Abstract
TEL gene rearrangement is the most common genetic lesion in pediatric acute lymphoblastic leukemia (ALL), occurring in about 25% of B-lineage cases. We previously showed that, among patients treated on St Jude protocols, TEL rearrangement independently conferred an excellent prognosis. To extend these results to patients treated with antimetabolite-based therapy, we performed Southern blot analysis to determine the TEL gene status of 104 cases of B-lineage ALL treated on Pediatric Oncology Group 8602, matched on age, gender, and leukocyte count. There were 52 failures among the 77 patients with germline TEL, compared with only 8 failures among 27 patients in the rearranged group. Based on a two-sided logistic regression analysis, stratified for age (subdivided at 10 years), leukocyte count (subdivided at 50,000), and gender, the estimated odds of failing by 4 years in the germline TEL group is 5.4 times that of the rearranged TEL group, with 95% confidence from 1.9 to 15.6, two-sided P = .0009. Thus, the presence of a rearranged TEL gene is also associated with an improved survival among patients treated with antimetabolite-based therapy. Our results indicate that all newly diagnosed ALL patients should be screened for TEL gene rearrangements and suggest that these patients are candidates for less intensive therapy.
CONTEMPORARY TREATMENT protocols for pediatric acute lymphoblastic leukemia (ALL) attempt to tailor the intensity of therapy to a patient's risk of relapse. Risk classification schemes use clinical features, such as age and leukocyte count, combined with biologic features to predict outcome. Certain genetic features, including a DNA index greater than 1.16 and the presence of trisomies of chromosomes 4 and 10, have been shown to correlate with good outcome, whereas specific chromosomal translocations, including the t(9; 22), t(1; 19), and t(4; 11), are associated with inferior outcomes.1-13 Although genetic features are the best predictors of outcome, only about one third of patients have prognostically important genetic lesions.
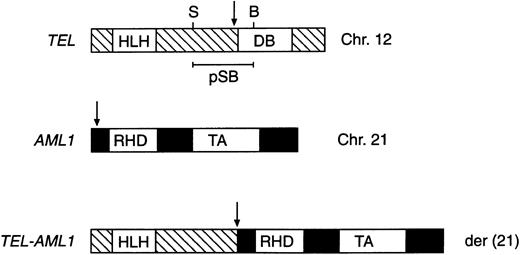
We and others have recently shown that a TEL-AML1 fusion, created by a cryptic t(12; 21), is the most common genetic alteration in pediatric ALL, occurring in about 25% of cases with a B-precursor phenotype.14,15 The t(12; 21) fuses the helix-loop-helix portion of TEL in-frame to AML1, resulting in expression of a TEL-AML1 chimeric message (Fig 1).16,17 In an attempt to improve our ability to risk classify patients, we recently determined the clinical significance of TEL gene rearrangements among pediatric ALL patients treated at St Jude Children's Research Hospital.18 We showed that TEL rearrangement independently predicted an excellent prognosis even after adjusting for age and leukocyte count.18 However, these results were limited to patients treated on St Jude Total Therapy Study XI19 and XII20 protocols, which emphasize multiagent chemotherapy, including epipodophyllotoxins. By contrast, a recent report suggests that expression of the TEL-AML1 chimeric transcript is not a favorable prognosis factor in pediatric ALL.21 These conflicting reports thus raise important therapeutic questions concerning the outcome of these patients when treated on different protocols. Therefore, to determine the impact of TEL rearrangement among patients treated with antimetabolite-based therapy, we analyzed the clinical significance of TEL gene status among 104 patients treated on the Pediatric Oncology Group (POG) 8602 protocol.
Schematic representation of the TEL/AML1 fusion formed by the t(12; 21). The TEL gene, located at chromosome 12, band p13 (Chr. 12), encodes a protein containing helix-loop-helix (HLH) and DNA-binding (DB) domains. AML1, located on chromosome 21 (Chr. 21), encodes a protein that contains a transactivation domain (TA) and a runt homology domain (RHD). Sac I (S) and BamHI (B) sites, as well as probe pSB, are shown in TEL. The der(21) product fuses the 5′ portion of TEL, containing the helix-loop-helix domain, to nearly all of AML1. Arrows indicate the t(12; 21) breakpoints in each gene and the site of fusion.
Schematic representation of the TEL/AML1 fusion formed by the t(12; 21). The TEL gene, located at chromosome 12, band p13 (Chr. 12), encodes a protein containing helix-loop-helix (HLH) and DNA-binding (DB) domains. AML1, located on chromosome 21 (Chr. 21), encodes a protein that contains a transactivation domain (TA) and a runt homology domain (RHD). Sac I (S) and BamHI (B) sites, as well as probe pSB, are shown in TEL. The der(21) product fuses the 5′ portion of TEL, containing the helix-loop-helix domain, to nearly all of AML1. Arrows indicate the t(12; 21) breakpoints in each gene and the site of fusion.
PATIENTS AND METHODS
Patient selection and statistical methods.Because rearranged TEL cases rarely have hyperdiploid karyotypes,14,18 we studied cases from a prioritized list of nonhyperdiploid B-precursor ALL patients treated on POG 8602, which accrued patients from January 1986 to January 1991. The details of this protocol have been previously described.2 Briefly, induction therapy consisted of vincristine, prednisone, and L-asparaginase, with triple intrathecal therapy comprising methotrexate, hydrocortisone, and cytarabine administered for subclinical central nervous system leukemia. The intensification phase consisted of intermediate-dose methotrexate, administered alone, with asparaginase, or with cytarabine. Continuation therapy included daily oral 6-mercaptopurine and weekly low-dose methotrexate with pulses of vincristine and prednisone.
We conducted a case-control retrospective study of samples obtained from the POG Cell Bank, per Breslow and Day,22 using case frequency matching per Shuster.23 Cases were patients failing treatment before 4 years, whereas controls were patients in continuous complete remission greater than 4 years. Patients failing treatment after 4 years were excluded. We also excluded hyperdiploid cases (DNA index >1.16), cases with either the t(9; 22) or t(4; 11), and patients for whom cells were never available in the cell bank. Patients were subdivided into eight strata, as shown in Table 1. A stratified random sample of 80 cases (failures) were selected according to its natural distribution, along with a stratified random sample of 80 controls in the identical number per stratum. Failures were intentionally overrepresented as 50%, rather than the natural of distribution of 29%, to increase statistical power to answer the study question. Under the assumptions that the frequency of rearranged TEL would be approximately 25% and the difference in 4-year event-free survival would be 25% for germline versus rearranged TEL, ie, 55% to 65% versus 80% to 90%, a sample size of 104 patients would be needed to have 80% power to detect the difference (P < .05, two-sided). The exact conditional Mantel-Haenszel test was used to compare the difference.24
TEL Results by Stratum
| Age (yr) . | Leukocyte Count (× 109/L) . | Sex . | TEL Status . | No Cells . | Unsatisfactory . | Total . | |
|---|---|---|---|---|---|---|---|
| . | . | . | Germline . | Rearranged . | . | . | . |
| <9.99 | ≤50 | M | 15/19* | 3/9 | 1/10 | 2/4 | 21/42 |
| >10.0 | ≤50 | M | 9/14 | 0/3 | 2/4 | 1/1 | 12/22 |
| <9.99 | >50 | M | 5/10 | 1/4 | 3/4 | 1/2 | 10/20 |
| >10.0 | >50 | M | 5/7 | 0/0 | 0/1 | 0/1 | 5/9 |
| <9.99 | ≤50 | F | 5/7 | 2/7 | 1/3 | 1/1 | 9/18 |
| >10.0 | ≤50 | F | 2/3 | 0/0 | 0/1 | 1/2 | 3/6 |
| <9.99 | >50 | F | 8/12 | 2/4 | 0/2 | 0/0 | 10/18 |
| >10.0 | >50 | F | 3/5 | 0/0 | 0/0 | 0/0 | 3/5 |
| Total | 52/77 | 8/27 | 7/25 | 6/11 | 73/140 | ||
| Age (yr) . | Leukocyte Count (× 109/L) . | Sex . | TEL Status . | No Cells . | Unsatisfactory . | Total . | |
|---|---|---|---|---|---|---|---|
| . | . | . | Germline . | Rearranged . | . | . | . |
| <9.99 | ≤50 | M | 15/19* | 3/9 | 1/10 | 2/4 | 21/42 |
| >10.0 | ≤50 | M | 9/14 | 0/3 | 2/4 | 1/1 | 12/22 |
| <9.99 | >50 | M | 5/10 | 1/4 | 3/4 | 1/2 | 10/20 |
| >10.0 | >50 | M | 5/7 | 0/0 | 0/1 | 0/1 | 5/9 |
| <9.99 | ≤50 | F | 5/7 | 2/7 | 1/3 | 1/1 | 9/18 |
| >10.0 | ≤50 | F | 2/3 | 0/0 | 0/1 | 1/2 | 3/6 |
| <9.99 | >50 | F | 8/12 | 2/4 | 0/2 | 0/0 | 10/18 |
| >10.0 | >50 | F | 3/5 | 0/0 | 0/0 | 0/0 | 3/5 |
| Total | 52/77 | 8/27 | 7/25 | 6/11 | 73/140 | ||
Number of failures/cases studied.
The list of 160 patients, which was prioritized in random order, was sent blinded as to outcome to the lead investigator, who performed Southern blot analysis for TEL rearrangement until 104 successful results were obtained. This led to 140 attempted cell bank searches, of which 115 had frozen cells available and 104 had successful assay results.
It is important to note that one cannot use data from a retrospective study that relies on the use of cryopreserved materials to obtain valid Kaplan-Meier25 estimates, irrespective of whether one distorted the natural distribution or not, if a substantial number of patients samples are missing. Even in this study, a potential bias might have occurred if past studies had disproportionately removed cryopreserved samples from patients in long-term remission with germline TEL or samples from rearranged cases that had an adverse event.
Molecular analysis.Genomic DNA obtained from FicollHypaque–enriched leukemic blasts was analyzed for TEL gene rearrangements as previously described.14 Briefly, 10 μg of high molecular weight DNA was digested with BamHI, separated electrophoretically in 0.8% agarose gels, and transferred to nylon membranes. Membranes were then hybridized with an [α-32P]dCTP-labeled 466-bp Sac I/BamHI TEL cDNA fragment (probe pSB).14 After high stringency washes, membranes were analyzed by autoradiography.
RESULTS
Hyperdiploid DNA content is associated with an excellent prognosis in pediatric ALL.12 To determine if TEL rearrangement can identify another subgroup of patients with a good prognosis, we performed Southern blot analysis on 104 nonhyperdiploid B-lineage ALL cases selected as described above. All blots were hybridized with probe pSB (Fig 1), which detects the majority of TEL rearrangements. A representative blot is shown in Fig 2. Overall, 27 cases showed rearrangements of TEL. Because our previous study18 showed that nearly all cases with TEL gene rearrangements also express the TEL-AML1 chimeric transcript, the present 27 cases were not analyzed for TEL-AML1 expression.
Southern blot of genomic DNA. DNA was digested with BamHI and the blot was hybridized with pSB. Control lane contains nonleukemic DNA. Lanes 1 through 10 contain DNA from 10 ALL cases. Molecular weight markers are shown on the left and germline TEL (G) is indicated. In this example, cases no. 2, 3, 4, and 9 show rearrangements of TEL.
Southern blot of genomic DNA. DNA was digested with BamHI and the blot was hybridized with pSB. Control lane contains nonleukemic DNA. Lanes 1 through 10 contain DNA from 10 ALL cases. Molecular weight markers are shown on the left and germline TEL (G) is indicated. In this example, cases no. 2, 3, 4, and 9 show rearrangements of TEL.
Table 1 describes the results of the study according to stratum and TEL results, including those cases with no cells available or unsatisfactory results. There were 52 failures among the 77 patients with germline TEL, compared with only 8 failures among the 27 patients in the rearranged group. Based on a two-sided Mantel-Haenszel stratified analysis,24 the estimated odds ratio (ie, the ratio of failure in germline TEL cases to that for rearranged TEL cases) is 5.4 (95% confidence interval, 1.9 to 15.6; P = .0009, two-sided). Under a worst case scenario to check for bias, we also grouped the “No Cells” group with the germline group and the unsatisfactory group with the rearranged group. The estimated odds ratio would then be 2.3 (95% confidence interval, 0.98 to 5.3; P = .055, two-sided).
DISCUSSION
Risk-based treatment protocols for childhood ALL tailor the intensity of therapy to the risk of relapse. Patients predicted to be at lower risk of relapse are generally treated with antimetabolite-based therapies, which are well-tolerated and associated with few long-term side effects. Higher-risk patients are treated more intensively, often with chemotherapy regimens that emphasize epipodophyllotoxins, anthracyclines, or alkylating agents. Although these intensive treatment regimens are more efficacious, patients are at increased risk of developing secondary acute myeloid leukemia or cardiomyopathy.26-28
The National Cancer Institute recently sponsored a workshop that adapted uniform age and leukocyte criteria to define clinical risk groups for children with B-lineage ALL.29 By consensus, the participants recommended an age of 1.00 to 9.99 years and a presenting leukocyte count of less than 50 × 109/L as standard-risk criteria, with all other combinations of these two features considered higher-risk. Four-year event-free survival estimates for these two categories were 80% and 65%, respectively. We recently showed that TEL rearrangement is associated with an exceptionally good outcome and provides independent prognostic information even among the workshop consensus risk groups.18 Among patients with a favorable age and leukocyte count by the workshop criteria, TEL status distinguished those with an excellent 5-year event-free survival from those with an intermediate outcome.18 It also identified long-term event-free survivors among patients with an unfavorable age or leukocyte count; only 1 of 11 patients with rearranged TEL in this group failed therapy.18 Likewise, in the present study, the prognostic impact of TEL rearrangement was not affected by age or leukocyte count (Table 1).
Because our previous studies included only patients treated on St Jude protocols, it was necessary to determine the impact of TEL status among patients treated less intensively, because treatment effects may dramatically alter the clinical significance of prognostic factors. For example, the t(1; 19) is associated with a poor outcome among patients treated with conventional antimetabolite-based therapies.2 However, when these patients are treated more intensively, their prognosis is similar to that of patients who lack this translocation.
In the present study, we have studied the prognostic significance of TEL gene rearrangements among patients treated with conventional antimetabolite-based therapy. We analyzed only nonhyperdiploid cases that lacked the t(4; 11) and t(9; 22) so that we could determine the impact of TEL status among cases that did not have other prognostically important genetic features. Furthermore, our previous results indicate that TEL rearrangements generally occur only in nonhyperdiploid cases that lack other detectable translocations.18 We have now shown that, among 104 B-lineage ALL cases treated on POG 8602, TEL gene rearrangement conferred a significantly improved prognosis. Germline TEL cases had an estimated 5.4-fold increased risk of relapse compared with the rearranged TEL cases. These results, together with our previous observations,18 confirm the excellent prognosis of patients whose leukemic blasts carry TEL gene rearrangements.
TEL status should thus be used as one of the key prognostic factors for treatment assignment in ALL, but must be confirmed in prospective studies, because retrospective studies may be biased by the availability of diagnostic bone marrow specimens. In this regard, we are now conducting a prospective study of the impact of TEL gene status on all newly diagnosed B-lineage ALL patients entered on POG protocols. Studies are also underway to determine the basis of the chemoresponsiveness of blasts carrying rearranged TEL. In addition, several laboratories are studying the biologic properties of the TEL-AML1 chimeric protein. For example, Hiebert et al30 have shown that TEL converts AML1 from an activator to a repressor of transcription. Future studies should further elucidate the mechanisms by which TEL-AML1 leads to oncogenic transformation.
In summary, our results indicate that patients with rearranged TEL are candidates for less intensive therapy. In addition, these studies should lead to an improved genetically based risk classification scheme for pediatric ALL. Ultimately, all patients should be classified by genetic features, leading to further improvements in outcome and more rational selection of therapy based on risk of treatment failure.
Supported in part by National Institutes of Health (NIH) Cancer Center (CORE) Grant No. CA-21765; Leukemia Program Project Grant No. CA-20180; NIH Grants No. CA-29139, CA-31566, CA-30969, CA-33603, CA-15989, and CA-32053; and the American Lebanese Syrian Associated Charities (ALSAC). J.E.R. was supported in part by a Career Development Award from the American Society of Clinical Oncology.
Address reprint requests to Frederick G. Behm, MD, Department of Pathology, St Jude Children's Research Hospital, 575 St Jude Place, Memphis, TN 38105.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal