MANY STUDIES HAVE led to the concept that membrane phospholipid asymmetry is ubiquitous. In general terms, the outer leaflet of eukaryotic plasma membranes is formed predominantly with the cholinephospholipids (sphingomyelin and phosphatidylcholine [PC]), whereas the majority of the aminophospholipids (phosphatidylserine [PS] and phosphatidylethanolamine [PE]) are confined to the membrane's inner leaflet. This selective localization dictates that asymmetric biomembranes are assembled and maintained by specific mechanisms that control transbilayer lipid sidedness. In 1984, it became clear that asymmetry was generated by the activity of an adenosine triphosphate (ATP)-dependent aminophospholipid translocase that specifically transports PS and PE between bilayer leaflets.1 This discovery underscored the prevailing concept that membrane lipid asymmetry was of major physiologic importance, because it showed that cells invest energy to catalyze lipid movement in order to maintain a specific transmembrane phospholipid distribution.
Although asymmetry is the rule for normal cells, loss of asymmetry, especially the appearance of PS at the cell surface, is associated with many physiologic and pathologic phenomena. Bevers et al2,3 were the first to report that the asymmetric orientation of phospholipids in blood platelets was rapidly lost upon influx of calcium during their activation, a finding that suggested a critical role for PS in thrombosis.4,5 Apoptotic6-9 and tumorigenic cells10,11 also express relatively large amounts of outer-leaflet PS that may serve as a trigger for macrophage recognition and promote the cells' phagocytosis.12-15
In this essay, we summarize recent observations on the perturbation of membrane phospholipid asymmetry and present a somewhat stochastic view of the patho-physiologic implications of surface-exposed PS. We also review briefly the mechanisms believed to be responsible for the regulation of phospholipid distributions across plasma membranes. Other details of transbilayer lipid distributions and the various techniques used in this field will not be discussed; several recent reviews of these and related topics have been published elsewhere.16-21
REGULATION OF MEMBRANE PHOSPHOLIPID TOPOGRAPHY
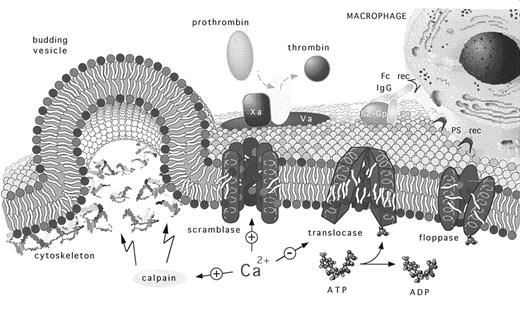
At least three distinct activities are involved in the regulation of membrane lipid sidedness. Two energy-requiring activities seem to work in concert to maintain a nonrandom transbilayer phospholipid orientation. Inhibition of these activities stops lipid movement, but it does not result in loss of asymmetry for at least several days in vitro. Influx of Ca2+ into the cytoplasm, on the other hand, activates a scramblase activity that results in rapid transbilayer phospholipid mixing that leads to a nearly symmetric distribution of phospholipids across the membrane bilayer (Fig 1).
The regulation and physiology of membrane phospholipid asymmetry. This model describes how membrane phospholipid asymmetry is generated, maintained, and perturbed as a prerequisite to various phosphatidylserine-related pathophysiologies. Membrane lipid asymmetry is regulated by the cooperative activities of three transporters. The ATP-dependent aminophospholipid-specific translocase, which rapidly transports PS and PE from the cell's outer-to-inner leaflet; the ATP-dependent nonspecific lipid floppase, which slowly transports lipids from the cell's inner-to-outer leaflet; and the Ca2+-dependent nonspecific lipid scramblase, which allows lipids to move randomly between both leaflets. The model predicts that the translocases are targets for Ca2+ that directly regulates the transporter's activities. The figure shows that elevated intracellular Ca2+ induces PS randomization across the cell's plasma membrane by providing a stimulus that positively and negatively regulates scramblase and translocase activities, respectively. At physiologic Ca2+ concentrations, PS asymmetry is promoted because of an active translocase and floppase but inactive scramblase. Depending on the type of cell, elevated intracellular Ca2+ levels can be achieved by cellular activation that generally results in the concomitant influx and accumulation of extracellular Ca2+ and by its release from intracellular stores. Increased cytosolic Ca2+ can also result in calpain activation, which facilitates membrane blebbing and the release of PS-expressing procoagulant microvesicles. Exposure of PS at the cell's outer leaflet. The appearance of PS at the cell's outer leaflet promotes coagulation and thrombosis by providing a catalytic surface for the assembly of the prothrombinase and tenase (not shown) complexes and marks the cell as a pathologic target for elimination by phagocytes. Recognition of the PS-expressing targets can occur by both antibody-dependent and direct receptor-mediated pathways. (Aminophospholipids are shown with red polar headgroups and cholinephospholipids with blue polar headgroups, see cover photo; β2-Gp, β2-glycoprotein-I; rec, receptor).
The regulation and physiology of membrane phospholipid asymmetry. This model describes how membrane phospholipid asymmetry is generated, maintained, and perturbed as a prerequisite to various phosphatidylserine-related pathophysiologies. Membrane lipid asymmetry is regulated by the cooperative activities of three transporters. The ATP-dependent aminophospholipid-specific translocase, which rapidly transports PS and PE from the cell's outer-to-inner leaflet; the ATP-dependent nonspecific lipid floppase, which slowly transports lipids from the cell's inner-to-outer leaflet; and the Ca2+-dependent nonspecific lipid scramblase, which allows lipids to move randomly between both leaflets. The model predicts that the translocases are targets for Ca2+ that directly regulates the transporter's activities. The figure shows that elevated intracellular Ca2+ induces PS randomization across the cell's plasma membrane by providing a stimulus that positively and negatively regulates scramblase and translocase activities, respectively. At physiologic Ca2+ concentrations, PS asymmetry is promoted because of an active translocase and floppase but inactive scramblase. Depending on the type of cell, elevated intracellular Ca2+ levels can be achieved by cellular activation that generally results in the concomitant influx and accumulation of extracellular Ca2+ and by its release from intracellular stores. Increased cytosolic Ca2+ can also result in calpain activation, which facilitates membrane blebbing and the release of PS-expressing procoagulant microvesicles. Exposure of PS at the cell's outer leaflet. The appearance of PS at the cell's outer leaflet promotes coagulation and thrombosis by providing a catalytic surface for the assembly of the prothrombinase and tenase (not shown) complexes and marks the cell as a pathologic target for elimination by phagocytes. Recognition of the PS-expressing targets can occur by both antibody-dependent and direct receptor-mediated pathways. (Aminophospholipids are shown with red polar headgroups and cholinephospholipids with blue polar headgroups, see cover photo; β2-Gp, β2-glycoprotein-I; rec, receptor).
Aminophospholipid translocase.The discovery of an ATP-dependent aminophospholipid translocase in red blood cells has provided direct evidence for the existence of mechanisms that generate and maintain membrane asymmetry through the transport of specific lipids across the cell's membrane.1,22,23 This activity is distinguished by its ability to transport PS and PE from the outer to inner leaflet of plasma membranes against the concentration gradient. Cholinephospholipids are not moved. Competition experiments have shown that the same protein transports both PS and PE, although PS is transported much faster, with half-times of 5 to 10 minutes.24,25 This process consumes one molecule of ATP per molecule of lipid transported.26 Transport is stereospecific for naturally occurring L-isomers of the glycero-backbone27 and is inhibited by vanadate,1 sulfhydryl-reactive reagents,28,29 and the histidine-reactive reagent bromophenacylbromide.25 In addition, activity is abrogated when cytoplasmic Ca2+ levels reach micromolar concentrations.30 31
Although these observations clearly indicate that lipid transport is catalyzed by one or more membrane proteins, its identity is still uncertain. A 110-kD Mg2+-ATPase has been partially purified32-34 and reconstituted into artificial lipid vesicles with at least a fraction of its active center at the outer face.35 These vesicles transported a spin-labeled PS analog from the inner to outer leaflet upon the addition of Mg2+-ATP, suggesting that this ATPase is responsible for aminophospholipid translocation. However, it was recently reported that, similar to previous purification strategies,33 the active fraction was not homogenous and contained several proteins ranging from 35 kD to 120 kD.36 These observations preclude assignment of the transporter to a single protein and are not inconsistent with studies implicating the involvement of a 30-kD to 32-kD band 7 transmembrane polypeptide in aminophospholipid transport.28,37,38 This protein, which may be complexed to Rh polypeptides, was preferentially labeled in erythrocyte membranes with a photoactivatable PS analog only under conditions conducive to PS transport.25,39,40 Consistent with these observations, aminophospholipid transport activity was suggested to require the coordinated and complimentary participation of a Mg2+-ATPase and a 32-kD protein,16 a motif not uncommon for the ATP-binding cassette (ABC) family of proteins to which the aminophospholipid translocase might belong.41 42
Aminophospholipid translocase activity has been observed in other membranes, including intracellular chromaffin granule membranes43 and endoplasmatic reticulum.44 Activity has also been shown in various cells,31,45-50 including endothelial cells, in which its expression is regulated by basic fibroblast growth factor.51 Mutants defective in PS transport have been isolated.52
ATP-dependent floppase.Another ATP-dependent translocating activity has been described that, in conjunction with the aminophospholipid translocase, may regulate the differential transbilayer orientation of phospholipids in complex biologic membranes. A less specific ATP-requiring floppase, first discovered in red blood cells, transports both aminophospholipids and cholinephospholipids from the inner to the outer leaflet with half-times about 10 times longer than those of the translocase-mediated inward movement of PS and PE.25,53-55 Similar to inward transport of aminophospholipids, outward movement was found to be abrogated by ATP depletion, sulfhydryl oxidation, and histidine modification, indicating that this process is also energy- and protein-dependent.25 Whether outward transport is an intrinsic property of the aminophospholipid translocase or is caused by the activity of a distinct membrane protein or protein complex is not known. Because rapid inward translocation of aminophospholipids does not accelerate outward migration of all phospholipids, both processes may be mediated by independent mechanisms.25 Nonetheless, both lipid-transporting activities seem to act in concert and establish a dynamic asymmetric steady-state in which all phospholipids are slowly but continuously moved to the outer membrane surface, whereas the aminophospholipids are shuttled directly back to the inner leaflet.21,25 Thus, the combined action of translocase and floppase seems to equip the cell with a mechanism that corrects for alterations in lipid distributions to avoid potential pathologic consequences. Whereas the maintenance of membrane lipid is asymmetry is stable and resistant to the mechanical stresses56 likely endured in the peripheral circulation, rapid perturbations in lipid asymmetry could be coincident to different membrane fusion events that accompany endocytosis and exocytosis.17,31 57-60
Lipid scramblase.Platelet plasma membranes harbor a Ca2+-dependent mechanism that can rapidly move phospholipids back and forth between the two membrane leaflets (flip-flop), leading within minutes to a loss of membrane lipid asymmetry.2,3 Because the influx of Ca2+ also abrogates aminophospholipid translocase activity,30,31,46 Ca2+-dependent loss of membrane phospholipid asymmetry is not corrected. Considering that spontaneous transbilayer migration of lipids is thermodynamically unfavorable, Ca2+-induced lipid randomization is likely to depend on a protein or proteins with lipid scramblase activity.61 Indeed, the existence of an inherited bleeding disorder (Scott syndrome, see below), characterized by an impairment of scramblase activity, reinforces the notion that specific membrane proteins are involved in this process.62-64 Ca2+-induced scramblase activity has also been found in other cells, but its activity is usually lower than in blood platelets (reviewed previously16 18 ).
Scramblase activity requires the continuous presence of cytoplasmic calcium.65 Provided the aminophospholipid translocase is not irreversibly inactivated by intracellular calpain, Ca2+ efflux can lead to restoration of lipid asymmetry.57 Lipid scrambling is bidirectional, and all major lipid classes move back and forth at comparable rates.46,65,66 Unlike the energy-dependent translocase and floppase, the lipid scramblase does not require hydrolyzable ATP. However, its activity partially decreases during prolonged ATP depletion.67,68 Proteins fractionated from platelet membranes have been reconstituted into artificial lipid vesicles, which exhibited Ca2+-dependent lipid-scrambling activity that was pronase-, heat-, and sulfhydryl-sensitive.69 Similar experiments performed with proteins from red blood cell membranes suggested that a 37-kD protein may be responsible for lipid scrambling activity.70 Although these data indicate that a protein is responsible for scramblase activity, it has also been proposed that lipid scrambling is the result of a complex between phosphatidylinositol 4,5-bisphosphate and calcium.67 In addition to the protein reconstitution studies, other experiments also indicate that such a mechanism cannot account for the scramblase activity observed in platelets.71
Loss of membrane lipid asymmetry is often accompanied by blebbing and subsequent shedding of lipid-symmetric microvesicles from the cell surface.57,72-75 Fusion between opposing segments of plasma membrane before the release of microvesicles was proposed to cause a localized collapse of lipid asymmetry.72 However, recent evidence indicates that this is not the mechanism responsible for lipid scrambling because (1) Ca2+-induced randomization of lipids can occur in the absence of microvesicle formation under conditions in which activation of intracellular calpain is prevented75-77 and (2) lipid scrambling and microvesicle formation are deficient in Scott syndrome72 but calpain activity is normal.62 Thus, at least both lipid scrambling and calpain activation are required for shedding of microvesicles (Fig 1).
In summary, the synchronous and cooperative action of the aminophospholipid translocase and the nonspecific floppase contribute to the generation and maintenance of membrane phospholipid asymmetry, whereas lipid scramblase activity results in its collapse. At physiologic (ie, low) cytoplasmic Ca2+ levels, both aminophospholipid translocase and nonspecific floppase are active, and phospholipid asymmetry is maintained. Conversely, high cytoplasmic Ca2+ concentrations activate lipid scramblase and block the cooperative action of translocase and floppase, leading to randomization of phospholipids across the membrane lipid bilayer. Conceivably, intermediate Ca2+ levels could lead to a circumstance in which both mechanisms are active and oppose each other.21 These situations can accommodate a wide range of steady-state distributions of membrane phospholipids commonly seen in in vitro stored red blood cells,78-80 sickle cells,81-84 blood cells from diabetics,85,86 aged red blood cells,87,88 and undifferentiated tumorigenic cells.10 11
Other mechanisms.Many studies have suggested that cytoskeletal proteins assist in the maintenance of membrane phospholipid asymmetry by selectively interacting with aminophospholipids.89-91 However, the interaction between PS and cytoskeletal proteins is thermodynamically weak and there is evidence that lipid asymmetry can be generated and maintained in artificial membrane preparations that lack cytoskeletal proteins.26,92,93 Moreover, spherocytic erythrocytes fully conserve lipid asymmetry despite markedly diminished levels of spectrin.94 Although these observations do not unequivocally rule out the cytoskeleton's function in the maintenance of lipid asymmetry, it is presumably not of major importance.
Another group of proteins that translocate lipid or lipid-soluble compounds across the plasma membrane have recently received wide attention. Unidirectional transport of PC is catalyzed by a member of the P-glycoprotein family in the canalicular domain of murine hepatocyte plasma membranes to provide PC for bile production.95,96 This membrane glycoprotein is encoded by the murine multidrug resistance gene mdr2 and belongs to the family of ABC proteins. Although its behavior resembles that of the ATP-dependent translocase and floppase, its lipid specificity is clearly different. Moreover, its presence may be restricted to hepatocyte membranes, precluding a role in establishing lipid asymmetry in other cells. However, P-glycoprotein encoded by the mdr1 gene is abundantly expressed in drug-resistant tumor cells, where it nonselectively expels lipid-soluble compounds from the inner to the outer membrane leaflet.97 98 Although the properties of the mdr1 P-glycoprotein resemble those of the nonselective red blood cell floppase, their possible relationship remains to be explored.
A recent proposal is that, unlike phospholipid scrambling in red blood cells, PS exposure in activated platelets is caused by a vectorial inward-outward aminophospholipid-specific transport mechanism.99,100 This conclusion contrasts with observations from other laboratories that show Ca2+-induced lipid scrambling involves nonspecific flip-flop of all lipid classes.3,46,65,66,72,101 Platelets are unlikely to have a specific mechanism different from that of red blood cells because the hereditary abnormality in PS exposure in Scott syndrome equally affects both platelets and erythrocytes.102 Moreover, insurmountable shape changes would be produced by the large mass-imbalance generated when outward transport of aminophospholipids is not compensated by inward transport of other lipids.18 Indeed, less than 1% of a mass imbalance produces large shape changes in giant unilamellar liposomes.103 Although the cell might be able to compensate for bilayer imbalances by releasing microvesicles, PS exposure in platelets can occur without microvesicle release.77
HEMOSTASIS AND THROMBOSIS
Lipids and coagulation.Membrane phospholipids propagate the proteolytic reactions that result in thrombin formation by promoting the assembly of coagulation factors on their surface. The most important pathway of coagulation is initiated by tissue factor, an integral membrane protein expressed on the surface of activated or disrupted cells.104-107 Tissue factor interacts with factor VII or VIIa, and this complex rapidly converts the zymogen factor IX, factor X, and factor VII itself into their active forms. Although assembly and catalytic activity of the tissue factor/factor VIIa complex is effective in the absence of anionic phospholipids, activity is increased by PS.108-110 However, anionic phospholipids are indispensable in promoting membrane binding and catalytic activity of the two subsequent coagulation factor complexes in the cascade that leads to thrombin formation.106,111 The tenase complex is initiated by the interaction of factor VIIIa with negatively charged lipid to create a high-affinity binding site for the enzyme factor IXa in the presence of Ca2+. This complex rapidly activates factor X into Xa. Likewise, in the prothrombinase complex, binding of factor Va to an anionic lipid surface promotes Ca2+-dependent binding of factor Xa, which converts prothrombin to thrombin (Fig 1). In both complexes, PS is the most effective anionic phospholipid.112 Binding of factors Va and VIIIa to naturally occurring phosphatidyl-L-serine is stereospecific and occurs with lower affinity to phosphatidyl-D-serine and other anionic phospholipids.113,114 PS is equally important in promoting the anticoagulant protein C pathway that provides feedback inhibition of thrombin formation. Protein C effectively inactivates factor Va when both are bound to the same lipid surface, which leads to disassembly of the prothrombinase complex.105 115
Procoagulant activity of blood platelets.Surface exposure of PS in platelet membranes provides for efficient propagation and control of the hemostatic process. Kalafatis et al106 have argued that specific protein receptors for factors Va and VIIIa may, in addition to PS binding sites, be present in cellular membranes. However, it should be noted that phospholipases116 and annexin V117 inhibit platelet prothrombinase activity. Although this does not eliminate the putative existence of protein receptors, these data prove that PS exposure is critical to coagulation. However, because platelets do not contain tissue factor they cannot initiate the coagulation cascade. Moreover, annexin V inhibits prothrombin activation only in cells that express both tissue factor- and prothrombinase-binding sites.118 These data indicate that surface exposure of PS is required for propagation but not necessarily for initiation of the coagulation process.
The extent to which membrane phospholipid asymmetry becomes perturbed during platelet activation correlates with the cells' ability to promote tenase and prothrombinase activity119 and depends on the type of agonist.3,72,120,121 Ca2+-ionophore is the most effective followed by complement membrane attack complex C5b-9, collagen + thrombin, collagen, and thrombin. ADP and epinephrine have no effect. The same order of agonist activity is observed for the extent of lipid-symmetric microvesicle shedding from the platelet surface.72 75
Platelet activation after vascular damage involves adhesion to subendothelial structures and aggregation of platelets to form a primary hemostatic plug at the wound site. The exposure of PS on aggregated platelets restricts and controls thrombin formation at the site of injury by providing a catalytic membrane surface for both procoagulant (tenase and prothrombinase) and anticoagulant (protein C) reactions. The physiologic significance of platelet-derived microvesicles is not clear. Because microvesicles tend to circulate rather than stick to the platelet aggregate, it is thought that they may be associated with thrombotic conditions. Indeed, increased amounts of circulating microvesicles have been observed in patients suffering from various disorders associated with secondary activated coagulation122 and in patients with such primary thrombotic disorders as transient ischemic attacks and myocardial infarction.123,124 It has also been shown that platelet microvesicles bind to and activate neutrophils, suggesting that hemostasis and inflammation may be linked.125 Indeed, it has recently been shown that the leukocyte adhesion molecule L-selectin binds PS.126 Thus, activation and aggregation of platelets at the site of injury could recruit leukocytes to the site of inflammation via the binding of platelet-exposed PS to leukocyte L-selectin. Unlike platelet-derived microvesicles, microparticles released from other cells may also contain tissue factor activity and initiate undesired coagulation in the circulation.118,127 128
Scott syndrome, a disorder of Ca-induced lipid scrambling.Scott syndrome, which was first described by Weiss et al,129 is a rare, moderately severe, bleeding disorder characterized by a deficiency in platelet procoagulant activity that is not associated with decreased coagulation factor levels.63 Although activation of these platelets results in normal secretion and aggregation, they express a relatively low number of factor Va and VIIIa binding sites.130,131 These cells exhibit decreased surface exposure of PS, reduced ability to promote both tenase and prothombinase activity in response to agonists,62 and impaired capacity to shed membrane-derived microvesicles.72 Studies of a recently discovered family in France indicated that Scott syndrome is an inherited bleeding disorder transmitted as an autosomal recessive trait.64 Remarkably, the defect in Ca2+-induced lipid scrambling is not restricted to platelets but can also be shown in the patients' erythrocytes, erythrocyte ghosts,102 and in Epstein-Barr virus-transformed B-lymphocytes.64,132 Experiments with single-cell clones of transformed lymphocytes have suggested that the reduced exposure of PS affects all cells to the same extent. Fusion of the lymphoblasts with a myeloma cell line restored PS exposures to normal levels.132 Platelets and erythrocytes from patients with Scott syndrome have normal phospholipid composition and show no obvious protein abnormality when examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.102 Taken together, these studies support the notion that Scott syndrome results from a deletion or mutation in multiple hematologic lineages that either affects the putative phospholipid scramblase directly or alters the Ca2+-induced activation mechanism.
Procoagulant activity of red blood cells.Although red blood cells lose phospholipid asymmetry and stimulate prothrombinase activity after the addition of Ca2+-ionophore,54,57,66,68,133 they are not considered significant in hemostasis and thrombosis. However, the possibility that perturbations in PS exposure contribute to thrombotic events commonly seen in diabetes mellitus and sickle cell crisis should not be dismissed. Indeed, increased procoagulant activity associated with loss of lipid asymmetry has been observed in erythrocytes incubated in hyperglycemic buffers,86 in platelets from diabetic patients,134 and in vesicles shed from reversibly sickled cells by repeated hypoxia-induced sickling.91 Interestingly, Ca2+-induced transbilayer movement of PS and the generation of red blood cell procoagulant activity is strongly inhibited by high-density lipoprotein (HDL) and apolipoprotein A-1135; this raises the possibility that the protective effect of HDL against arterial thrombosis may be due, in part, to HDL-dependent inhibition of thrombogenic surface expression.
Procoagulant activity of white blood cells.Although lipid sidedness has not been investigated in white blood cells, functional assembly of the prothrombinase complex on monocytes, neutrophils, and lymphocytes has been found to be kinetically equivalent to artificial PS-PC vesicles.136,137 The prothrombinase activity of monocytes is enhanced by endotoxin activation, a result that supports the notion that their ability to contribute to thrombin formation is important in the thrombotic events associated with inflammation and healing.138,139 Interestingly, the prothrombinase activity of activated monocytes can be enhanced by a reduction in their ATP levels and reduced by inhibition of protein synthesis. These observation may be directly related to the appearance of PS at the cell surface. Hypothetically, reduction of ATP would result in inhibiting the aminophospholipid translocase and prevent recovery from the cell's procoagulant state. Conversely, inhibition of protein synthesis would limit expression of the lipid scramblase and prevent PS exposure. Because stimulated monocytes also release microvesicles that express tissue factor activity,138-141 they have the capacity both to initiate and propagate coagulation in response to an inflammatory stimulus.
Complement activation of endothelial cells.Consistent with the principal anticoagulant function of endothelial cells, prothombinase assembly, even when stimulated with Ca2+-ionophore, is poorly supported on these cells.142 However, an interesting exception is observed upon insertion of complement membrane attack complex C5b-9. In this case, the assembled complement pore is removed from the cell by shedding into procoagulant microvesicles. The remnant cell, having discarded most of the complex, remains mainly noncoagulant.142 A similar mechanism of complement-mediated formation of procoagulant microvesicles occurs in platelets.121 This raises the possibility that complement-induced release of microvesicles into the circulation could contribute to inflammation-associated disseminated intravascular coagulation.
Complement-induced loss of lipid asymmetry in cell-derived microvesicles may be an intrinsic property of complement pore formation because incubation of lipid vesicles with C5b-9 causes transbilayer lipid exchange between both leaflets.143 Such pore-forming peptides as mellitin have also been shown to promote fast flip-flop of lipids in liposomes.144 These findings raise the possibility that the mechanism of Ca2+-induced phospholipid scrambling in plasma membranes requires the assembly of pore-forming membrane proteins. This model (Fig 1) envisions pore-mediated flip-flop of phospholipids to involve movement of polar headgroups through a central aqueous channel while the fatty acid moieties diffuse along a hydrophobic interface between subunits.144 145 Such a bidirectional transport mechanism implies that the size of the lipid headgroup, rather than its chemical composition, determines which molecules take part in this process and accounts for its lack of specificity.
Neoplasia and thrombosis.Laboratory evidence of hypercoagulability found in cancer patients is likely to be responsible for the symptomatic thromboembolic episodes frequently observed in these patients. The principal problematic features of blast cells are increased expression of tissue factor activity146-148 and the presence of a tumor-specific cysteine protease149-151 that directly activates factor X independent of the tissue factor pathway. In addition, different tumorigenic cells also express a catalytic surface that promotes the assembly and catalysis of the prothrombinase complex.10,11,118,152 Unlike tissue factor activity, the prothrombinase activity of tumorigenic cells is inhibited by annexin V118,153 because of competition for PS expressed at the cell surface. Tumor cells also release microvesicles that catalyze prothrombinase activity,152,154 a process similar to phospholipid scrambling and microvesicle release in other cells. The expression of both tissue factor and PS in the tumor cells and in their shed microvesicles128 could facilitate platelet-independent initiation and propagation of coagulation, and it may be responsible for fibrin deposits often seen in solid tumors.
Antiphospholipid syndome and thrombosis.Autoantibodies to phospholipid-binding proteins, which include lupus anticoagulants, comprise a heterogeneous group of circulating Igs that are associated with increased risk of arterial and venous thrombosis, thrombocytopenia, and recurrent abortions.155,156 Although these antibodies were first believed to recognize anionic phospholipids directly, recent evidence indicates that the antibodies are directed against various plasma proteins, particularly when these proteins are bound to anionic phospholipid surfaces.157-162 These include antibodies to lipid-bound β2-glycoprotein-I, prothrombin, protein C, and protein S. Interaction occurs whether the protein antigens are bound to model membranes, activated platelets, or platelet-derived microvesicles.163 Some patients have antibodies against only one of the phospholipid-binding proteins, whereas others display a variety of antigenic specificities. The relationship of these antibodies to thrombosis is not clear, especially when one considers that many antibody-positive individuals are asymptomatic. Whereas the presence of antibodies to β2-glycoprotein-I seems to be frequently associated with thrombosis, this relationship is less evident for antibodies against prothrombin, protein C, and protein S.162 This is rather unexpected considering the high thrombotic tendency of patients with hereditary protein C deficiency.
The mechanisms that elicit production of antibodies to lipid-bound serum proteins is unclear. Rather than an aberrant autoimmune response, the production of these antibodies may be a normal response against an antigenic epitope formed by interaction of the plasma proteins with a PS-expressing thrombogenic surface.9,159 Whatever the mechanism, antibodies against lipid-bound β2-glycoprotein-I or prothrombin usually prolong clotting times in vitro by preventing the assembly of prothrombinase or by inhibiting the proteolysis of prothrombin.157-159,161 It should be stressed that such a response does not necessarily lead to a bleeding tendency but may only reduce the propensity for thrombosis. Indeed, whereas thrombotic events are frequently observed in cancer, cancer patients who have lupus anticoagulant antibodies have been reported not to suffer from thromboembolic complications.164
ROLE OF PS IN CELL-CELL RECOGNITION
Recognition of PS by macrophages.Another feature of PS-expressing cells is their propensity to be recognized by the reticuloendothelial system.165,166 This was first shown by insertion of controlled amounts of fluorescent PS analogs into mouse red blood cells. Reinjection of these cells into syngeneic animals resulted in their rapid removal from the peripheral circulation and accumulation in splenic macrophages and Kupffer cells.13 Cell clearance depended on the amount of exogenously-inserted PS, and it occurred when the cells contained only about ∼1 mol% of the PS analog. However, clearance was incomplete. This was likely the result of aminophospholipid translocase activity, which continuously pumped PS to the inner leaflet of the circulating cells, thereby eliminating the putative PS ligand and preventing its recognition by macrophages. Anionic lipid-dependent binding to macrophages has also been observed with PS-containing liposomes in vivo167 and in vitro.168 169 These results underscore the potential significance of PS in cell-cell recognition and have led to the notion that specific receptor ligand interactions mediate the elimination of PS-expressing cells.
Recognition of PS-expressing cells.Normal cells do not expose significant amounts of PS, but pathologic cells seem to have undergone lipid rearrangements that result in PS exposure. Reorientation of PS has been observed, for example, in sickle cell disease79,81,83,84,91,120,170 and β-thalassemia,171 possibly because of decreased aminophospholipid translocase activity172 and alterations in passive diffusion rates of lipids between membrane leaflets,173 respectively. Apart from the thrombogenic state associated with these diseases, PS exposure might also explain the cells' increased susceptibility to phagocytosis. Indeed, macrophages bind PS-expressing deoxygenated sickle cells174 and leukemic cells10 by a mechanism that is PS-dependent. Recovery to a normal, non–PS-expressing, phenotype by reoxygenation of sickle cells174 or chemically induced differentiation of leukemic cells results in the disappearance of cell surface PS and reduction in macrophage binding.10 175
Normal red blood cells seem to have an intrinsic property whereby they accumulate small amounts of surface-exposed PS over their lifespan.79,87,88 Given that macrophages are able to recognize PS, exposure of this lipid in aging red blood cells presumably contributes to their removal from the circulation. In vitro stored red blood cells also suffer from the gradual appearance of PS at the outer surface in an amount proportional to the duration of storage.79 Because aging cells progressively lose ATP-dependent enzymatic activities,176,177 both Mg2+-ATP–dependent aminophospholipid translocase and the Ca2+ pump will be affected. Conceivably, this condition leads to increased cytoplasmic Ca2+ levels that stimulate lipid scramblase and suppress aminophospholipid translocase. Indeed, aminophospholipid transport activity decreases upon storage of red blood cells78,178 and platelets.179 Because oxidation affects the activity of membrane lipid transporters,28,178,180-182 age-related alterations in the cells' redox state183 may also contribute to PS exposure and cell recognition.
PS expression during apoptosis.Apoptosis, defined by characteristic morphologic alterations and DNA fragmentation, is also accompanied by exposure of PS at the cell's outer surface. This was first shown by the ability of apoptotic lymphocytes to shorten clotting times in the PS-dependent Russell viper venom coagulation assay and by labeling of surface exposed PS with fluorescamine.6 The observation was later confirmed by direct measurement of annexin V binding to different apoptotic cells.7,8,184 Recent studies have shown convincingly that PS exposure is one of the earliest manifestations of apoptosis, and that it precedes DNA fragmentation, plasma membrane blebbing, and loss of membrane integrity.7 The process has been shown to be Ca2+-dependent and to involve bidirectional, nonspecific flip-flop of phospholipids.185 Although a PS-specific reverse translocase has been suggested to be responsible for this process,7 there is no evidence that such a transporter exists. Because the appearance of PS in apoptosis shares features typical of the collapse of membrane phospholipid asymmetry in activated platelets, it is most likely that apoptotic cell membrane lipid asymmetry is compromised by the combined actions of an activated scramblase and inhibited translocase.21 Conceivably, these events could be accompanied by membrane unpacking, which has also been shown to precede DNA fragmentation.186,187 This proposed mechanism is also consistent with the observation that calpain inhibitors prevent the characteristic blebbing and microvesiculation common to both platelet activation72 and apoptosis.188
PS receptors in macrophages.The mechanisms of PS-mediated cellular recognition by macrophages probably involve several distinct pathways (Fig 1). Inflammatory macrophages can recognize PS-expressing apoptotic lymphocytes via a specific PS receptor that is inhibited by liposomes containing phosphatidyl-L-serine but not by other anionic phospholipids, including phosphatidyl-D-serine.6 Whether this receptor is the same as macrosialin (CD68),189 the 94- to 97-kD membrane protein that binds PS-expressing cells and oxidized low-density lipoproteins,190,191 remains to be explored. A seemingly distinct macrophage receptor belonging to the class B scavenger receptor I and CD36 has recently been described.192-194 Gene transfer of this receptor to nonphagocytic cells confers recognition for PS192 and apoptotic cells.193 This less-specific receptor also recognizes a variety of modified proteins, including oxidized LDL. Antibodies could also contribute to the removal of PS-expressing cells.13 For example, antiphospholipid syndrome antibodies that recognize plasma proteins bound to PS-expressing cells (or their microvesicles) can be expected to bind avidly to macrophages via the cells' Fc receptor. Other data indicate that members of the selectin family of adhesion molecules bind PS,126 suggesting that they can also function as PS receptors. In addition, β2-glycoprotein-I binds to intravenously injected PS-containing liposomes,195 suggesting that it could play a direct, antibody-independent role in the clearance of PS-expressing cells. It should be noted that PS-independent recognition mechanisms, including the vitronectin receptor (αVβ3 integrin) on bone marrow macrophages, are also involved in recognition and sequestration of apoptotic cells.14,196 The complexity of these recognition mechanisms is further illustrated by the observation that bone marrow macrophages can be stimulated to express the PS-binding characteristics of inflammatory macrophages.15 Lastly, it was recently reported that smooth muscle cells also recognize PS-expressing cells,8 suggesting that PS-dependent cell recognition is not limited to professional phagocytes.
CONCLUSIONS
The lipid transporter-controlled emergence of PS in the cell's outer leaflet results in the expression of altered surface properties that influence and regulate the cell's interaction with its environment. PS clearly plays a pivotal role in maintaining the delicate balance between hemostasis and thrombosis as its overexpression generates potentially dangerous thrombogenic surfaces. It is therefore essential that distinct, albeit cooperative mechanisms for the recognition and removal of PS-expressing cells exist. Understanding these mechanisms, as well as those that generate and regulate membrane lipid sidedness and those that promote a collapse of phospholipid asymmetry, is a starting point from which the role of lipid transporters in disease can be assessed.
ACKNOWLEDGMENT
The authors dedicate this article to the memory of Mrs Mary Ann Scott. We thank Drs E.M. Bevers, Y. Killion, and P. Comfurius for comments and critical review of the manuscript and are grateful to L. Feldman for her editorial assistance.
Supported in part by National Institutes of Health Grant No. DK41714.
Address reprint requests to Alan J. Schroit, PhD, Department of Cell Biology, Box 173, The University of Texas M.D. Anderson Cancer Center, 1500 Holcomble Blvd, Houston, TX 77030.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal