7 and 14 days (the ELISA plates were coated with DNP-bovine serum albumin (BSA). A T-cell–independent response was measured by injection of five HSA-deficient and five C57BL/6 mice IP with 10 mg DNP-ficoll. After 5 days, the serum anti-DNP titers were measured.
For Listeria infections, virulent Listeria monocytogenes (strain EGD) were grown in trypticase-soy broth (Difco, Detroit, MI). Aliquots of log-phase cultures were stored at −70°C until use. For each experiment, a vial was thawed, bacteria were washed once in saline, and diluted in endotoxin-free PBS before injection. Infections (4 × 103 bacteria in 200 μL PBS) were performed by injection into the tail vein of five HSA-deficient and five wild-type C57BL/6 mice (7 weeks old). After 5 days, the bacterial titer in extracts of the liver of each mouse was determined by plating 10-fold serial dilutions on trypticase-soy broth agar plates. Plates were incubated at 37°C, and the number of colony-forming units (CFU) counted after 24 hours.
Borrelia infections were performed as previously described.38 Briefly, three HSA-deficient and three wild-type mice, all females, were infected with Borrelia burgdorferi strain ZS7 by SC injection at the tailbase of 100 μL PBS containing 1 × 108 bacteria. The mice were monitored for clinical signs of arthritis once per week beginning the second week after infection. After 10 weeks, all mice were killed and histopathology was performed on sections from lymph nodes, spleen, thymus, heart, liver, kidney, skeletal muscle, ear, brain, tongue, and knee joints. Serum was examined for titers of anti-B burgdorferi antibodies and skin biopsy from the ear was cultured to recover B burgdorferi as previously described.
For malaria infections, groups of five HSA-deficient and wild-type mice were infected with Plasmodium chabaudi chabaudi by IP injection of 1 × 105 infected erythrocytes (taken from an infected donor and diluted in 0.9% NaCl to give 1 × 106 infected erythrocytes/mL). To monitor parasitaemia, tail-blood thin smears on a glass slide were fixed with methanol and stained with 5% Giemsa, pH 7.2, for 15 minutes. Infected erythrocytes were counted and expressed as the percent of total erythrocytes counted. A minimum of 1,000 red blood cells (RBCs) were counted per sample. Mice were kept on a normal day/night cycle, infected at 12:00 noon, and samples were taken every day thereafter, also at 12:00 noon.
Erythrocyte analysis.For measuring the blood sedimentation rate, approximately 100 μL of fresh blood was collected from the tail vein into heparinized glass capillary tubes (Clay Adams, Parsippany, NJ; 75 mm long, 1.50 mm outer diameter [OD], 1.10 mm inner diameter [ID]). The tubes were plugged at the bottom with modeling clay and placed at approximately a 60° angle. At various times, the position of the RBC meniscus was measured.
For hypotonic lysis, heparinized blood was collected from the tail vein and 10 μL was added to 100 μL H2O containing NaCl at the following concentrations: 55, 60, 64, 68 72, 75, 79, 83, 85, 88, 90, 94, 97, and 103 mmol/L. After incubation for 2 minutes at room temperature, the samples were centrifuged at 1,500 rpm for 5 minutes and the absorbance of the supernatants was measured in a spectrophotometer at 550 nm.
To measure the half-life of RBCs, approximately 2 mL blood was collected from three anesthetized mice and immediately mixed with 200 μL acid citrate dextrose (45 mmol/L sodium citrate, 23 mmol/L citric acid, 74 mmol/L glucose, pH 5.0). Cells were pelleted by centrifugation and washed twice in PBS. An aliquot of 1 × 109 cells was stained with the red fluorescent cell linker PKH-26 as described by the supplier (Sigma, St Louis, MO; PKH26-GL) and then washed four times with PBS. Cells were resuspended in 0.2 mL PBS, mixed with 0.2 mL serum from the donor mice and injected via the tail vein into a recipient mouse. Stained erythrocytes were monitored by flow cytometry using a sample of heparinized tail blood taken at various times after injection.
Fluorescence-activated cell sorting (FACS) analysis of erythrocyte aggregation was measured by collecting blood from HSA + and - mice directly into FACS buffer (PBS, 3% fetal calf serum [FCS], 0.1% sodium azide). Cells were then analyzed on a FACStarPlus (Becton Dickinson) with a sheath pressure of 10 and a sample differential of either 0.5 or 3.0.
Bone marrow (BM) reconstitution of irradiated mice.Six groups of three Rag2-deficient mice each were irradiated in a single dose of 400 rad (whole body irradiation from an x-ray tube source, Phillips, Hamburg, Germany, MCN 321, 250 kV 10 mA, filters used were 1 mm Cu and 2.5 mm Al) and 3 hours later, 2 × 106 BM cells were injected via the tail vein (see Table 2). Donor BM cells were prepared by pooling the cells from four femurs of two HSA-deficient, C57BL/6, Ly5.1 mice and from four femurs of two age-matched C57BL/6, HSA-positive, Ly5.2 mice. BM cells were mixed in several proportions before injection into recipients. The chimerism of T and B cells in the blood was measured by labeling the lymphocytes for FACS analysis as described above from blood collected at 4, 28, and 119 days after repopulation. All animals were obtained from the specific pathogen-free breeding facility of the Max Planck Institute and all procedures were approved and performed according to the animal protection guidelines of the Institute. Irradiated mice were given autoclaved food pellets and the drinking water was supplemented with 0.16% Neomycin-sulfate. All irradiated mice not injected with BM died within 5 weeks after irradiation.
Reconstitution of Irradiated Rag2- Mice With HSA +/+ and HSA −/− BM
| Group* . | Day 28 B Cells . | Day 28 T Cells . | Day 117 B Cells . | Day 117 T Cells . | ||||
|---|---|---|---|---|---|---|---|---|
| . | 5.1 . | 5.2 . | 5.1 . | 5.2 . | 5.1 . | 5.2 . | 5.1 . | 5.2 . |
| A | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 |
| B | 94 | 6 | 83 | 17 | 95 | 5 | 92 | 8 |
| C | 60 | 40 | 43 | 57 | 64 | 36 | 56 | 44 |
| D | 8 | 92 | 11 | 89 | 14 | 86 | 9 | 91 |
| E | 0 | 100 | 0 | 100 | 1 | 99 | 1 | 99 |
| F | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 |
| G | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Group* . | Day 28 B Cells . | Day 28 T Cells . | Day 117 B Cells . | Day 117 T Cells . | ||||
|---|---|---|---|---|---|---|---|---|
| . | 5.1 . | 5.2 . | 5.1 . | 5.2 . | 5.1 . | 5.2 . | 5.1 . | 5.2 . |
| A | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 |
| B | 94 | 6 | 83 | 17 | 95 | 5 | 92 | 8 |
| C | 60 | 40 | 43 | 57 | 64 | 36 | 56 | 44 |
| D | 8 | 92 | 11 | 89 | 14 | 86 | 9 | 91 |
| E | 0 | 100 | 0 | 100 | 1 | 99 | 1 | 99 |
| F | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 |
| G | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| *BM Mix Injected | HSA- Ly5.1 BM Cells | HSA+ B6Ly5.2 BM Cells |
| A | 6 × 106 | 0 |
| B | 5.4 × 106 | 0.6 × 106 |
| C | 3 × 106 | 3 × 106 |
| D | 0.6 × 106 | 5.4 × 106 |
| E | 0 | 6 × 106 |
| F | Unirradiated C57BL/6-Ly5.2 mice | |
| G | Unirradiated Rag2 −/− mice | |
| *BM Mix Injected | HSA- Ly5.1 BM Cells | HSA+ B6Ly5.2 BM Cells |
| A | 6 × 106 | 0 |
| B | 5.4 × 106 | 0.6 × 106 |
| C | 3 × 106 | 3 × 106 |
| D | 0.6 × 106 | 5.4 × 106 |
| E | 0 | 6 × 106 |
| F | Unirradiated C57BL/6-Ly5.2 mice | |
| G | Unirradiated Rag2 −/− mice | |
RESULTS
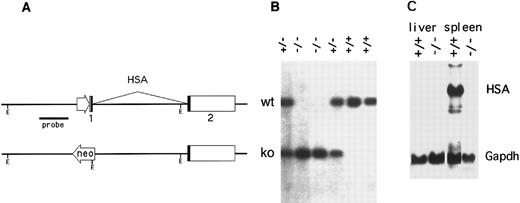
Construction of CD24-targeted mice.The mouse genome contains three HSA genes (CD24a, b, and c), two of which (CD24b and CD24c) appear to be inactive, intronless pseudogenes.27 Using the strategy and targeting vectors previously described,25 the functional murine CD24a gene was disrupted in C57BL/6 embryonic stem (ES)26 cells by replacing the promoter and first exon with a neomycin resistance expression cassette (Fig 1A). Chimeric offspring, produced by injecting blastocysts with ES cells carrying the targeted CD24a gene were then mated to C57BL/6 mice and germline transmission of the CD24 mutation was obtained. Correct targeting was confirmed by Southern and Northern blotting (Fig 1B and C). While HSA mRNA in the spleen of wild-type mice was strongly expressed, no HSA-specific mRNA was detected in the spleen of HSA-/- mice (Fig 1C).
Inactivation of the murine CD24a (HSA) gene. (A) The promoter and first exon of the CD24a gene was replaced by a neomycin-resistance expression cassette in mouse embryonal stem cells by homologous recombination. (B) A Southern blot of EcoRI digested tail DNA was hybridized with a HincII-BamHI genomic DNA probe derived from the region immediately 5′ to the targeted replacement. Mice heterozygous for the mutation (+/−) show bands from both the intact allele (upper band) and the targeted allele. (C) Northern blots of total RNA extracted from the spleen and liver of both wild type (+/+) and HSA-deficient mice (-/-) was sequentially hybridized with HSA and glyceraldehyde phosphate dehydrogenase cDNA probes.
Inactivation of the murine CD24a (HSA) gene. (A) The promoter and first exon of the CD24a gene was replaced by a neomycin-resistance expression cassette in mouse embryonal stem cells by homologous recombination. (B) A Southern blot of EcoRI digested tail DNA was hybridized with a HincII-BamHI genomic DNA probe derived from the region immediately 5′ to the targeted replacement. Mice heterozygous for the mutation (+/−) show bands from both the intact allele (upper band) and the targeted allele. (C) Northern blots of total RNA extracted from the spleen and liver of both wild type (+/+) and HSA-deficient mice (-/-) was sequentially hybridized with HSA and glyceraldehyde phosphate dehydrogenase cDNA probes.
Breeding.Mice homozygous for the targeted HSA locus were obtained and showed no gross physical or behavioral abnormalities. There was however, some distortion in the frequency with which the targeted allele was transmitted to the offspring. In matings where both parents were heterozygous for the deletion, the litter size was reduced from an average of 7.3 mice/litter in heterozygotes mated to wild-type mice (21 litters and 154 offspring) to 4.7 mice/litter (86 litters and 402 offspring). When both parents were homozygous for the HSA deletion, the litter size was also smaller (5.4 mice/litter, 47 litters and 255 mice). In addition, in heterozygote matings, only one of eight of the offspring were homozygous for the ko allele instead of the expected one of four. The reason for this reduced transmission frequency is not known.
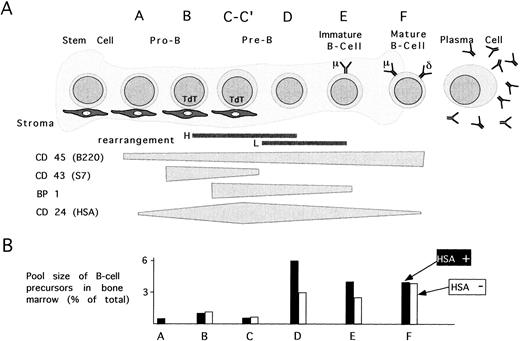
B-cell development in HSA-deficient mice.Because most hematopoietic lineages express HSA in immature stages and loose this expression during maturation, we first used FACS to characterize various hematopoietic lineages in the BM and thymus of HSA-deficient mice. Based on staining with several antibodies, B-cell development in the BM has been resolved into the ordered stages A-F according to increasing maturity (see Fig 3A).6 Stages A-C express the marker S7 (CD43) and stage C is also positive for BP-1. The later stages D-F are negative for S7 where stages E and F also express surface immunoglobulin. Ongoing immunoglobulin gene rearrangement occurs primarily in the late B to D stages for the heavy-chain and stages D-E for the light-chain. HSA expression is either absent or very low in stage A, appears in stage B, and increases to a maximum in late stage C cells. Lower levels are present on stages D-F and on peripheral, naive B cells (see Fig 3A for summary).
The effect of HSA-deficiency on B-cell maturation. (A) B-cell maturation in BM is schematically divided into stages A-F according to Hardy et al6 and is based on the regulated expression of the surface markers CD45, CD43, BP1, CD24, IgM, and IgD. (B) The cellular pools size for each maturation stage is depicted by bars representing the mean % of total BM cells in 20 HSA-sufficient (▪) and 24 HSA-deficient (□) mice.
The effect of HSA-deficiency on B-cell maturation. (A) B-cell maturation in BM is schematically divided into stages A-F according to Hardy et al6 and is based on the regulated expression of the surface markers CD45, CD43, BP1, CD24, IgM, and IgD. (B) The cellular pools size for each maturation stage is depicted by bars representing the mean % of total BM cells in 20 HSA-sufficient (▪) and 24 HSA-deficient (□) mice.
Although the total yield of BM cells from a femur of HSA-deficient mice was the same as in wild-type mice (Table 1), the ratio of cells with forward and orthogonal light scattering characteristic of immature lymphocytes in HSA-deficient mice compared with wild-type mice was reduced to 0.7 ± 0.2 (average from 44 mice, see Fig 2A for an example). Since in normal mice 4 to 16 weeks old, roughly 90% of these cells are immature B cells of the D-F stages, these results suggest that it is these stages of B-cell development that are reduced in HSA-deficient mice. This was confirmed by staining BM-derived B-cell precursors with a set of antibodies recognizing CD43, CD45/B220, BP1, HSA, IgM, and IgD. When used in different combinations, this set distinguishes the stages of B-cell development (see Figs 2 and 3). When the pool sizes for each of the maturation stages A-F were determined, HSA-deficient mice showed a reduction in the number of B-cell precursors in stages D and E, but normal numbers of A-C and F stage cells (Fig 3B shows the average pool sizes in 24 HSA-deficient and 20 HSA-sufficient mice from eight different experiments.) These results suggest that the lack of HSA partially interferes with the stage C to D transition, but that the remaining capacity for B-cell maturation in the BM is sufficient to generate normal numbers of mature B cells (see analysis of peripheral B cells below).
Summary of Additional Parameters Tested in HSA-Deficient Mice
| Parameter . | HSA-Positive Mice . | HSA-Deficient Mice . | ||
|---|---|---|---|---|
| . | 2 weeks old . | 9-12 weeks old . | 2 weeks old . | 9-12 weeks old . |
| Cellularity | ||||
| BM (total*) | 1.7 × 107 ± 0.3 (10) | 2.1 × 107 ± 0.6 (10) | 1.5 × 107 ± 0.3 (6) | 2.4 × 107 ± 0.9 (8) |
| Spleen (total) | 4.5 × 107 ± 2 (10) | 1.8 × 108 ± 0.2 (4) | 5.1 × 107 ± 2.5 (6) | 2.0 × 108 ± 0.3 (4) |
| B cells | 56% ± 8 (8) | 63% ± 4 (4) | 46% ± 4 (3) | 56% ± 9 (4) |
| T cells | 29% ± 6 (8) | 28% ± 5 (4) | 31% ± 3 (3) | 30% ± 10 (4) |
| Gr1+ cells | 7% ± 1 (8) | ND | 8% ± 0.5 (3) | ND |
| Thymus | 2.2 × 108 ± 1.5 (8) | 1.3 × 108 ± 0.7 (8) | 2.5 × 108 ± 0.4 (3) | 1.5 × 108 ± 0.4 (4) |
| Erythrocytes cell number (×106/μL) | 8.1 ± 0.4 | 7.7 ± 0.2 | ||
| MCV (fL) | 48 ± 1 | 50 ± 1 | ||
| MCH (pg) | 16.1 ± 0.4 | 17.9 ± 0.1 | ||
| Hematocrit (%) | 38.9 ± 2.2 | 38.8 ± 1.8 | ||
| Reticulocytes (×103/μL) | 22.2 ± 4.8 | 17.0 ± 2.2 | ||
| Leukocytes (×103/μL) | 6.0 ± 1.5 | 4.9 ± 0.8 | ||
| Thrombocytes (×103/μL) | 708 ± 82 | 742 ± 162 | ||
| Monocytes (% leukocytes) | 3.2 ± 1.6 | 2.8 ± 0.8 | ||
| Segmented leukocytes (% leukocytes) | 7.2 ± 3.3 | 12.4 ± 2.7 | ||
| B1 (Ly-1+) B cells in peritoneum | 10% of total | 7% of total | ||
| Serum isotype levels for IgM, IgG1, IgG2b, IgG3, IgA in preimmune mice | No differences | |||
| Immunization | ||||
| TNP-Ficoll 1° | No difference | |||
| TNP-Ova, primary response | No difference | |||
| TNP-Ova, secondary response | No difference | |||
| Infection | ||||
| Listeria (liver titer) 5 days after infection | 2 × 105 ± 2 (5) | 4 × 105 ± 4 (4) | ||
| Borrelia | Up to 71 days after infection, no clinical signs of arthritis in either group (3) Borrelia could be isolated from skin biopsies of all animals at day 71. | |||
| No difference in inflammatory damage as judged by histological sections of lymph nodes, spleen, thymus, heart, liver, kidney, brain or knee joint. | ||||
| P chabaudi chabaudi (malaria) | Higher titers of infected erythrocytes in HSA-deficient mice but mice were able to clear infection and are immune to a second infection. | |||
| Parameter . | HSA-Positive Mice . | HSA-Deficient Mice . | ||
|---|---|---|---|---|
| . | 2 weeks old . | 9-12 weeks old . | 2 weeks old . | 9-12 weeks old . |
| Cellularity | ||||
| BM (total*) | 1.7 × 107 ± 0.3 (10) | 2.1 × 107 ± 0.6 (10) | 1.5 × 107 ± 0.3 (6) | 2.4 × 107 ± 0.9 (8) |
| Spleen (total) | 4.5 × 107 ± 2 (10) | 1.8 × 108 ± 0.2 (4) | 5.1 × 107 ± 2.5 (6) | 2.0 × 108 ± 0.3 (4) |
| B cells | 56% ± 8 (8) | 63% ± 4 (4) | 46% ± 4 (3) | 56% ± 9 (4) |
| T cells | 29% ± 6 (8) | 28% ± 5 (4) | 31% ± 3 (3) | 30% ± 10 (4) |
| Gr1+ cells | 7% ± 1 (8) | ND | 8% ± 0.5 (3) | ND |
| Thymus | 2.2 × 108 ± 1.5 (8) | 1.3 × 108 ± 0.7 (8) | 2.5 × 108 ± 0.4 (3) | 1.5 × 108 ± 0.4 (4) |
| Erythrocytes cell number (×106/μL) | 8.1 ± 0.4 | 7.7 ± 0.2 | ||
| MCV (fL) | 48 ± 1 | 50 ± 1 | ||
| MCH (pg) | 16.1 ± 0.4 | 17.9 ± 0.1 | ||
| Hematocrit (%) | 38.9 ± 2.2 | 38.8 ± 1.8 | ||
| Reticulocytes (×103/μL) | 22.2 ± 4.8 | 17.0 ± 2.2 | ||
| Leukocytes (×103/μL) | 6.0 ± 1.5 | 4.9 ± 0.8 | ||
| Thrombocytes (×103/μL) | 708 ± 82 | 742 ± 162 | ||
| Monocytes (% leukocytes) | 3.2 ± 1.6 | 2.8 ± 0.8 | ||
| Segmented leukocytes (% leukocytes) | 7.2 ± 3.3 | 12.4 ± 2.7 | ||
| B1 (Ly-1+) B cells in peritoneum | 10% of total | 7% of total | ||
| Serum isotype levels for IgM, IgG1, IgG2b, IgG3, IgA in preimmune mice | No differences | |||
| Immunization | ||||
| TNP-Ficoll 1° | No difference | |||
| TNP-Ova, primary response | No difference | |||
| TNP-Ova, secondary response | No difference | |||
| Infection | ||||
| Listeria (liver titer) 5 days after infection | 2 × 105 ± 2 (5) | 4 × 105 ± 4 (4) | ||
| Borrelia | Up to 71 days after infection, no clinical signs of arthritis in either group (3) Borrelia could be isolated from skin biopsies of all animals at day 71. | |||
| No difference in inflammatory damage as judged by histological sections of lymph nodes, spleen, thymus, heart, liver, kidney, brain or knee joint. | ||||
| P chabaudi chabaudi (malaria) | Higher titers of infected erythrocytes in HSA-deficient mice but mice were able to clear infection and are immune to a second infection. | |||
All comparisons were in age- and sex-matched C57BL/6 mice. The number of mice used is given in parentheses.
Abbreviation: ND, not done.
Total cells from two femurs.
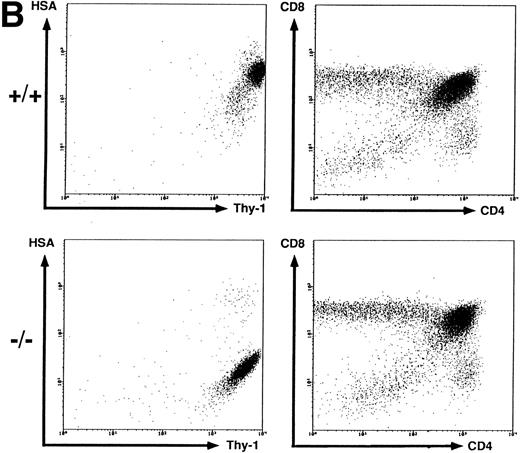
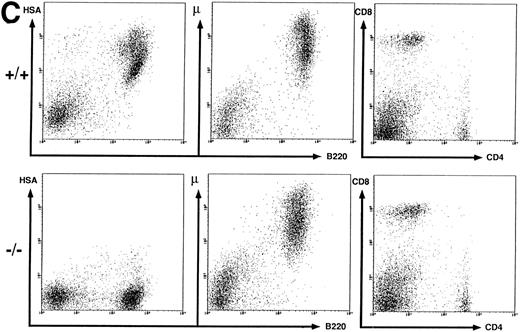
Flow cytometric analysis of spleen, thymus, and BM cells. BM (A), thymus (B), and spleen (C) cells from wild-type (+/+) and HSA-deficient (-/-) mice were labeled with fluorescent antibodies recognizing the B-cell surface markers B220, IgMb and the T-cell markers CD4, CD8, and Thy-1 in different combinations of three antibodies. BM cells were also anlayzed by forward (FSC) versus orthogonal (SSC) light scattering and by labeling with the antibody S7, recognizing CD43. The number of cells in the oval lymphocyte gate in the FSC versus SSC plot is given as percentage of total BM cells analyzed. The rectangular gates correspond to the B-cell maturation stages A-F according to Hardy et al6 (see Fig 3) and the number of cells present in each gate is given as percentage of total B220+ cells. The results presented are representative of eight independent experiments with a total of 20 HSA-sufficient and 24 HSA-deficient mice.
Flow cytometric analysis of spleen, thymus, and BM cells. BM (A), thymus (B), and spleen (C) cells from wild-type (+/+) and HSA-deficient (-/-) mice were labeled with fluorescent antibodies recognizing the B-cell surface markers B220, IgMb and the T-cell markers CD4, CD8, and Thy-1 in different combinations of three antibodies. BM cells were also anlayzed by forward (FSC) versus orthogonal (SSC) light scattering and by labeling with the antibody S7, recognizing CD43. The number of cells in the oval lymphocyte gate in the FSC versus SSC plot is given as percentage of total BM cells analyzed. The rectangular gates correspond to the B-cell maturation stages A-F according to Hardy et al6 (see Fig 3) and the number of cells present in each gate is given as percentage of total B220+ cells. The results presented are representative of eight independent experiments with a total of 20 HSA-sufficient and 24 HSA-deficient mice.
BM repopulation of irradiated mice.Because the lack of HSA seems to interfere with B-cell maturation, the influence of HSA on the ability of BM hematopoietic stem cells to repopulate irradiated recipients was also examined. To do this, mixtures of BM from HSA+/+, C57BL/6-Ly5.2+ mice and HSA-/-, C57BL/6-Ly5.1+ mice were injected into the tail vein of groups of three Rag2- mice that were irradiated with 400 rad. Peripheral, donor derived, B and T cells were examined at 4 days, 28 days, and 119 days after repopulation. The Ly5 allotypic markers are particularly useful to follow peripheral T cells derived from each of the two donors since HSA is not expressed on this population. As shown in Table 2, no difference was seen in the ability to repopulate irradiated mice with BM derived from HSA-deficient donors. Similar results were obtained when the irradiated recipients were wild-type, rather than Rag2-deficient (data not shown).
T-cell development in HSA-deficient mice.Most thymocytes and peripheral B cells express HSA. Using several markers for T cells (Thy-1, CD4, and CD8), Fig 2B shows that the disruption of CD24a had no effect on the proportion of immature T cell single- and double-positive subpopulations in the thymus, even though they completely lack HSA staining. Thus, T-cell maturation can proceed in the absence of HSA.
Peripheral B and T cells in HSA-deficient mice.Using antibodies specific for B cells (B220 and IgM) and T cells (see above), no significant difference in the absolute number or proportion of B- and T-cell subpopulations in spleen (Fig 2C) or lymph nodes (data not shown) was observed, even though the B cells completely lacked HSA staining in HSA-/- mice. The stainings for HSA were performed with four different anti-HSA monoclonal antibodies (M1/69,28 J11d,8 79,18 and 20C920) with the same results. In heterozygotes, the mean fluorescent intensity of HSA-staining on peripheral B cells or on thymocytes is reduced by a factor of 2, suggesting a gene dosage effect (data not shown). Staining for macrophage and granulocyte lineages (Mac 1 and Gr-1) in spleen, lymph nodes, and BM also showed no difference between HSA-/- and HSA+/+ mice (data not shown). Normal numbers of B1 (Ly1+) B cells39 were also found in the peritoneum of HSA-deficient mice (data not shown).
In summary, despite a modest block in B-cell development in HSA-/- mice at the C to D transition, B cells are able to mature and fill the peripheral lymphoid organs to normal levels. No reduction in splenic or lymph node B-cell numbers has been observed in HSA-/- mice, even in 2-day-old or 1.5-year-old mice (data not shown). Thus, the production of other major hematopoietic lineages is not affected by the lack of HSA. These results are in agreement with earlier results obtained in chimeric mice made by injecting blastocysts with ES cells lacking both functional CD24a alleles.25
The immune response in HSA-deficient mice.Having established that, despite a mild block in B-cell development, the quantities of peripheral B and T cells were normal, we wished to test whether the inability to express HSA had an effect on their function. Peripheral B cells normally express HSA, while peripheral T cells are negative, but can reexpress HSA when activated.24 Since HSA has been linked to lymphocyte costimulation,20-24 and costimulation has been shown to be important in a variety of immunological responses including protein immunization and bacterial infections,40 41 the immune response of HSA-/- mice was examined in a variety of ways (see Materials and Methods for details) including measuring antigen-specific serum antibody titers following immunizing mice with T-cell–dependent, as well as T-cell–independent antigens, and clearance of bacterial infection with Borrelia and Listeria. Basal levels of serum immunoglobulin isotypes in unimmunized mice were also measured. No differences were observed between HSA-deficient and control C57BL/6 mice in these experiments.
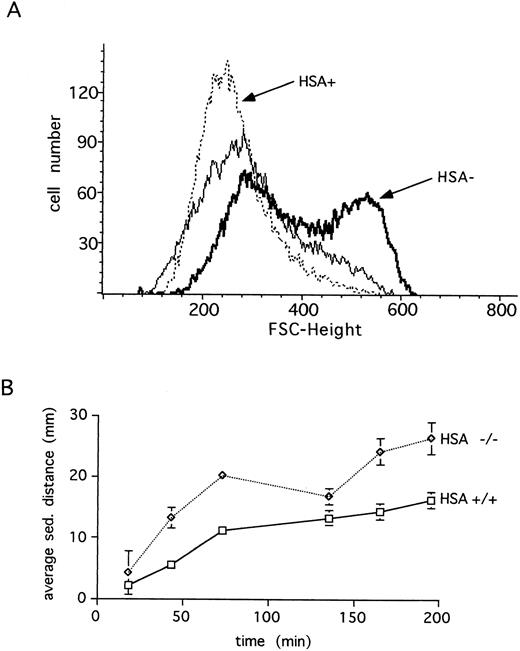
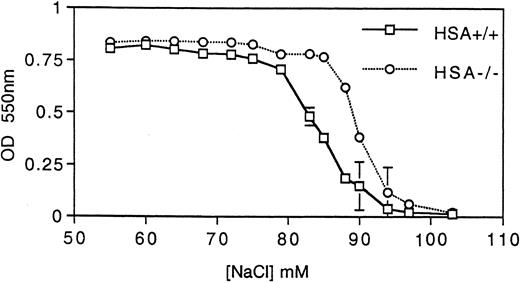
HSA expression and erythrocytes.Unlike many other hematopoietic lineages, HSA expression on mature erythrocytes remains at high levels.3 For this reason, we examined the erythrocytes in HSA-deficient mice for possible defects. Erythrocytes lacking HSA showed an increased tendency to aggregate, as indicated by the increased forward light scatter in FACS analysis (Fig 4A). This aggregation was reversible since increased shear-forces associated with higher flow rates during analysis lead to disaggregation of the HSA-deficient erythrocytes (Fig 4A). Consistent with increased aggregation, erythrocytes lacking HSA also showed a faster sedimentation rate (Fig 4B). A further alteration in mutant erythrocytes was indicated by an increased tendency to lyse when incubated under hypotonic conditions in vitro (Fig 5). Despite these in vitro differences, however, no difference in hematocrit, erythrocyte hemoglobin content, blood reticulocytes numbers, or serum erythropoietin (EPO) levels could be measured (Table 1). Differential leukocyte counts, platelet counts, and gross cellular morphology were also not different. Interestingly, the color of freshly prepared spleens from HSA-deficient mice was darker than spleens from control mice, suggesting perhaps that there are more erythrocytes per spleen in HSA-deficient mice. Since the spleen is believed to play a major role in removing old or damaged erythrocytes, this could mean that erythrocytes lacking HSA could have a shorter half-life. To test this, isolated erythrocytes from HSA+/+ and HSA-/- mice were stained with a fluorescent dye and reinjected into HSA+/+ and HSA-/- recipient mice (Table 3). The apparent mean half-life of normal erythrocytes in HSA+/+ mice was estimated to be about 15 days. In contrast, the apparent half-life of HSA-/- erythrocytes injected into HSA+/+ mice was reduced to about 4 days. Mutant-stained erythrocytes injected into HSA-/- mice showed an apparent half-life of about 11 days (see Table 3 and discussion). To rule out the possibility that the reduced half-life of mutant erythrocytes in normal mice was due to an immune reaction mounted against the HSA-deficient erythrocytes, the same experiment was repeated in Rag2-deficient recipients. In these recipients, an immune reaction can be ruled out, as Rag2-deficient mice completely lack mature, functional T and B lymphocytes. Here again, the mutant erythrocytes showed a reduced half-life compared with wild-type erythrocytes (Table 3).
Erythrocytes from HSA-deficient mice show increased aggregation. (A) The forward (FSC) light scattering of blood erythrocytes is shown for wild-type (HSA+, dotted line) and HSA-deficient (HSA-, thick line) mice at a flow rate of 130 events/s (sample differential 0.5). Increasing the flow rate to 600 events/s (sample differential 3.0) results in disaggregation of the HSA- erythrocytes (thin solid line). (B) The average blood sedimentation distance, with standard deviations, from five wild-type (+/+) and five HSA-deficient (-/-) mice over time is shown.
Erythrocytes from HSA-deficient mice show increased aggregation. (A) The forward (FSC) light scattering of blood erythrocytes is shown for wild-type (HSA+, dotted line) and HSA-deficient (HSA-, thick line) mice at a flow rate of 130 events/s (sample differential 0.5). Increasing the flow rate to 600 events/s (sample differential 3.0) results in disaggregation of the HSA- erythrocytes (thin solid line). (B) The average blood sedimentation distance, with standard deviations, from five wild-type (+/+) and five HSA-deficient (-/-) mice over time is shown.
HSA-deficient erythrocytes are more susceptible to hypotonic lysis. Hemoglobin (measured by absorbance at 550 nm) released from erythrocytes incubated at various tonicities was determined for both HSA+/+ and HSA-/- erythrocytes. The data points are the averages and standard deviations of three mice and the results are representative of three independent experiments (a total of nine mice).
HSA-deficient erythrocytes are more susceptible to hypotonic lysis. Hemoglobin (measured by absorbance at 550 nm) released from erythrocytes incubated at various tonicities was determined for both HSA+/+ and HSA-/- erythrocytes. The data points are the averages and standard deviations of three mice and the results are representative of three independent experiments (a total of nine mice).
Mean Erythrocyte Half-Life
| HSA Genotype of Erythrocyte3-150 . | Phenotype of Recipient Mouse . | No. of Mice . | T1/2 (d) . | |
|---|---|---|---|---|
| . | HSA . | Rag2 . | . | . |
| + | + | + | 5 | 15.4 ± 3.6 |
| − | + | + | 5 | 3.8 ± 0.8 |
| − | − | + | 3 | 10.7 ± 1.2 |
| + | + | − | 4 | 25.0 ± 10 |
| − | + | − | 4 | 9.0 ± 2 |
| HSA Genotype of Erythrocyte3-150 . | Phenotype of Recipient Mouse . | No. of Mice . | T1/2 (d) . | |
|---|---|---|---|---|
| . | HSA . | Rag2 . | . | . |
| + | + | + | 5 | 15.4 ± 3.6 |
| − | + | + | 5 | 3.8 ± 0.8 |
| − | − | + | 3 | 10.7 ± 1.2 |
| + | + | − | 4 | 25.0 ± 10 |
| − | + | − | 4 | 9.0 ± 2 |
Fluorescently-stained normal (+) or HSA-deficient (−) erythrocytes were injected into wild-type, C57BL/6, HSA-deficient, or Rag2-deficient mice and the mean half-life was determined (see Materials and Methods).
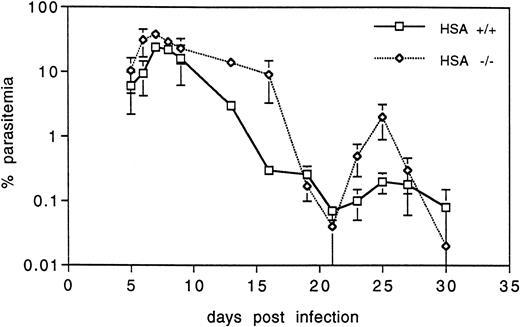
Malaria.Because erythrocytes in HSA-deficient mice appeared to be altered, we investigated whether this might affect the in vivo growth and development of an intraerythrocytic parasite such as Plasmodium. To test this, groups of five control and five HSA-/- mice were infected with P chabaudi chabaudi (a nonlethal rodent malaria parasite) and the parasitemia was monitored in the blood at regular intervals during the primary infection. Both groups showed the typical rise in blood-born parasites peaking at about 8 to 10 days postinfection (one typical example of three independent experiments is shown in Fig 6), after which the infection was cleared. Interestingly, HSA-/- mice consistently had a higher proportion of infected erythrocytes, both in the acute phase immediately following the peak of infection (>10-fold higher than in the control mice) and in the recrudescence phase at about 25 days after infection. However, consistent with the results presented above suggesting that the immune system of HSA-mutant mice is largely intact, HSA-/- mice were able to clear the malaria infection with the same kinetics as control mice. Reinfection of both groups 1 month later did not lead to further episodes of parasitemia (data not shown), indicating that both groups carried effective immunity to malaria.
HSA-deficient mice infected with P chabaudi chabaudi have higher blood parasitemia, but are able to clear the infection as well as wild-type mice. The percent of infected erythrocytes is shown at various days after infection. The values are the averages and standard deviations of five mice in each group and are representative of three independent experiments.
HSA-deficient mice infected with P chabaudi chabaudi have higher blood parasitemia, but are able to clear the infection as well as wild-type mice. The percent of infected erythrocytes is shown at various days after infection. The values are the averages and standard deviations of five mice in each group and are representative of three independent experiments.
DISCUSSION
FACS analysis of B-cell precursors in the BM showed a leaky block in B-cell development (Fig 3). This block leads to an overall reduction in the number of B-cell precursors in the BM, which varied from mouse to mouse between 10% and 50%. These results are completely consistent with the reduction in B-cell precursors previously reported in mice chimeric for HSA expression.25 This partial block appears to be at the stage C to D transition since stages A-C are normal. The number of more mature cells in fraction F is relatively normal. Maturation from fraction C to D involves a strong expansion in cell numbers and presumably is induced by the successful rearrangement of the immunoglobulin heavy chain and its expression on the surface of the cell in association with the surrogate light chain proteins lambda 5 and VpreB.42,43 Conceivably, HSA could be involved in augmenting this proliferative signal. Despite the lack of HSA, B cells are able to mature in HSA-deficient mice to such an extent that the peripheral B-cell compartment is normal in number and function. The phenotype of the HSA-deficient mouse concerning B-cell development is similar to, but less severe than the lambda 5 and Jak3 mutant mice where a leaky block at the C to D transition is also seen.44-46 In analogy to costimulation in mature, peripheral lymphocytes, perhaps HSA acts synergistically with the activating signal from the pre-B–cell antigen receptor to stimulate proliferation and maturation to more mature B cells. Interestingly, HSA expression reaches a maximum at the C to D transition during B-cell development.6
We previously reported that chimeric mice made by injecting blastocysts with ES cells lacking a functional HSA gene resulted in mice with a considerably elevated chimerism in hematopoietic tissue compared with chimeric mice made by injecting HSA+/+ ES cells. We speculated that the lack of HSA expression could lead to a more efficient colonization of the BM or hematopoietic differentiation from stem cells. In competitive repopulation experiments with irradiated recipients, we were unable to observe any difference in peripheral lymphocyte chimerism between HSA-/- and HSA+/+ BM donors. This suggests either that the colonization potential of adult BM stem cells is different from that of stem cells produced during embryogenesis, that the presence of chimeric BM stroma is important, or that the effect of HSA loss on BM seeding is earlier in embryonic development and not relevant for irradiated, adult recipients.
The fact that HSA is expressed on immature cells of all major hematopoietic lineages, and in most cases is also regulated during cellular maturation, suggests that HSA could be important for maturation. For this reason, we expected that deletion of the functional gene for HSA could lead to defects in the maturation of numerous hematopoietic lineages. This does not appear to be the case. While there is a leaky block in B-lymphocyte development, the maturation of all other major hematopoietic cell types seems to be normal.
For mature lymphocytes, HSA has been implicated to play a role both in cellular adhesion processes and in signaling, which leads to lymphocyte activation. Nevertheless, the normal immune responses to antigen and to bacterial infection indicate that the peripheral immune system is not grossly affected by the absence of HSA. As has been the case for numerous other genes examined by targeted mutation in mice, we are forced to postulate that compensatory changes and/or functionally overlapping systems are responsible for the mild change in phenotype of HSA-/- mice. This conclusion is consistent with the fact that for cellular adhesion, as well as costimulation, several ligand-receptor pairs have been identified on lymphocytes and antigen-presenting cells, which can independently mediate these functions (vascular cell adhesion molecule-1 [VCAM-1]/very late antigen-4 [VLA-4], lymphocyte function associated-1 [LFA-1]/intercellular adhesion molecule-1 [ICAM-1],-2, CD28,CTLA-4/B7-1,B7-2, CD-2/LFA-3) (for a review, see Guinan et al40 ). Other than an occasional 1.5- to 2-fold increase in the mean fluorescence intensity of B220 on B cells, no change (suggesting compensation) in other surface antigens was observed (in addition to those described above, anti VLA-4, major histocompatability complex [MHC] I, IgD, Pgp-1 and T-cell receptor antibodies were also tested).
Compensation or redundancy is also the most likely explanation for the lack of a gross effect on other tissues/organs in the mouse where HSA expression has been reported, which include developing neurons in the central nervous system,13,14embryonic intestinal, nasal, salivary gland, and renal epithelium in the rat,13 rat odontoblasts and hair follicles in rat,13 and mouse (P.J. Nielsen, unpublished data, September 1992), epidermal Langerhans cells,22 and thymic and BM-derived dendritic cells in the mouse.16 17 The fact that HSA is expressed in a variety of cell types during embryonic development may, however, explain the reduced frequency of homozygous mutant mice derived from matings of heterozygous parents and the reduced litter size in heterozygote and homozygote matings.
In contrast to lymphocytes, HSA-deficient erythrocytes showed clear alterations when compared with normal erythrocytes. Interestingly, these changes, which included an increased aggregation and susceptibility to osmotic lysis in vitro (Figs 4 and 5), have been associated with aging of normal erythrocytes.47 This could suggest that HSA-deficient erythrocytes age more rapidly. Consistent with this, in vivo, HSA-deficient erythrocytes have a shorter half-life than normal (Table 3), but the mice are not anemic, indicating that erythrocyte replacement is not a problem. The half-life of 15 to 25 days measured here for normal erythrocytes is in the range of 10 to 30 days measured by radioactive labelling in several reports.48 Mutant erythrocytes appear to have a twofold to threefold shorter half-life. The half-life measured in Rag2-deficient mice for both normal and mutant erythrocytes was somewhat longer that in normal mice. This may be due to differences in genetic backgrounds of the recipients (the Rag2-deficient mice were a mixture of C57BL/6 and 129 strains) or due to the fact that Rag2-deficient mice are immunodeficient. The longer half-life of mutant erythrocytes in HSA-/- recipients may be explained by a competition between the endogenous mutant erythrocytes and the stained donor erythrocytes for a limiting clearance mechanism.
Conceivably, changes in the erythrocyte due to loss of HSA may have more acute consequences under circumstances of unusual physiological stress. This may be the explanation for the increased levels of erythrocytes carrying parasites in HSA-/- mice infected with P chabaudi chabaudi (Fig 6). In some malarial species, including P chabaudi chabaudi, the number of infected erythrocytes visible in the blood falls shortly before the erythrocytes rupture to release progeny.49 This disappearance, termed sequestration, is thought to involve adhesive interactions between parasite-modified erythrocyte plasma membranes and either endothelium or liver Kupfer cells, depending on the parasite strain.50 One explanation for the increased levels of infected erythrocytes in the HSA-deficient mice could be that sequestration functions poorly in the absence of HSA. Alternatively, the absence of HSA from the surface of erythrocytes could increase the efficiency with which the parasite can enter the erythrocyte or reduce the generation time of the parasite.
To learn more about which genes overlap or compensate for HSA in function, it may be helpful to produce mice also defective at a second locus. For B-cell development, possible candidate genes could be lambda 5, CD43, or VLA-4. In the case of costimulation in mature lymphocytes, mutants in LFA-1, CD28, CD2, or ICAM-1 would be interesting to combine with HSA mutants. We have begun to generate HSA/CD28 double mutants to learn more about the relative importance of these two costimulatory molecules during an immune response.
ACKNOWLEDGMENT
The authors thank I. Voerenbach, C. Westphal, and S. Maier for blastocyst injections and mouse breeding, Dr H. Rodewald for the C57BL/6 Ly5.2 mice, H. Kohler and Drs R. Carsetti and M. Lamers for antibodies, as well as practical and theoretical help with the FACS analysis. Drs M. Gassmann and H. Marti and the Tierspital Zürich were very helpful with differential blood counts. We also thank T. Franz, K.H. Widmann, and J. Kury for help with maintenance and analysis of the HSA knockout strain and L. Lay for the photowork.
Submitted February 28, 1996; accepted September 16, 1996.
R.H.W. was supported by a fellowship from the Max-Planck Society (Munich, Germany).
This report is dedicated to the memory of Georges Köhler, who died on March 1, 1995.
Address reprint requests to P.J. Nielsen, PhD, Max Planck Institute for Immunobiology, Stübeweg 51, D-79108 Freiburg i. Br., Germany.
REFERENCES
Author notes
Deceased.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal