Abstract
Anaplastic large cell lymphoma (ALCL) is composed of large, frequently bizarre, cells of T- or null-cell phenotype that show a preferential sinusoidal growth pattern and consistent CD30 positivity. Whether these tumors represent a single entity or several, and what the exact cell origin, is controversial. Recently, granzyme B, a cytotoxic granule component, was reported in a small percentage of ALCL, suggesting that some cases may originate from cytotoxic lymphocytes. To further investigate this possibility, we performed an immunohistochemical study of 33 ALCLs of T- and null-cell type, using monoclonal antibodies to cytotoxic cell-associated antigens, including CD8, CD56, CD57, and the cytotoxic granular proteins perforin and TIA-1. In addition, CD4 expression was also evaluated. ALCL cases included 27 classical systemic forms and variants, 3 primary cutaneous (PC) forms, and 3 acquired immunodeficiency syndrome-associated forms. Cytotoxic antigen expression was also studied in 51 cases of Hodgkin's disease (HD) and 17 large B-cell lymphomas (LBCLs) with anaplastic cytomorphology and/or CD30 positivity. We found that 76% of ALCLs, representing all subtypes except the PC forms, expressed either TIA-1, perforin, or both proteins. Expression of TIA-1 and perforin were highly correlated (P < .001). On the basis of their immunophenotypic profiles, several subtypes of cytotoxic antigen positive and negative ALCL could be recognized. Fifty-five percent of ALCLs (18 of 33) displayed an immunophenotypic profile consistent with cytotoxic T cells. Six cases expressed cytotoxic granular proteins in the absence of lineage specific markers, and one case expressed both T-cell – and natural killer cell–like markers. These 7 cases (21%) were placed into a phenotypic category of cytotoxic lymphocytes of unspecified subtype. Twenty-four percent (8 cases) of ALCLs were cytotoxic granule protein negative. All but one of these displayed a T-cell phenotype. Cytotoxic granule protein expression did not correlate with the presence of the NPM-ALK fusion transcript. Only 10% of the 51 HD cases were found to be TIA-1+, and none expressed perforin. Cytotoxic antigen expression was absent in LBCL. The expression of cytotoxic granule proteins in the majority of ALCL implies a cytotoxic lymphocyte phenotype and suggests that most cases originate from lymphocytes with cytotoxic potential. Furthermore, the demonstration of cytotoxic cell related proteins may be a useful addition to the current panel of antibodies used to distinguish ALCL, HD, and anaplastic LBCL.
CYTOTOXIC lymphocytes of all types possess azurophilic cytoplasmic granules1-3 that contain a unique set of toxic proteins.3-9 Among these proteins are perforin,3,4,7 granzymes A and B,3,5,6,8-10 and a recently recognized effector protein called T-cell intracellular antigen-1 or TIA-1.11-13 Perforin, also called cytolysin or pore-forming protein, has the capacity to form pores in target cell membranes.4-7 Perforin colocalizes in the azurophilic granules with a subfamily of serine proteases called granzymes,5,6,8 and TIA-1.11-13 The granzymes and TIA-1 trigger a process leading to apoptotic DNA-fragmentation of the target cell.10,12 In addition to granule-associated cytotoxicity, a nonsecretory process involving the apoptosis inducing cell surface receptor Fas-antigen (CD95) is an additional mechanism of lymphotoxicity.14
Neoplastic proliferations of cytotoxic lymphocytes have been increasingly recognized as a result of progress in defining these cells and in the availability of antibodies to cytotoxic cell associated antigens, including the granule associated proteins.15-19 Leukemias composed of large granular lymphocytes generally show a cytotoxic T- or natural killer (NK)-cell phenotype.20,21 Occasional peripheral T-cell lymphomas, generally of extranodal origin, are reported to possess a phenotype consistent with derivation from either cytotoxic α/β or γ/δ T cells.15-19 Recently, the non-B–cell nasal lymphomas, formerly called lethal midline granuloma, have been found to be derived from granular lymphocytes of NK-cell type.18,22 Most recently, granzyme B positivity was reported in some cases of anaplastic large cell lymphoma (ALCL)16 raising the possibility that at least a subset of these tumors may also be derived from lymphocytes with cytotoxic potential.
The ALCLs were initially identified as a group of pleomorphic large cell non-Hodgkin's lymphomas with strong expression of Hodgkin's disease (HD) associated antigen ‘Ki-1’/CD30.23 Although the majority of cases show a T-cell phenotype or T-cell receptor (TCR) gene rearrangement,23-26 some fail to show either a B- or T-cell phenotype/genotype,21,24-26 and a small percentage have been reported to display a B-cell or mixed-cell phenotype.23,24 26 The B-cell cases are now believed to be distinct from the majority of ALCL, a view reflected in the Revised European-American Classification of Lymphoid Neoplasms (REAL). 21
There are two clinicopathological forms of primary ALCL.21,27 The most common form, the classical systemic (CS) type, typically involves multiple nodal and extranodal sites at the time of presentation.21,23,27 The other is primary cutaneous (PC) ALCL, which occurs as a localized disease and tends to remain restricted to the skin.21,27-29 Although the PC form of ALCL is histologically similar to the CS form, its distinct phenotypic characteristics, indolent clinical course and relationship with lymphomatoid papulosis suggest that primary cutaneous ALCL may represent a biologically different disease.27-30
A significant percentage of classical ALCLs possess a nonrandom chromosomal translocation t(2; 5)(p23; q35), which results in the juxtaposition of the nucleophosmin (NPM) gene on chromosome 5q35 to the anaplastic lymphoma kinase (ALK) gene on chromosome 2p23.31-34 The expression of an NPM-ALK chimeric product is believed to play a role in the pathogenesis of t(2; 5) carrying ALCLs.33
There is little known about the normal cellular counterpart of ALCL. Because ALCL is strongly CD30+, it has been postulated that this lymphoma may be related to the CD30+ large extrafollicular lymphoid cells often found in reactive lymphoid tissues.21,23 More recently, as a result of a recent report showing granzyme B expression in some cases of ALCL,16 it has also been suggested that this lymphoma may be derived from cytotoxic lymphoid cells.
To explore the possibility that ALCL may be derived from lymphocytes with cytotoxic potential, we undertook a systematic immunohistochemical analysis of 33 histologically and phenotypically confirmed ALCLs using monoclonal antibodies (MoAbs) against the cytotoxic granule-associated proteins TIA-1 and perforin as well as other antigens associated with cytotoxic cell subpopulations. We specifically focused on cytotoxic granule-associated proteins not only because they are specific to the cytotoxic cell family, but also because they are relatively resistant to routine tissue processing, enabling them to be easily identified in archival pathologic material. Because ALCL shows morphological and immunological overlap with HD35 and some large B-cell lymphomas (LBCLs),21 36 51 cases of HD as well as 17 anaplastic or CD30+ LBCLs were also included in this study.
MATERIALS AND METHODS
Tissue samples and immunophenotyping.Formalin or B5 fixed and paraffin embedded biopsies of 33 primary ALCLs of T- and null-cell type, 51 cases of HD and 17 LBCLs, were selected from the histopathology files of the Hematopathology Section, Laboratory of Pathology, National Cancer Institute, National Institutes of Health, (Bethesda, MD) and the Department of Pathology, Albert Szent-Gyorgyi Medical University (Szeged, Hungary) (see Table 1). All cases were classified according to the REAL Classification.21
Cytotoxic Granule Protein Expression in ALCL, HD, and LBCL
| Cases . | TIA-1 . | Perforin . | Overall Positivity (%) . |
|---|---|---|---|
| . | (%) . | (%) . | . |
| ALCL | 23/33 (70) | 22/33 (67) | 25/33 (76) |
| Classical systemic | 13/19 (68) | 14/19 (74) | 15/19 (79) |
| Histiocyte rich variant | 6/6 (100) | 6/6 (100) | 6/6 (100) |
| Small cell variant | 2/2 (100) | 2/2 (100) | 2/2 (100) |
| Primary cutaneous | 0/3 | 0/3 | 0/3 |
| AIDS-associated | 2/3 (67) | 0/3 | 2/3 (67) |
| HD | 5/51 (10) | 0/26 | 5/51 (10) |
| LP HD | 0/3 | 0/1 | 0/3 |
| NS HD | 2/33 (6) | 0/18 | 2/33 (6) |
| MC HD | 2/11 (18) | 0/5 | 2/11 (18) |
| LD HD | 1/4 (25) | 0/2 | 1/4 (25) |
| LBCL | 0/17 (0) | 0/12 (0) | 0/17 (0) |
| Diffuse LBCL | 0/10 | 0/8 | 0/10 |
| T-cell rich BCL | 0/3 | 0/1 | 0/3 |
| Mediastinal LBCL | 0/2 | 0/1 | 0/2 |
| Follicular LBCL | 0/1 | 0/1 | 0/1 |
| LBCL with LP HD | 0/1 | 0/1 | 0/1 |
| Cases . | TIA-1 . | Perforin . | Overall Positivity (%) . |
|---|---|---|---|
| . | (%) . | (%) . | . |
| ALCL | 23/33 (70) | 22/33 (67) | 25/33 (76) |
| Classical systemic | 13/19 (68) | 14/19 (74) | 15/19 (79) |
| Histiocyte rich variant | 6/6 (100) | 6/6 (100) | 6/6 (100) |
| Small cell variant | 2/2 (100) | 2/2 (100) | 2/2 (100) |
| Primary cutaneous | 0/3 | 0/3 | 0/3 |
| AIDS-associated | 2/3 (67) | 0/3 | 2/3 (67) |
| HD | 5/51 (10) | 0/26 | 5/51 (10) |
| LP HD | 0/3 | 0/1 | 0/3 |
| NS HD | 2/33 (6) | 0/18 | 2/33 (6) |
| MC HD | 2/11 (18) | 0/5 | 2/11 (18) |
| LD HD | 1/4 (25) | 0/2 | 1/4 (25) |
| LBCL | 0/17 (0) | 0/12 (0) | 0/17 (0) |
| Diffuse LBCL | 0/10 | 0/8 | 0/10 |
| T-cell rich BCL | 0/3 | 0/1 | 0/3 |
| Mediastinal LBCL | 0/2 | 0/1 | 0/2 |
| Follicular LBCL | 0/1 | 0/1 | 0/1 |
| LBCL with LP HD | 0/1 | 0/1 | 0/1 |
Abbreviations: LD, lymphocyte depletion; LP, nodular lymphocyte predominance; MC, mixed cellularity; NS; nodular sclerosis.
The 33 ALCL cases of T- and null-cell type were subclassified as follows using previously described criteria34: 19 CS forms, 6 histiocyte-rich (HR) variants, 3 PC forms, 3 acquired immunodeficiency syndrome (AIDS)-associated cases and 2 small cell-predominant (SCP) variants (see Table 1). All cases other than PC forms and SCP variants presented with lymph node enlargement, but 6 also had skin involvement. Both SCP variant cases occurred as a subcutaneous mass. The age of the patients ranged from 1 to 64, with a mean of 29 years. All but two cases had been previously immunophenotyped in paraffin sections with MoAbs of CD15/LeuM1 (Becton Dickinson [BD], Mountain View, CA), CD20c/L26 (DAKO Corp [D], Carpenteria, CA), CD30/BerH2 (D), CD43/Leu22 (BD), CD45R0/A6 (Zymed, South San Francisco, CA), CD68/KP1 (D), EMA/E29 (D), and with a polyclonal CD3 antibody (D), which detects an intracytoplasmic amino acid sequence of the CD3ε chain.37 Some cases were also studied in frozen sections using CD2/Leu5b (BD), CD3/Leu4 (BD), CD4/Leu3a (BD), CD5/Leu1(BD), CD7/Leu9 (BD), CD8/Leu2a (BD), CD11b/Leu15 (BD), CD11c/LeuM5 (BD), CD16/Leu11 (BD), CD19/Leu12 (BD), CD22/Leu14 (BD), CD25/ACT-1 (D), CD30/BerH2 (D), CD56/Leu19 (BD) and to TCR β- and δ-chains using βF1 and TCR-δ1 MoAbs (T-Cell Sciences, Cambridge, MA). All cases showed strong CD30 expression and epithelial membrane antigen (EMA) positivity was found in 16 out of 29 (55%) cases. Twenty-six ALCLs had a T-cell phenotype, expressing various combinations of T-cell antigens (CD2, CD3, CD5, CD7, CD43, CD45R0, and CD3ε chain) either in frozen or paraffin sections, without B-cell–associated antigen positivity. Seven cases in which neither T- nor B-cell–associated antigens could be detected were categorized as null-cell type.
The 51 Hodgkin's lymphomas consisted of 48 cases of classical (11 mixed cellularity [MC], 33 nodular sclerosis [NS] and 4 lymphocytic depletion [D]) and 3 nodular lymphocytic predominant (LP) HD (see Table 1). Immunophenotyping was performed in the majority of the cases using the paraffin section antibody panel above to confirm the histologic diagnosis. Of the 32 classical HD cases stained with the BerH2 antibody, 31 were positive. Of 21 cases studied with B- and T-cell specific antibodies, 6 displayed B-cell antigen positivity whereas none showed T-cell antigen positivity. All 3 LP cases were negative for both CD30, CD15, and T-cell–associated antigens, but positive for CD20, as expected.
The 17 LBCLs showed anaplastic cytomorphology and/or CD30 positivity. All cases were positive for either CD19, CD22, or CD20c/L26, and negative for all T-cell–associated antigens. Ten of the 17 cases were diffuse LBCLs with anaplastic cytomorphological features and included 6 with CD30 positivity. These 6 cases were consistent with the B-cell ALCL category according to the Updated Kiel Lymphoma Classification.38 The LBCL cases also included 3 T-cell rich B-cell lymphomas (TCRBCL; 2 were focally CD30+); 2 CD30+ primary mediastinal LBCLs; 1 follicular large cell lymphoma with CD30+ tumor cells and 1 LP HD with a syncytial large cell lymphoma component (Table 1).
Immunohistochemical detection of cytotoxic cell antigens.TIA-1 was detected using a previously described mouse anti-human MoAb11 (clone TIA-1; Coulter Immunology, Hialeah, FL; dilution: 1:1,000). For the demonstration of perforin protein, ascites fluid of a rat anti-mouse IgG2 MoAb denoted P1-8 (kindly provided by Hideo Yagita, Juntendo University School of Medicine, Tokyo, Japan; dilution: 1:1,000) was used. The P1-8 antibody had previously been shown to cross react with human perforin in both Western blot analysis and in immunocytochemistry.4 7 In an initial pilot study of this antibody, we confirmed its specific reactivity with human perforin by Western blot analysis, and showed identical staining patterns in both frozen and paraffin-embedded tissue specimens (data not shown).
Immunostains were also performed in paraffin embedded tissue sections for the cytotoxic cell-related surface antigens CD8, CD56, and CD57. In addition, staining for CD4 molecule was also performed on paraffin tissue sections. For detection of CD8 antigen, culture supernatant of MoAb C8/144B (kindly provided by Kevin C. Gatter, The John Radcliffe Hospital, Oxford, UK; dilution: 1:40) was used. For CD56, a MoAb called 123C3 was used that reacts with a fixation-resistant epitope of neural cell adhesion molecule39 (kindly provided by John Hilkens, The Netherlands Cancer Institute, Amsterdam, The Netherlands; dilution: 1:200). The CD57 molecule was demonstrated with MoAb Leu7 (BD; dilution: 1:40). For demonstration of CD4 antigen in paraffin sections, MoAb CD4/1F6 (Novocastra Laboratories Ltd, Newcastle upon Tyne, UK; dilution 1:20) was applied. The CD4/1F6 antibody was raised against recombinant CD4 protein and identifies a single band of expected size in Western blot analysis. The CD4/1F6 is effective in both frozen and paraffin embedded tissue sections (Dr Ian Milton, Novocastra Laboratories, personal communication).
All immunohistochemical reactions were performed using a streptavidin-biotin-peroxidase complex method, following antigen retrieval with microwave oven heating. Briefly, 5-μm thick paraffin sections were mounted on Fisherbrand/Plus Superfrost Precleaned slides (Fisher Scientific, Pittsburgh, PA) and attached by overnight heating at 58°C. Following deparaffinization and blocking of endogenous peroxidase activity, the sections to be stained with TIA-1, perforin/P1-8, CD8/C8/144B, and CD57/Leu7 were first placed in microwave pressure cooker (Nordic Ware, Minneapolis, MN) in 0.01 mol/L citrate buffer (pH 6.0) and heated in a microwave oven at maximum power (800 W) for 40 minutes. After removing the pressure cooker from the oven, the sections were left in the hot buffer for a further 30 minutes. For CD4/1F6, a 0.5 mol/L Tris base buffer (pH 10)40 was used, as above. For CD56/123C3, the sections were placed in hot citrate buffer and heated at maximum power for 8 minutes. Sections of both CD4/1F6 and CD56/123C3 were immediately cooled in TBS, following the heating. Thereafter, all sections were washed in 0.05 mol/L Tris-HCL saline (TBS; pH 7.6) containing 5% fetal calf serum (FCS; GIBCO Laboratories, Grand Island, NY) for at least 15 minutes, and incubated overnight with the primary antibodies at room temperature. All antibody dilutions were made with TBS containing 10% FCS and 0.1% (wt/vol) NaN3. The immunoreactions were detected with DAKO StreptABComplex/HRP Duet Kit, according to the instructions of the manufacturer with the following modification. For detection of the rat anti-perforin antibody P1-8, a biotinylated rabbit anti-rat IgG (Vector Laboratories, Burlingame, CA) was substituted at the secondary antibody step. The peroxidase reaction was developed in TBS containing 0.1% (wt/vol) 3,3′ diaminobenzidine tetrahydrochloride (Sigma, St Louis, MO)/0.03% (vol/vol) H2O2 and the sections were counterstained with Gills hematoxylin.
Reactions were classified as positive if 50% or more of the tumor cells stained, and focally positive if less than 50%, but more than 5%, of the tumor cells stained. Negative reaction was defined if less than 5% of the tumor cells stained.
The SU-DHL-1 ALCL prototypic cell line33 34 was found to be positive both for TIA-1 and perforin either in cytocentrifuged cells or formalin fixed, paraffin wax-embedded cell pellets, and was subsequently used as positive control for expression of these antigens. Paraffin embedded tissue sections from a CD56+ peripheral T-cell lymphoma of small intestinal origin were used as positive control for the CD56 MoAb 123C3.
Reverse transcriptase-polymerase chain reaction (RT-PCR) assay to fusion product of t(2; 5) translocation.The presence of the NPM-ALK fusion transcript was assessed by RT-PCR in a subset of 18 cases of ALCL as previously described.34 The 18 cases included 12 previously reported cases34 and 6 new cases.
Statistical analysis.The chi-square test was used to compare the prevalence of the cytotoxic protein expression in different lymphoma categories. Spearman Rank Order (Spearman R) test with pairwise deletion was performed to evaluate statistical correlations between different antigen expressions in ALCL, as well as to analyze their relationship with the presence of the NPM-ALK fusion product. The Mann-Whitney U test was used to correlate the prevalence of the cytotoxic protein expression with the age of the ALCL patients.
RESULTS
Anaplastic large cell lymphomas of T-cell and ‘null’-cell type.The results are summarized in Tables 1 and 2. Twenty-three (70%) of the 33 ALCL cases revealed TIA-1 positivity in the lymphoma cells. In 20 of the 23 positive cases virtually all tumor cells were positive, while in the remaining 3 cases less than half of the tumor cells showed TIA-1 positivity. Sixteen TIA-1+ cases displayed a T-cell phenotype, six revealed a null-cell phenotype, and one had an ambiguous phenotype staining with both T-cell and NK-cell markers. Positive staining was seen in 13 out of 19 (68%) CS forms, and all (100%) of the 6 HR and 2 SCP variants. The typical staining pattern was granular and cytoplasmic, with frequent paranuclear staining in the Golgi region (Fig 1). The 2 SCP cases showed homogenous granular TIA-1 reaction in both the small cell and the CD30+ large cell components. In addition, 2 of the 3 AIDS-associated cases (67%) showed positive staining, whereas none of the 3 PC forms marked for TIA-1. Beside neoplastic cell staining, small reactive lymphocytes with a similar typical granular staining pattern were seen in all cases investigated. These lymphocytes represented scattered interfollicular and intrafollicular small cells, but in some cases accounted for up to 40% of the non-neoplastic paracortical lymphocytes either in the involved or uninvolved lymph node regions. Cytoplasmic TIA-1 staining also occurred in normal granulocytes, but with a less distinct granular pattern than in positive lymphocytes. In some cases, occasional epithelioid histiocytes displayed weak, diffuse cytoplasmic staining. TIA-1+ tumor cells revealed a distinct granular staining pattern that was always easily distinguishable from reactions of granulocytes and epithelioid histiocytes.
Summary of Findings in ALCL of T- and Null-Cell Type
| Cases . | Age/Sex . | TIA-1 . | PRF . | CD4 . | CD8 . | CD56 . | CD57 . | Other Antigens* . | NPM-ALK . |
|---|---|---|---|---|---|---|---|---|---|
| 1. CS | 9/M | + | + | + | − | − | − | CD2/3/7+, CD5− | ND |
| 2. CS | 28/M | + | + | + | − | − | − | CD2/3/7/43/45R0+, CD5− | ND |
| 3. CS | 53/F | − | + | + | − | − | − | CD45R0+, CD3ε/43− | − |
| 4. CS | 64/M | − | (+) | + | − | − | − | CD3ε/43+, CD45R0− | ND |
| 5. CS | 16/M | + | + | − | + | − | − | CD3/3ε/7+, CD5− | + |
| 6. CS | 62/M | + | + | + | (+) | − | − | CD2/3/5/7+ | ND |
| 7. CS | 11/M | + | + | − | − | − | − | CD2/3/7/43/45R0+, CD5−, β−, δ− | + |
| 8. CS | 5/M | + | + | − | − | − | − | CD5+, CD3/3ε/43/45R0− | ND |
| 9. CS | 28/M | + | + | − | − | + | − | CD3ε/43+, CD2/3/5/7/16−, β−, δ− | + |
| 10. CS | 30/F | + | + | − | − | − | − | CD43+, CD3ε/45R0− | + |
| 11. CS | 35/F | + | + | ND | − | − | − | CD3ε/43/45R0− | + |
| 12. CS | 27/M | + | (+) | − | − | − | − | CD2/3/3ε/5/7−, β−, δ− | ND |
| 13. CS | 14/F | (+) | + | − | − | − | − | CD3ε/43/45R0− | + |
| 14. CS | 41/F | (+) | + | − | − | − | − | CD3ε/43/45R0− | − |
| 15. CS | 49/M | + | − | − | − | − | − | CD3ε/43/45R0− | − |
| 16. CS | 20/F | − | − | + | − | − | − | CD3ε/43/45R0+ | ND |
| 17. CS | 52/M | − | − | − | (+) | − | − | CD2/3/5/43/45R0+, CD7−, β−, δ− | ND |
| 18. CS | 43/M | − | − | + | (+) | − | − | CD3ε/43/45R0+ | + |
| 19. CS | 59/M | − | − | + | + | − | (+) | CD3ε/43+, CD45R0− | + |
| 20. HR | 18/M | + | + | + | − | − | − | CD43/45R0+, CD3ε− | ND |
| 21. HR | 13/M | + | + | + | − | − | − | CD43/45R0+, CD3ε− | + |
| 22. HR | 31/M | + | (+) | + | − | − | − | CD3/43/45R0+, CD2/5/7− | ND |
| 23. HR | 44/F | + | + | (+) | − | − | − | CD43/45R0+, CD3ε− | ND |
| 24. HR | 25/M | + | + | ND | + | ND | ND | CD43/45R0+, CD3ε− | ND |
| 25. HR | 13/M | (+) | + | − | − | − | − | CD2/5/7+, β+, CD3/3ε− | − |
| 26. SCP | 14/F | + | + | ND | + | − | − | CD3ε/43/45R0+ | ND |
| 27. SCP | 7/F | + | + | − | − | − | − | CD3ε/43/45R0+ | ND |
| 28. PC | 29/M | − | − | + | − | − | − | CD2/3/3ε/5/43+, CD7/45R0−, β−, δ− | − |
| 29. PC | 48/M | − | − | + | − | − | − | CD3ε/43/45R0+ | ND |
| 30. PC | 43/M | − | − | + | − | − | − | CD43+, CD3ε/45R0− | − |
| 31. HIV | 3/F | + | − | + | − | − | − | CD2/3/5/7/43+, β+, CD45R0−, δ− | − |
| 32. HIV | 1/M | + | − | − | − | − | − | CD3ε/43/45R0+ | − |
| 33. HIV | 13/F | − | − | − | − | − | − | LCA+, CD3ε/43/45R0/68− | − |
| Cases . | Age/Sex . | TIA-1 . | PRF . | CD4 . | CD8 . | CD56 . | CD57 . | Other Antigens* . | NPM-ALK . |
|---|---|---|---|---|---|---|---|---|---|
| 1. CS | 9/M | + | + | + | − | − | − | CD2/3/7+, CD5− | ND |
| 2. CS | 28/M | + | + | + | − | − | − | CD2/3/7/43/45R0+, CD5− | ND |
| 3. CS | 53/F | − | + | + | − | − | − | CD45R0+, CD3ε/43− | − |
| 4. CS | 64/M | − | (+) | + | − | − | − | CD3ε/43+, CD45R0− | ND |
| 5. CS | 16/M | + | + | − | + | − | − | CD3/3ε/7+, CD5− | + |
| 6. CS | 62/M | + | + | + | (+) | − | − | CD2/3/5/7+ | ND |
| 7. CS | 11/M | + | + | − | − | − | − | CD2/3/7/43/45R0+, CD5−, β−, δ− | + |
| 8. CS | 5/M | + | + | − | − | − | − | CD5+, CD3/3ε/43/45R0− | ND |
| 9. CS | 28/M | + | + | − | − | + | − | CD3ε/43+, CD2/3/5/7/16−, β−, δ− | + |
| 10. CS | 30/F | + | + | − | − | − | − | CD43+, CD3ε/45R0− | + |
| 11. CS | 35/F | + | + | ND | − | − | − | CD3ε/43/45R0− | + |
| 12. CS | 27/M | + | (+) | − | − | − | − | CD2/3/3ε/5/7−, β−, δ− | ND |
| 13. CS | 14/F | (+) | + | − | − | − | − | CD3ε/43/45R0− | + |
| 14. CS | 41/F | (+) | + | − | − | − | − | CD3ε/43/45R0− | − |
| 15. CS | 49/M | + | − | − | − | − | − | CD3ε/43/45R0− | − |
| 16. CS | 20/F | − | − | + | − | − | − | CD3ε/43/45R0+ | ND |
| 17. CS | 52/M | − | − | − | (+) | − | − | CD2/3/5/43/45R0+, CD7−, β−, δ− | ND |
| 18. CS | 43/M | − | − | + | (+) | − | − | CD3ε/43/45R0+ | + |
| 19. CS | 59/M | − | − | + | + | − | (+) | CD3ε/43+, CD45R0− | + |
| 20. HR | 18/M | + | + | + | − | − | − | CD43/45R0+, CD3ε− | ND |
| 21. HR | 13/M | + | + | + | − | − | − | CD43/45R0+, CD3ε− | + |
| 22. HR | 31/M | + | (+) | + | − | − | − | CD3/43/45R0+, CD2/5/7− | ND |
| 23. HR | 44/F | + | + | (+) | − | − | − | CD43/45R0+, CD3ε− | ND |
| 24. HR | 25/M | + | + | ND | + | ND | ND | CD43/45R0+, CD3ε− | ND |
| 25. HR | 13/M | (+) | + | − | − | − | − | CD2/5/7+, β+, CD3/3ε− | − |
| 26. SCP | 14/F | + | + | ND | + | − | − | CD3ε/43/45R0+ | ND |
| 27. SCP | 7/F | + | + | − | − | − | − | CD3ε/43/45R0+ | ND |
| 28. PC | 29/M | − | − | + | − | − | − | CD2/3/3ε/5/43+, CD7/45R0−, β−, δ− | − |
| 29. PC | 48/M | − | − | + | − | − | − | CD3ε/43/45R0+ | ND |
| 30. PC | 43/M | − | − | + | − | − | − | CD43+, CD3ε/45R0− | − |
| 31. HIV | 3/F | + | − | + | − | − | − | CD2/3/5/7/43+, β+, CD45R0−, δ− | − |
| 32. HIV | 1/M | + | − | − | − | − | − | CD3ε/43/45R0+ | − |
| 33. HIV | 13/F | − | − | − | − | − | − | LCA+, CD3ε/43/45R0/68− | − |
+, majority (>50%) of the tumor cells are positive; (+), less than 50% but more than 5% of the tumor cells are positive; −, tumor cells are negative.
Abbreviations: CS, classical systemic form of ALCL; F, female; HIV, human immunodeficiency virus-associated ALCL; HR, histiocyte-rich variant of ALCL; M, male; ND, not done; NPM-ALK, RT-PCR assay for NPM-ALK fusion transcript of t(2; 5) translocation; PC, primary cutaneous form of ALCL; PRF, perforin; SCP, small cell-predominant variant of ALCL; β and δ, T-cell receptor (TCR) beta and delta chains; CD3ε, CD3 epsilon chain as detected with DAKO polyclonal CD3 antibody.
All of the cases were CD30+. No one of these cases showed B-cell marker (CD19, CD22, or CD20/L26) positivity.
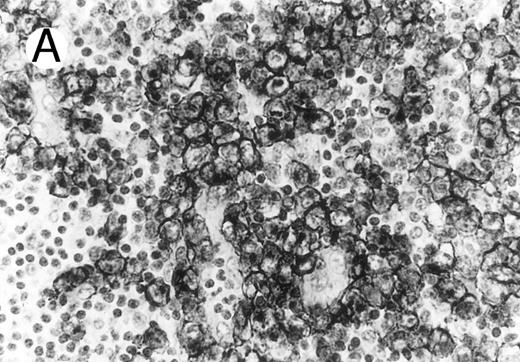
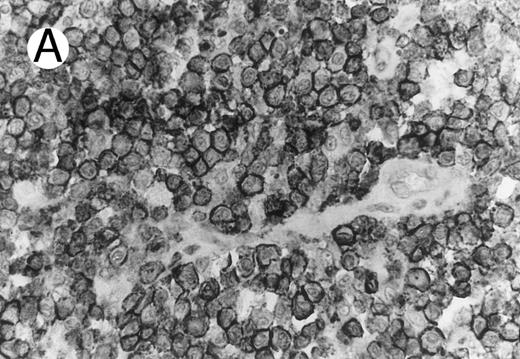
Cytotoxic granule antigen expression in a common form of ALCL (Table 2; case 11). (A) Immunostain for CD30 highlights sheets of large tumor cells. (B) The tumor cells of this case display granular cytoplasmic TIA-1 immunoreaction with paranuclear accumulation in the Golgi region. (C) Strong cytoplasmic perforin reaction is shown in the same case. (sABC-peroxidase technique, hematoxylin counterstain, original magnification × 400.)
Cytotoxic granule antigen expression in a common form of ALCL (Table 2; case 11). (A) Immunostain for CD30 highlights sheets of large tumor cells. (B) The tumor cells of this case display granular cytoplasmic TIA-1 immunoreaction with paranuclear accumulation in the Golgi region. (C) Strong cytoplasmic perforin reaction is shown in the same case. (sABC-peroxidase technique, hematoxylin counterstain, original magnification × 400.)
Twenty-two ALCLs (67%) showed perforin+ neoplastic cells. Three of them were focally positive showing perforin staining in less than half of the large atypical cells. The perforin+ cases comprised 14 of 19 CS forms (74%), 6 of 6 HR (100%), and 2 of 2 SCP variant cases (100%). Perforin+ tumor cells were not detected in any of the AIDS-associated and PC cases. The perforin+ neoplastic cells showed either a dot-like paranuclear or a diffuse cytoplasmic reaction, with frequent perinuclear accumulation outlining the nuclear membrane (Fig 1). Occasionally granular staining was also present in some tumor cells. In the SCP variants, both CD30− small atypical cells and CD30+ large lymphoma cells showed perforin positivity, although the large cells displayed a more intense homogeneous reaction. Scattered non-neoplastic, perforin+ small lymphocytes, displaying a granular reaction were detected in nearly every case in uninvolved sinuses and in the paracortex, providing a convenient endogenous positive control of the immunoreaction. The perforin+ lymphocytes were generally present in much smaller numbers than TIA-1 positive reactive lymphocytes.
Overall 25 (76%) of the 33 ALCLs revealed positivity for at least one of the two cytotoxic granule proteins. The majority of positive cases (20 out of 25; 80%) expressed both proteins. TIA-1 positivity alone was shown in 3 out of 25 cases (12%) including 2 out of 3 AIDS-associated ALCLs, whereas perforin expression in the absence of TIA-1 was found in 2 out of 25 (8%) CS ALCLs. TIA-1 and perforin expressions were highly correlated (P < .001; Spearman R test). Cytotoxic protein expression showed no statistically significant correlation with the patients age (P = .085; Mann-Whitney U test).
Thirty ALCLs were evaluated for CD4 antigen expression. In 17 cases, CD4 immunostaining was performed in paraffin sections, whereas frozen section immunophenotyping data could be obtained in 13 cases. Sixteen of the 30 cases (53%) were CD4+; all displayed a T-cell phenotype. From the 16 CD4+ cases, 10 (63%) coexpressed cytotoxic granule protein (Fig 2). CD4 and CD8 coexpression was shown in 3 out of 30 cases (10%).
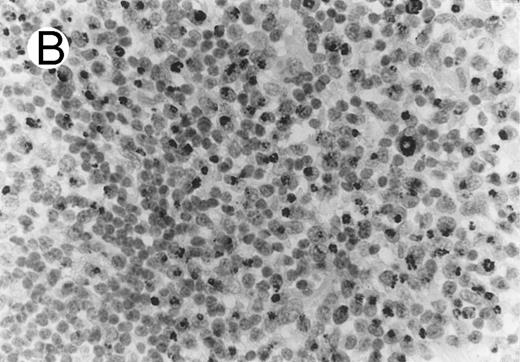
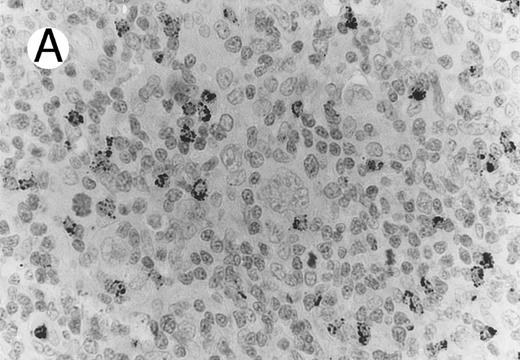
ALCL of cytotoxic T-cell type with CD4 positivity (Table 2; case 3). (A) Paraffin section immunostain shows homogenous CD4 positivity in tumor cells. (B) Perforin positivity is shown in same case. (sABC-peroxidase technique, hematoxylin counterstain, original magnification × 400.)
ALCL of cytotoxic T-cell type with CD4 positivity (Table 2; case 3). (A) Paraffin section immunostain shows homogenous CD4 positivity in tumor cells. (B) Perforin positivity is shown in same case. (sABC-peroxidase technique, hematoxylin counterstain, original magnification × 400.)
CD8 antigen expression was determined in each ALCL, including 20 cases those were studied in paraffin sections with CD8 MoAb C8/144B, and 13 cases immunophenotyped in frozen sections. Twenty-one percent (7/33) of the ALCLs demonstrated CD8 positivity in neoplastic cells. All CD8+ ALCLs had a T-cell phenotype, and 4 coexpressed the cytotoxic granular proteins (Fig 3). CD8+ reactive lymphocytes were seen in all cases investigated, with a geographical distribution closely resembling to distribution of TIA-1+ non-neoplastic lymphocytes.
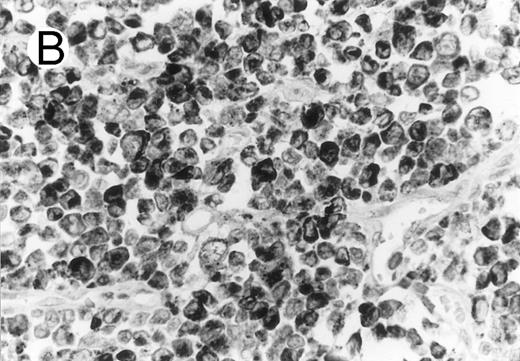
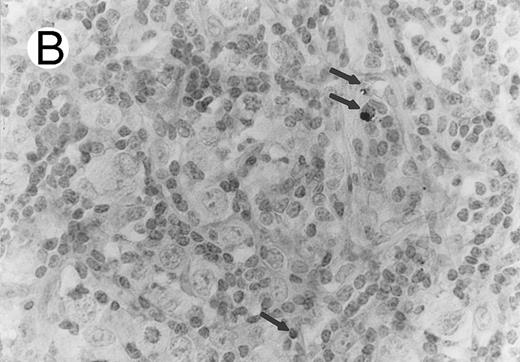
ALCL of CD8+ cytotoxic T-cell type (Table 2; case 5). (A) Tumor cells display strong CD8 positivity in paraffin section. (B) Immunostain for TIA-1 reveals granular staining in the majority of tumor cells. (sABC-peroxidase technique, hematoxylin counterstain, original magnification × 400.)
ALCL of CD8+ cytotoxic T-cell type (Table 2; case 5). (A) Tumor cells display strong CD8 positivity in paraffin section. (B) Immunostain for TIA-1 reveals granular staining in the majority of tumor cells. (sABC-peroxidase technique, hematoxylin counterstain, original magnification × 400.)
Of the 32 ALCLs evaluated for CD56, 1 CS case was positive. This case strongly coexpressed both cytotoxic granule proteins, but did not show surface T-cell antigen (CD2, CD3, CD5, and CD7) positivity. CD3ε chain, however, was found to be positive in this case.
CD57 was shown to be focally positive in 1 out of 32 ALCL. This case coexpressed CD4 and CD8 proteins, but did not express cytotoxic granule proteins.
The above findings allowed us to subclassify the ALCLs into several phenotypic subgroups (Table 3). Eighteen of the 33 cases were designated as having a cytotoxic T-cell phenotype on the basis of coexpression of of cytotoxic proteins and T-cell related antigens. These 18 cases could be further subdivided as follows: 9 CD4+/CD8−, 3 CD4−/CD8+, 5 CD4−/CD8−, and 1 CD4+/CD8+. Seven of the 33 cases (21%) had a phenotype of cytotoxic lymphocytes of unspecified subtype. Six of these expressed cytotoxic granular proteins in the absence of lineage specific markers. The seventh case coexpressed cytotoxic proteins, CD56 antigen and CD3ε, and was considered to have an ambiguous phenotype, consistent with either a T- or NK-cell–like phenotype. Eight of the 33 cases (24%) were cytotoxic protein negative and displayed either a T cell (7 cases) or an unspecified lymphoid cell phenotype (1 case).
Immunophenotypic Subtypes of ALCL
| Cytotoxic protein positive ALCL | 25/33 (76%) |
| CD30+ cytotoxic T cell | 18/33 (55%) |
| CD4+ cytotoxic T cell | 9/33 (27%) |
| CD8+ cytotoxic T cell | 3/33 (9%) |
| CD4/CD8− cytotoxic T cell | 5/33 (15%) |
| CD4/CD8+ cytotoxic T cell | 1/33 (3%) |
| CD30+ cytotoxic lymphocyte, unspecified subtype | 7/33 (21%) |
| CD56+ cytotoxic cell | 1/33 (3%) |
| Cytotoxic cell, unspecified subset | 6/33 (18%) |
| Cytotoxic protein negative ALCL | 8/33 (24%) |
| CD30+ T cell | 7/33 (21%) |
| CD4+ T cell | 4/33 (12%) |
| CD8+ T cell | 1/33 (3%) |
| CD4+/CD8+ T cell | 2/33 (6%) |
| CD30+ lymphoid cell, unspecified subtype | 1/33 (3%) |
| Cytotoxic protein positive ALCL | 25/33 (76%) |
| CD30+ cytotoxic T cell | 18/33 (55%) |
| CD4+ cytotoxic T cell | 9/33 (27%) |
| CD8+ cytotoxic T cell | 3/33 (9%) |
| CD4/CD8− cytotoxic T cell | 5/33 (15%) |
| CD4/CD8+ cytotoxic T cell | 1/33 (3%) |
| CD30+ cytotoxic lymphocyte, unspecified subtype | 7/33 (21%) |
| CD56+ cytotoxic cell | 1/33 (3%) |
| Cytotoxic cell, unspecified subset | 6/33 (18%) |
| Cytotoxic protein negative ALCL | 8/33 (24%) |
| CD30+ T cell | 7/33 (21%) |
| CD4+ T cell | 4/33 (12%) |
| CD8+ T cell | 1/33 (3%) |
| CD4+/CD8+ T cell | 2/33 (6%) |
| CD30+ lymphoid cell, unspecified subtype | 1/33 (3%) |
NPM-ALK fusion product was present in 9 out of 18 (50%) ALCLs and revealed no statistically significant correlation with the TIA-1, perforin, CD4, CD8, CD56, and CD57 expression (P = .346, P = .063, P = .216, P = .063, P = .332 and P = .332; Spearman R test), respectively.
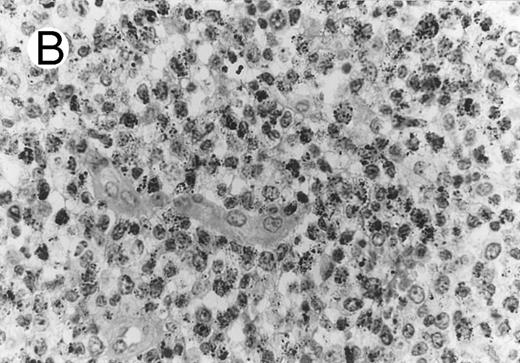
HD.Five of the 51 HD cases (10%), including 2 cases of NS, 2 of MC, and 1 of LD, contained significant numbers of TIA-1-positive tumor cells. Three of the 51 cases (2 NS and 1 MC) showed positivity in the majority of the tumor cells, whereas the remaining two displayed weak focal positivity. Positive Reed-Sternberg (RS) cells displayed either a granular cytoplasmic or a paranuclear dot-like staining. None of 26 cases tested for perforin expression (Fig 4) showed positivity, including 3 TIA-1+ cases. The difference in prevalence of TIA-1 expression in ALCL and HD was statistically significant (P < .001; chi-square test). All TIA1+ HD cases showed a null-cell immunophenotype. Two of the 5 TIA-1+ Hodgkin's lymphomas were studied for the presence of the NPM-ALK fusion product and negative results were obtained for each.
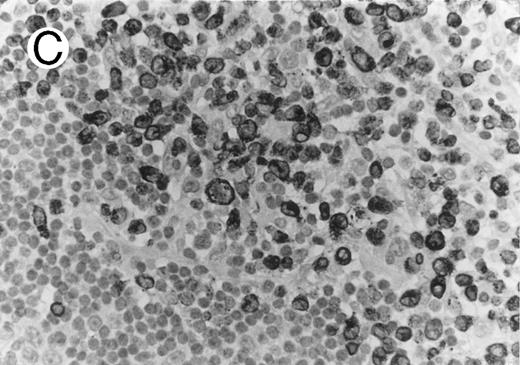
Case of HD stained for cytotoxic granule proteins. (A) Numerous reactive lymphocytes display granular TIA-1 positivity, whereas tumor cells are negative. (B) Rare lymphocytes reveal perforin immunoreaction (arrows). (sABC-peroxidase technique, hematoxylin counterstain, original magnification × 400.)
Case of HD stained for cytotoxic granule proteins. (A) Numerous reactive lymphocytes display granular TIA-1 positivity, whereas tumor cells are negative. (B) Rare lymphocytes reveal perforin immunoreaction (arrows). (sABC-peroxidase technique, hematoxylin counterstain, original magnification × 400.)
Characteristic granular cytoplasmic TIA-1 immunoreactivity occurred in up to 40% of the small lymphocytes in virtually all cases of HD. Variable numbers of these lymphocytes could be found in the vicinity of RS cells. In addition to the reactive lymphocytes and granulocytes, occasional epithelioid histiocytes showed weak diffuse cytoplasmic TIA-1 staining. Perforin positivity was detected in 20% or fewer reactive lymphocytes (Fig 4).
B-cell ALCLs and other large B-cell lymphomas.None of the 17 LBCLs including the 6 B-ALCLs revealed cytotoxic protein expression in the tumor cells. The cytotoxic granular protein expression in T- and null-cell ALCLs and LBCLs revealed a statistically significant difference (P < .001; chi-square test).
DISCUSSION
In this study, we have identified cytotoxic cell related proteins in over 75% of T- and null-cell ALCLs, and in 83% of primary noncutaneous cases. Cytotoxic effector proteins TIA-1 and perforin were identified in 70% and 67% of the cases, respectively. The majority of cases (61%) coexpressed both cytotoxic granule proteins. These data indicate that most primary systemic ALCLs are derived from lymphocytes with cytotoxic potential.
ALCL is generally divided into two main categories based on clinical presentation.21,27 PC ALCL presents in the skin and generally remains localized and indolent for many years.21,27-29 CS cases present as predominantly nodal disease and are generally aggressive tumors, although they often respond to therapy.21,23,27,41 Although both forms show expression of CD30 and have similar anaplastic morphology, their disparate clinical presentation and course, as well as the low frequency of t(2; 5) translocation in the PC form have suggested that these similar immunomorphologic diseases are biologically distinct.27,29,30 34 Our data support the distinct nature of the two diseases, since none of the 3 PC cases expressed the cytotoxic cell constituents perforin and TIA-1.
Within the systemic group of ALCL, there are several morphologic variants, including a small cell (predominant) variant (SCP)42 and an HR variant.43 Both the SCP variant and the HR variant, despite their distinctive morphologies, share clinical and pathologic features with classical systemic ALCL, possess the characteristic t(2:5) translocation, and have a tendency to transform into classical morphology.34,42 43 All eight variant cases (2 SCP and 6 HR) showed expression of both cytotoxic proteins, again supporting their biological relationship to ALCL of classical anaplastic morphology. Furthermore, the presence of cytotoxic granule constituents in both the CD30− small atypical and the CD30+ large cell component of SCP may indicate that both cell populations are part of the neoplastic process. Of the 3 AIDS-associated cases studied, 2 stained for TIA-1 and none stained for perforin, suggesting that some of these cases may also be derived from cytotoxic lymphocytes.
The majority of the ALCL cases (25 out of 33; 76%) expressed cytotoxic granule constituents. Twenty of these expressed both perforin and TIA-1, whereas 5 cases expressed only one of the cytotoxic proteins. The reason for this may be due to the existence of cytotoxic cell subpopulations that are capable of expressing only one or the other of these two proteins or, alternatively, to a loss of expression of one, as a result of the neoplastic process.
Most of the cases which expressed cytotoxic granule proteins coexpressed T-cell associated antigens (18 out of 25; 72%), and were commonly CD4+ (9/25; 36%). CD8 was expressed by 3 out of 25 (12%) cytotoxic granule protein positive cases. One additional case coexpressed both CD4 and CD8 antigens. Six cases did not display lineage restricted markers and one ambiguous case coexpressed both CD56 and T-cell markers. Thus, phenotypically, the majority of cases resemble cytotoxic T lymphocytes. Although most cytotoxic T cells generally express CD8 antigen, a subset of CD4+ T cells has been reported that may acquire cytotoxic activity on activation.2 7
Cytotoxic lymphocytes represent a heterogeneous but functionally related subpopulation of lymphocytes, which includes NK cells, cytotoxic α/β T cells and γ/δ T cells.3-5,7,9 These lymphocyte subsets differ from each other in origin, phenotype and target cell recognition, but share a common contact-dependent nonphagocytic killing mechanism.5,9 Perforin, granzymes, and TIA-1 comprise the major toxic constituents of functionally active cytotoxic lymphocytes.3-7,11,13 Perforin is able to lyse target cells through the formation of membrane channels, which allow passage of large proteins and ions.9 The presence of perforin correlates with cytotoxic activity of the lymphocytes.7 The granzymes, a serine protease subfamily, are also present in secretory granules of cytotoxic lymphocytes.5,6,8 TIA-1 can be recognized by an MoAb raised against a cytoplasmic antigen of permeabilized T cells.11,13 Using this antibody, the TIA-1 antigen has been found to be restricted to lymphocytes with cytotoxic capacity, regardless of their activation status.11,13 The function of TIA-1 and the granzymes has been linked to the induction of DNA-fragmentation during target cell killing.10 12
Most cytotoxic effector cells show granular lymphocyte cytomorphology because of the presence of azurophilic cytoplasmic granules.1-4 These specialized granules contain effector proteins that are important in the cytotoxic activity.3-9 Using antibodies to two of these cytotoxic cell restricted granular proteins, TIA-1 and perforin, we found that the majority of the ALCLs display a cytotoxic cell-like phenotype. Both the positive neoplastic cells and positive reactive lymphocytes present in the tumors revealed a typical granular cytoplasmic staining using the TIA-1 MoAb. By immuno-electron microscopy, TIA-1 has been found in dense-core granules, multivesicular bodies, and in the Golgi apparatus of CTLs.11 Despite numerous light microscopic and ultrastructural studies in ALCL, neither azurophilic nor dense-core granules, the ultrastructural equivalents of azurophilic granules, have been observed, although occasionally the presence of some dense lysosomes and granules have been reported.44 45 Nevertheless the light microscopic immunohistochemical staining pattern of TIA-1 suggests the existence of equivalent structures in ALCL. The diffuse cytoplasmic appearance of the perforin we frequently found in the tumor cells suggests that this protein compartment is only partially organized into granules.
The perforin MoAb P1-8, used in this study, was developed to mouse perforin and was shown to be reactive to denatured protein.46 It also shows a strong and specific reaction to human perforin.4,7 This interspecies cross-reactivity is due to the close to 70% amino acid sequence homology between the human and mouse perforin proteins.47 In our initial pilot studies using this antibody we had confirmed its reported reactivity with human perforin by Western blot analysis and showed its ability to mark both frozen and paraffin tissue sections equally well. The present study shows the utility of the P1-8 antibody in identifying perforin containing cells in fixed paraffin embedded tissues.
The nonrandom chromosomal translocation t(2; 5)(p23; q35) and its molecular counterpart the fusion of the NPM and ALK genes has been reported in up to 60% of classical ALCLs.30-34 In the present study, we found that cytotoxic granule protein+ cases may display either NPM-ALK positivity or negativity. Our data suggest that both the cytotoxic phenotype and t(2; 5) translocation are common, but independent phenomena in ALCL of T- and null-cell type.
There is very little additional information in the literature regarding the presence of cytotoxic cell associated proteins in lymphomas. TIA-1 has been previously reported in the reactive T cells of AIDS-related and follicular lymphomas.48,49 Recently, TIA-1 has been reported to be consistently present in certain NK cell-like lymphomas.18 Finally, hepatosplenic γ/δ T-cell lymphomas may also be derived from cytotoxic lymphocytes, because all express TIA-1 and CD56.19 Perforin-positive malignant lymphomas have been occasionally described, but the findings are restricted to CD56+ cases.15,17 Granzyme B expression has been studied in a large series of peripheral T-cell lymphomas,16 where it was detected at highest prevalence in extranodal tumors, particularly in those involving mucosa-associated tissues. Of the 25 cases of ALCL studied, 16% (4 cases) were found positive.16 In contrast, our study focusing on TIA-1 and perforin revealed a 76% positivity rate for cytotoxic antigen expression. Although the high prevalence of cytotoxic antigen expression in ALCL implies derivation from cells with cytotoxic potential, the exact nature of this cell, whether a precursor or a mature cytotoxic effector cell, cannot be discerned from this investigation.
ALCL shows morphological and immunological overlaps with classical HD.35 Because of this, it has been suggested that the two entities are histogenetically related.21,23,35 In the present study, 10% of HD cases showed cytotoxic granule protein positivity, and those were positive only for TIA-1. Furthermore, 2 of the 5 positive cases stained only focally. Similar findings have been published by Oudejans et al,50 who described granzyme B expression in 11 out of 61 (18%) HD cases.50 In the latter study, cytotoxic antigen expression was reported as weak in 10 of the 11 positive cases. This weak and focal staining is in contrast to most ALCLs, which show strong and generalized cytotoxic granule protein positivity. These findings are consistent with the accumulating evidence from biologic and molecular genetic studies, which support the notion that ALCL and most HD are not biologically related.34 Although the presence of cytotoxic protein expression alone does not exclude a diagnosis of HD, the evaluation of cytotoxic protein expression, as part of a larger panel of diagnostic antibodies, should facilitate the histomorphological differential diagnosis of ALCL and HD.
Occasional ALCLs are reported to have a B-cell immunophenotype,23,26 and the updated Kiel Classification recognizes an entity of B-cell ALCL.38 However, B-cell ALCL is a controversial category and its frequency and relationship to non-B–cell ALCLs is uncertain. Although rare B-cell lymphomas have been reported to have molecular or cytogenetic evidence of the t(2; 5) translocation (the characteristic genetic lesion of the non-B–cell ALCL group), including two cases of anaplastic B-cell lymphoma,51-56 neither of the latter anaplastic cases were shown to express the NPM/ALK fusion message. Furthermore, in the single largest molecular study of B-ALCL to date, none of 10 cases examined by RT-PCR expressed the NPM/ALK fusion message.57 The presence of the t(2; 5) or the NPM/ALK fusion message in B-cell lymphomas also does not appear to define a specific histologic or immunophenotypic entity, since the t(2; 5) has been reported in both CD30 positive and negative cases, and in both common large B-cell lymphomas as well as in morphologically anaplastic B-cell lymphomas. In addition, cases reported as B-ALCL usually do not show the relatively favorable prognosis of the primary non-B–cell CD30+ lymphomas, and are clinically indistinguishable from the CD30− aggressive LBCLs.36,41 For these reasons, B-cell ALCL is not recognized as a separate entity in the REAL classification and was designated only as a morphologic variant of diffuse LBCL.21 The 17 morphologically anaplastic LBCLs, in the present study, which included 6 cases consistent with B-cell ALCL (according to the Updated Kiel Classification38; anaplastic morphology and CD30 positivity), were consistently negative for cytotoxic cell granular proteins. Interestingly, 3 of the latter 6 cases studied for expression of p80, the protein product of the NPM/ALK fusion message, were negative (data not shown). These findings support the concept that despite their immunomorphologic similarities with typical T- and null-cell ALCLs, anaplastic B-cell lymphomas are biologically distinct and best grouped with LBCLs.21 36
CTLs and NK cells show unidirectional killing of target cells without causing self-destruction or damaging bystander cells. When the killing process is completed, the cytotoxic lymphocytes remain viable and they are able to recycle and initiate a new lytic interaction.58 Both CTLs and NK cells have been found to be highly resistant in vitro to cytoplasmic granule-associated killing mediated by either other cytotoxic lymphocytes or their own secreted granules, or by perforin itself.59 60 It is interesting to speculate that should this resistance extend to neoplastic cells possessing a cytotoxic cell phenotype, it may help these tumors escape from control of cellular immunity, and, as a consequence, play a role in lymphomagenesis. Further investigations will need to be performed to define the functional significance of the cytotoxic phenotype in the pathogenesis of T- and null-cell ALCL.
ACKNOWLEDGMENT
The authors are grateful to Cynthia A. Harris, Sarah E. Delay-Brown, Maria Labdy, and Agnes Dudas for their expert technical assistance, and to Hideo Yagita, Kevin C. Gatter, and John Hilkens for providing us with the perforin, CD8 and CD56 antibodies, respectively. In addition, we thank Joseph M. Tuscano, Gyorgy Petrovics and Tibor Krenacs for their contributions to this study, and Ralph L. Isenberg for his photographic assistance.
Address reprint requests to Mark Raffeld, MD, Hematopathology Section, Laboratory of Pathology, Bldg 10, Room 2N112, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal