Abstract
Based on anatomic and developmental findings characterizing hematopoietic cells in close approximation with endosteal cells, we have begun an analysis of osteoblast/hematopoietic cell interactions. We explore here the functional interdependence between these two cell types from the standpoint of de novo cytokine secretion. We determined that, over a 96-hour period, CD34+ bone marrow cells had no significant effect on osteoblast secretion of granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, or transforming growth factor-β1 , but in some experiments minor increases in leukemia inhibitory factor levels were observed. However, when CD34+ bone marrow cells were cocultured in direct contact with osteoblasts, a 222% ± 55% (range, 153% to 288%) augmentation in interleukin-6 (IL-6) synthesis was observed. The accumulation of IL-6 protein was most rapid during the initial 24-hour period, accounting for nearly 55% of the total IL-6 produced by osteoblasts in the absence of blood cells and 77% of the total in the presence of the CD34+ cells. Cell-to-cell contact does not appear to be required for this activity, as determined by coculturing the two cell types separated by porous micromembranes. The identity of the soluble activity produced by the CD34+ cells remains unknown, but is not likely due to IL-1β or tumor necrosis factor-α, as determined with neutralizing antibodies. To our knowledge, these data represent the first demonstration that early hematopoietic cells induce the production of molecules required for the function of normal bone marrow microenvironments, in this case through the induction of hematopoietic cytokine (IL-6) secretion by osteoblasts.
THE BONE MARROW is responsible for two fundamental processes: the production of mature blood cells from hematopoietic stem cells and supporting bone formation via the generation of osteoblasts. Despite the intriguing anatomic and developmental findings characterizing hematopoietic cells in close approximation with endosteal osteoblasts, surprisingly little is known relating to the role of osteoblasts in blood development.1,2 Therefore, we have begun an analysis of osteoblast/hematopoietic cell interactions in the bone marrow microenvironment. Using osteoblasts derived from human bone explants3 and osteosarcoma cell lines, we and others have determined that human osteoblasts produce granulocyte colony-stimulating factor (G-CSF ),4 granulocyte-macrophage colony-stimulating factor (GM-CSF ),4 interleukin-1β (IL-1β),5 IL-6,5 leukemia inhibitory factor (LIF ),6 macrophage colony-stimulating factor (M-CSF ),7-9 and tumor necrosis factor-α (TNF-α)10 protein. Furthermore, we have observed that osteoblasts maintain hematopoietic progenitors and long-term culture-initiating cells (LTC-IC) in vitro.11
Recently, intriguing data have emerged that hematopoietic cells might cooperate with the marrow stromal populations to assemble the various hematopoietic microenvironments. The induction of stromal cell-derived cytokines in response to either factors produced by the hematopoietic cells or after the adhesion of blood cells to stromal cells are mechanisms believed to be involved in orchestrating this process.12-14 Given our findings with enriched populations of human osteoblasts on early hematopoietic cells in vitro, and the possible importance of osteoblasts in hematopoiesis, we therefore asked whether hematopoietic cells contribute to the hematopoietic supportive activity of primary human osteoblasts by activating the production of cytokines by osteoblasts.
We report here that IL-6 and, to a lesser degree, LIF, but not G-CSF, GM-CSF, and transforming growth factor-β1 (TGF-β1 ) are specifically secreted by osteoblasts in response to hematopoietic progenitor cells. The maximal accumulation of secreted IL-6 occurs during the initial 24-hour period of coculture and is correlated with human CD34+ bone marrow cell number. Furthermore, cell-to-cell contact does not appear to be required for this activity. The identity of the soluble activity produced by the CD34+ cells remains unknown, but is not likely due to IL-1β or TNF-α, as determined with neutralizing antibodies. To our knowledge, these data represent the first demonstration that normal hematopoietic progenitor cells induce the production molecules required for the production of a normal bone marrow microenvironment by cells of the osteoblast lineage.
MATERIALS AND METHODS
Human osteoblasts.Enriched human osteoblast cultures were established using modifications of methods described by Robey and Termine.3 Normal human trabecular bone was obtained from patients undergoing orthopedic surgery in accordance with the University of Michigan's Investigational Review Board. Bone cleaned of loosely adherent tissue was ground to produce a uniform particle size (size, ≤1 mm2; BioComp Minimill, W. Lorenz, Jacksonville, FL) and incubated in 1 mg/mL bacterial collagenase (Type P; Boehringer Mannheim Biologicals, Indianapolis, IN). The explants were placed into culture until confluent monolayers were produced in a 1:1 (vol/vol) mixture of Ham's F12/Dulbecco's minimal essential medium (DMEM; Biofluids, Rockville, MD) with low Ca+2 and 10% fetal bovine serum. Thereafter, cultures were maintained in calcium replete Ham's F12/DMEM (1:1 vol/vol) medium containing 10% heat-inactivated fetal bovine serum (FBS; Life Technologies, Grand Island, NY), antibiotics, 10 mmol/L β-glycerol phosphate, and 10 mg/mL L-ascorbate. To verify that the cells expressed an osteoblast phenotype, the cultures were screened for the expression of the osteoblast-specific protein osteocalcin (osteocalcin+) and the absence of c-kit ligand (c-kit ligand−) by reverse transcriptase polymerase chain reaction (RT-PCR), as previously detailed.4 Using morphologic and RNA criteria, we can detect contamination of our osteoblast preparations by marrow stromal elements to levels of 1% of the total population (data not presented).
Isolation of human CD34+ bone marrow cells.Human bone marrow cells were obtained from healthy adult volunteers by iliac crest puncture and aspiration into preservative-free heparin under a protocol approved by the University of Michigan's Investigational Review Board. Mononuclear cells were isolated by density separation on Ficoll-Hypaque (specific gravity, 1.077). Plastic adherence at 37°C was performed in modified Dexter's medium (Iscove's modified Dulbecco's medium [IMDM], 10% FBS, 10% equine serum, 1 μmol/L hydrocortisone, penicillin/streptomycin [Life Technologies]). After overnight adherence, the nonadherent cells were recovered and CD34+ bone marrow cells were isolated by positive immunomagnetic selection using the QUIND/10 antibody (Miltenyi Biotec Inc, Sunnyvale, CA). In some experiments, mononuclear cells isolated by positive immunomagnetic selection were stained with a phycoerythrin conjugate of the anti-CD34 antibody, HPCA-2 (Becton Dickinson, San Jose, CA), to evaluate purity. Under these conditions, 92.5% ± 3.5% (n = 2) of the recovered cells expressed the CD34 antigen. Where indicated, these cells were further isolated by fluorescence-activated cell sorting (FACS; EPICS C; Coulter Corp, Hialeah, FL).
Liquid coculture of CD34+ bone marrow cells and primary human osteoblasts.Primary human osteocalcin+, c-kit ligand− osteoblasts at confluence were harvested by trypsinization (20 minutes at 37°C, 0.05% trypsin/0.5 mmol/L EDTA [Life Technologies]) and seeded into 96-well or 24-well flat-bottomed tissue culture plates to a final density of 2 × 104/cm2 in Ca+2 replete Ham's F12/DMEM (1:1 vol/vol) containing 10% heat-inactivated FBS, antibiotics, 10 mmol/L β-glycerol phosphate, and 10 mg/mL L-ascorbate. After 7 days, the osteoblast monolayers were washed twice and 1 × 104 CD34+ bone marrow cells were seeded onto the osteoblast monolayers. Thereafter, conditioned medium was collected over the first 96 hours of the osteoblast/blood cell coculture. Where indicated, serum concentrations were reduced from 10% to 1% (vol/vol).
To determine whether viable osteoblasts were required for the production of cytokines, osteoblasts grown to confluence were fixed in situ with 2% paraformaldehyde in phosphate-buffered saline for 10 minutes at 25°C. Subsequently, the osteoblast cell monolayers were washed four times with medium before the initiation of coculture experiments. For direct cell-cell contact investigations, CD34+ bone marrow cells (1.0 × 104) were seeded into the top chamber of TransWell (Corning Costar Corp, Cambridge, MA) dual-chambered 24-well plates (0.4-μm pore size), with confluent primary human osteoblasts in the bottom chamber. To determine whether IL-1β or TNF-α was responsible for the soluble activity produced by the CD34+ cells that stimulates IL-6 production by human osteoblasts, IL-1β and TNF-α neutralizing monoclonal antibodies (murine IgG1 monoclonal; Genzyme Corp, Cambridge, MA), isotype-matched control (MOPC-21; Sigma, St Louis, MO), or vehicle were added daily to a final concentration of 10 μg/mL.
Production of bone marrow CD34+ cell-conditioned medium.CD34+ bone marrow cells (1 × 105) were seeded into the top chamber of TransWell (Corning Costar Corp) dual-chambered 24-well plates (0.4-μm pore size) with 0.7 mL total of a 1:1 mixture of DMEM/F12 medium supplemented with 10% fetal calf serum, Pen/Strep, 10 mmol/L L-ascorbate, and 10 mg/mL β-glycerol phosphate in 24-multiwell culture plates. At 24, 48, and 72 hours, the conditioned medium was harvested, spun at 12,000 rpm at 4°C for 15 minutes, and frozen at −80°C.
RT-PCR.Total cellular RNA was recovered by lysing cells directly in Stat-60 according to the directions of the manufacturer (Tel-Test Inc, Friendswood, TX). RNA integrity and purity was evaluated by electrophoresis with ethidium bromide and absorbance at A260/A280 . RNA (1.0 μg), 10× RT buffer (1× RT buffer: 50 mmol/L Tris HCl, pH 8.3, 50 mmol/L KCl, 8.0 mmol/L MgCl2 , and 10 mmol/L dithiothreitol), 25 mmol/L dXTP mix (25 mmol/L of each dXTP [adenosine, cytosine, thymidine, guanidine (ACGT)]), 3.0 μg oligo d(T), and 2.5 U reverse transcriptase (M-MLV Reverse Transcriptase; Life Technologies, Grand Island, NY) were incubated together at 38°C for 1 hour. One-fifth of the double-stranded product was mixed with 10× Taq buffer (1× Taq/RT buffer: 10 mmol/L Tris, pH 8.3, 50 mmol/L KCl, 1.5 mmol/L MgCl2 , 0.01% gelatin, and 2.0 mmol/L dithiothreitol), 1 mmol/L dXTP mix, 500 ng of each sense and antisense oligonucleotide, and 2.5 U Taq polymerase (Taq polymerase; GIBCO-BRL). Sense and antisense primers were prepared by the oligonucleotide synthesis core at the University of Michigan and were designed to cross intron/exon boundaries: c-kit ligand (sense, GAAGGGATCTGCAGGAATCGTGTG; antisense, GCCCTTGTAAGACCTGGCTGTCTC; expected size, 665 bp15 ), osteocalcin (sense, GGCAGCGAGGTAGTGAAGAG; antisense, GATGTGGTCAGCCAACTCGT; expected size, 137 bp16 ), IL-6 (sense, GGATCCTCCTTCTCCACAAGCGCCTTCGGTCCA; antisense, AAGCTTGTTCCTCACTACTCTCAAATGTGTTCTG; expected size, 398 bp17 ), actin (sense, GTGGGGCGCCCCCAGGCACCA; antisense, CTCCTTAATGTCACGCACGATTTC; expected size, 548 bp18 ), as previously detailed.4 The samples underwent thermal cycling at 94°C for 1 minute and at 72°C for 3 minutes for 35 cycles, followed by 10 minutes of extension at 72°C (Perkin Elmer Cetus DNA thermal cycler; Perkin Elmer Cetus, Norwalk, CT). The products were electrophoresed in 3% agarose and visualized using ethidum bromide. To control for false-positives due to overamplification or DNA contamination, reverse transcriptase was omitted from the reaction (data not presented) and primers were designed to cross intron\exon boundaries.
Cytokine enzyme-linked immunosorbent assays (ELISAs).Conditioned medium was collected at the times indicated and stored at −80°C until assayed for cytokine levels by double-antibody sandwich method with commercially available ELISA kits according to the directions of the manufacturer: IL-1β (sensitivity, 0.1 pg/mL; range, 3.9 to 250 pg/mL; R&D Systems, Minneapolis, MN), IL-6 (sensitivity, 0.7 pg/mL; range, 3.33 to 300 pg/mL; R&D Systems), G-CSF (sensitivity, 7.2 pg/mL; range, 39 to 2,500 pg/mL), GM-CSF (sensitivity, 1.5 pg/mL; range, 7.8 to 5,000 pg/mL; R&D Systems), LIF (sensitivity, 2.0 pg/mL; range, 31 to 2,000 pg/mL; R&D Systems), TGF-β1 (sensitivity, 0.05 ng/mL; range, 0.1 to 4 ng/mL; Genzyme), and TNF-α (sensitivity, 3 pg/mL; range, 6 to 1,024 pg/mL; Genzyme). Cytokine levels are presented as the mean ± standard error cytokine per milliliter for triplicate determinations.
Statistical analyses.Each experiment was repeated a minimum of three times. Analysis of variance (ANOVA) was used to determine statistical significance.
RESULTS
The effect of human bone marrow CD34+ cells on cytokine synthesis by human osteoblasts.In previous investigations, we have shown that normal human osteoblasts constitutively produce a variety of cytokine mRNAs under basal conditions.4 In the present investigations, we examined whether untransformed human osteoblast-like cells would respond to normal hematopoietic cells by augmenting their secretion of soluble G-CSF, GM-CSF, IL-6, LIF, and TGF-β1 . We chose these particular cytokines as representatives of proteins with largely stimulatory (IL-6, GM-CSF, and G-CSF ) and inhibitory (LIF and TGF-β1 ) activity on hematopoietic cells.19-23 Explant-derived osteoblast monolayers were seeded with CD34+ bone marrow cells for 4 days in Ca+2 replete Ham's F12/DMEM (1:1 vol/vol) containing 10% heat-inactivated FBS, antibiotics, 10 mmol/L β-glycerol phosphate, and 10 mg/mL L-ascorbate. Under these conditions, we have previously determined that osteoblasts maintain hematopoietic progenitors and LTC-IC activity for at least 2 weeks in vitro.11
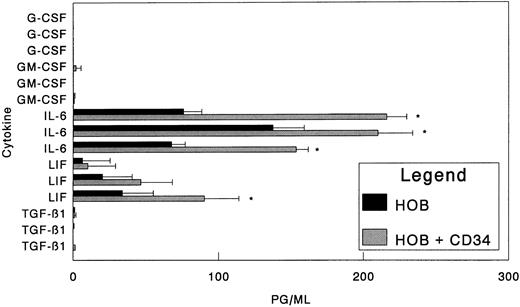
Over a 96-hour period, we observed that CD34+ cells had no significant effect on the production of osteoblast-derived, soluble G-CSF to the limits of the assay (7.2 pg/mL; Fig 1). During the same period, no significant alterations in the levels of GM-CSF produced were noted, and the production of TGF-β1 was also not significantly altered (Fig 1). In contrast, the presence of CD34+ cells induced osteoblasts in some experiments to secrete enhanced levels of LIF. However, more striking was the effect that CD34+ cells had on IL-6 levels. Under basal conditions (no CD34+ cells), osteoblasts produced levels of IL-6 that were easily detected by the ELISA, ranging from 67 to 137 pg/mL under the 10% serum conditions. When CD34+ cells were placed in direct contact with the osteoblasts, an average increase in IL-6 production of 222% ± 55% (range, 153% to 288%) was observed (Fig 1). To verify that CD34+ bone marrow cells and not a small number of contaminating cells were responsible for the IL-6 stimulation, CD34+ cells were isolated by positive immunomagnetic selection and further purified by FACS using a phycoerythrin conjugate of the anti-CD34 antibody, HPCA-2 (Becton Dickinson). Under these conditions, the FACS-sorted CD34+ cells exhibit the same IL-6 stimulatory activity on human osteoblasts as do the immunoselected cells (Table 1).
Effect of human bone marrow CD34+ cells on cytokine synthesis by human osteoblasts. Bone marrow CD34+ cells (1.0 × 104) were seeded directly onto confluent human osteoblast monolayers (HOB) as described in the Materials and Methods in 24-well tissue culture plates with 10% FBS. At 96 hours, conditioned medium was collected and assayed for G-CSF, GM-CSF, IL-6, LIF, or TGF-β1 by ELISA. Individual data from three independent experiments are presented as the mean ± standard deviation for triplicate determinations. *Significant difference from no CD34+ (HOB) control to a statistical significance of P < .05.
Effect of human bone marrow CD34+ cells on cytokine synthesis by human osteoblasts. Bone marrow CD34+ cells (1.0 × 104) were seeded directly onto confluent human osteoblast monolayers (HOB) as described in the Materials and Methods in 24-well tissue culture plates with 10% FBS. At 96 hours, conditioned medium was collected and assayed for G-CSF, GM-CSF, IL-6, LIF, or TGF-β1 by ELISA. Individual data from three independent experiments are presented as the mean ± standard deviation for triplicate determinations. *Significant difference from no CD34+ (HOB) control to a statistical significance of P < .05.
Effect of Sorted CD34+ Bone Marrow Cells on IL-6 Production by Human Osteoblast Cells
| Osteoblast Isolate . | No CD34+ Cells . | 1 × 104 CD34+ Cells . |
|---|---|---|
| 1 | 10,131 ± 635 | 16,230 ± 832* |
| 2 | 12,072 ± 1,819 | 18,987 ± 2,453† |
| 3 | 4,204 ± 338 | 17,006 ± 4,253† |
| Osteoblast Isolate . | No CD34+ Cells . | 1 × 104 CD34+ Cells . |
|---|---|---|
| 1 | 10,131 ± 635 | 16,230 ± 832* |
| 2 | 12,072 ± 1,819 | 18,987 ± 2,453† |
| 3 | 4,204 ± 338 | 17,006 ± 4,253† |
Nonadherent bone marrow mononuclear CD34+ cells were isolated by positive immunomagnetic selection and prepared for fluorescence-activated cell sorting using a phycoerythrin conjugate of the anti-CD34 antibody, HPCA-2 (Becton Dickinson, San Jose, CA). Selected CD34+ cells (1.0 × 104) were seeded directly onto confluent human osteoblast monolayers in 96-well tissue culture plates containing 1% FBS. At 96 hours, conditioned medium was collected and assayed for IL-6 by ELISA. Data from a representative of two independent experiments are presented as the mean ± standard deviation for triplicate determinations.
Significant difference from no CD34+ cells (osteoblasts only) to a statistical significance of P < .05.
Significant difference from no CD34+ cells (osteoblasts only) to a statistical significance of P < .01.
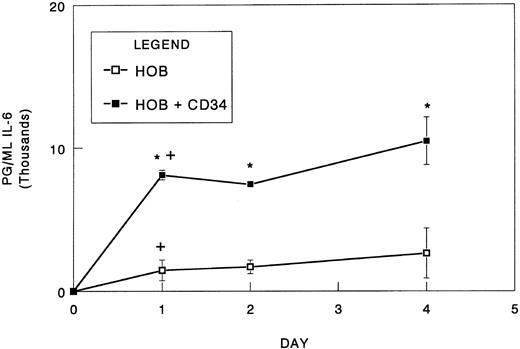
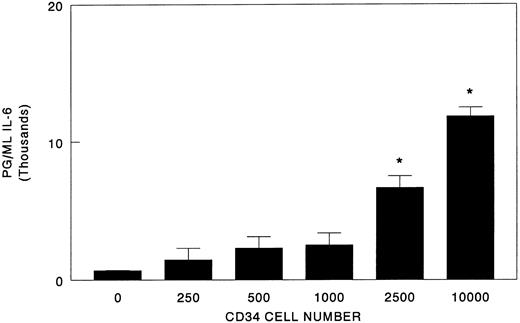
To determine the time frame in which IL-6 synthesis occurs in response to blood cells, osteoblast/CD34+ cell cocultures were established and conditioned medium was collected over the course of 4 days. To minimize the effects that unknown serum components may have on IL-6 synthesis, we reduced the serum concentrations of our cultures from 10% to 1%. Under these conditions, immunodetectable levels of IL-6 increased nearly 10-fold relative to those observed in the higher serum concentrations, suggesting the possibly of serum inhibitor (Figs 1 and 2). For osteoblasts cultured in the absence of the hematopoietic cells, the majority of IL-6 accumulated during the initial 24-hour period (55% of total IL-6 produced; Fig 2). Subsequently, the mean IL-6 levels continued to accumulate but were not significantly different from the initial 24-hour period. The inclusion of CD34+ bone marrow cells significantly increased the levels of IL-6 detected at all time points relative to the osteoblasts alone (Fig 2). Curiously, after the initial 24 hours, the IL-6 levels plateaued rather than continuing to accumulate, possibly representing consumption by the CD34+ cells. In addition, IL-6 levels in vitro correlated with increasing CD34+ cell numbers (Fig 3).
Rate of IL-6 synthesis in coculture of CD34+ bone marrow cells with osteoblasts. Bone marrow CD34+ cells (1.0 × 104) were seeded directly onto confluent human osteoblast monolayers (HOB) in 96-well tissue culture plates containing 1% FBS. At 24, 48, and 96 hours, conditioned medium was collected and assayed for IL-6 by ELISA. Data from a representative of three independent experiments are presented as the mean ± standard deviation for triplicate determinations. *Significant difference from no CD34+ (HOB). +Significant difference from previous time point to a statistical significance of P < .05.
Rate of IL-6 synthesis in coculture of CD34+ bone marrow cells with osteoblasts. Bone marrow CD34+ cells (1.0 × 104) were seeded directly onto confluent human osteoblast monolayers (HOB) in 96-well tissue culture plates containing 1% FBS. At 24, 48, and 96 hours, conditioned medium was collected and assayed for IL-6 by ELISA. Data from a representative of three independent experiments are presented as the mean ± standard deviation for triplicate determinations. *Significant difference from no CD34+ (HOB). +Significant difference from previous time point to a statistical significance of P < .05.
Effect of CD34+ bone marrow cell number on IL-6 synthesis in coculture with osteoblasts. Bone marrow CD34+ cells (0 to 1.0 × 104) were seeded directly onto confluent human osteoblast monolayers (HOB) in 96-well tissue culture plates containing 1% FBS. At 96 hours, conditioned medium was collected and assayed for IL-6 by ELISA. Data from a representative of three experiments are presented as the mean ± standard deviation for triplicate determinations. *Significant difference from no CD34+ cell control (HOB) to a statistical significance of P < .05.
Effect of CD34+ bone marrow cell number on IL-6 synthesis in coculture with osteoblasts. Bone marrow CD34+ cells (0 to 1.0 × 104) were seeded directly onto confluent human osteoblast monolayers (HOB) in 96-well tissue culture plates containing 1% FBS. At 96 hours, conditioned medium was collected and assayed for IL-6 by ELISA. Data from a representative of three experiments are presented as the mean ± standard deviation for triplicate determinations. *Significant difference from no CD34+ cell control (HOB) to a statistical significance of P < .05.
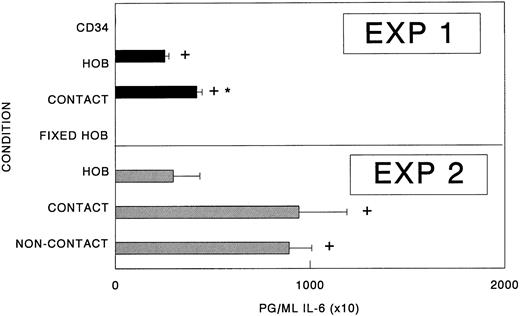
Cell-to-cell contact between CD34 cells is not required for increased IL-6 synthesis.To evaluate whether direct contact between osteoblasts and hematopoietic cells is required for the augmented production of IL-6, CD34+ bone marrow cells were seeded either directly onto osteoblast monolayers or into the top chamber of dual-chambered culture plates that facilitate the free exchange of soluble molecules but physically separate the heterologous populations. As reported previously, significant increases in IL-6 production were observed when osteoblasts were cultured with CD34+ cells (Fig 4). Under conditions in which osteoblasts and hematopoietic cells were separated, a significant, albeit somewhat smaller increase in IL-6 production was observed relative to controls. These results suggest that either the osteoblasts or the hematopoietic cells produce soluble factor(s) that mediates the production of IL-6 by human osteoblasts (Fig 4).
Cell- to-cell contact is not required for elevated IL-6 synthesis. CD34+ bone marrow cells (CD34+; 1.0 × 104) were seeded either directly onto confluent osteoblast monolayers (HOB; contact cultures), the top chamber of dual-chambered 24-well plates with confluent primary human osteoblasts in the bottom chamber (noncontact), or alone (CD34+) in Ham's F12/DMEM (1:1 vol/vol) containing 1% FBS. In some cases, 2% paraformaldehyde was used to mildly fix the osteoblasts before the initiation of coculture experiments (fixed HOB). At 96 hours, conditioned medium was collected and assayed for IL-6 by ELISA. Data from a representative of three experiments are presented as the mean ± standard deviation for triplicate determinations. *Significant difference from HOB. +Significant difference from CD34+ or fixed HOB cultures to a statistical significance of P < .05.
Cell- to-cell contact is not required for elevated IL-6 synthesis. CD34+ bone marrow cells (CD34+; 1.0 × 104) were seeded either directly onto confluent osteoblast monolayers (HOB; contact cultures), the top chamber of dual-chambered 24-well plates with confluent primary human osteoblasts in the bottom chamber (noncontact), or alone (CD34+) in Ham's F12/DMEM (1:1 vol/vol) containing 1% FBS. In some cases, 2% paraformaldehyde was used to mildly fix the osteoblasts before the initiation of coculture experiments (fixed HOB). At 96 hours, conditioned medium was collected and assayed for IL-6 by ELISA. Data from a representative of three experiments are presented as the mean ± standard deviation for triplicate determinations. *Significant difference from HOB. +Significant difference from CD34+ or fixed HOB cultures to a statistical significance of P < .05.
Osteoblasts are primarily responsible for IL-6 synthesis in coculture with CD34+ bone marrow cells.Alone, osteoblasts constitutively produce IL-6, but in coculture with CD34+ bone marrow cells, increased levels of IL-6 are observed. We next wanted to determine which of the cell populations were responsible for the enhanced IL-6 synthesis. We therefore determined whether viable osteoblasts were required for the augmented IL-6 levels. For these experiments, 2% paraformaldehyde was used to fix the osteoblasts before the initiation of the cocultures. As shown in Fig 4, alone or in the presence of fixed osteoblasts, CD34+ bone marrow cell-conditioned medium contained no immunodetectable IL-6.
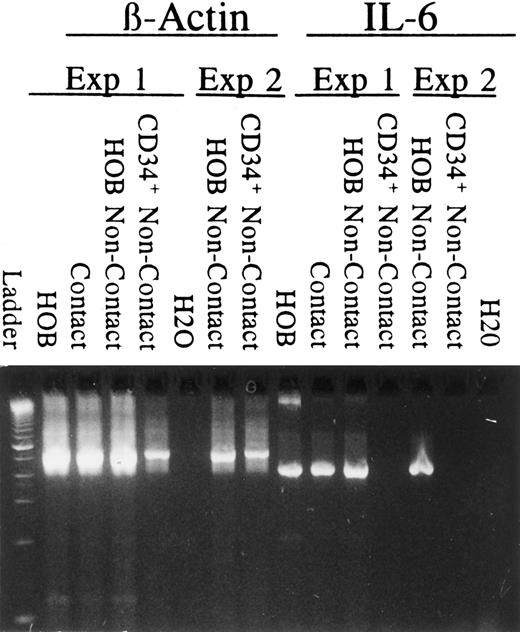
To rule out the possibility that osteoblasts furnish signal(s) to the hematopoietic cells that indirectly stimulates the hematopoietic cells to produce IL-6, two types of experiments were performed. First, RT-PCR was performed to detect IL-6 mRNA on cells recovered from dual-chambered plates in which IL-6 protein levels are significantly elevated relative to osteoblasts cultured alone (Fig 4). As shown in Fig 5, IL-6 message was observed (1) in osteoblasts alone, (2) in osteoblasts and CD34+ cultured in direct contact, and (3) in osteoblasts isolated from the bottom of the dual-chambered culture wells. IL-6 mRNA was not observed in CD34+ bone marrow cells isolated from the top of the dual-chambered culture wells in which enhanced IL-6 protein synthesis was observed (Fig 4). In the second type of experiment, CD34+ cell-conditioned medium was collected and placed on osteoblasts. Under these conditions, CD34+ cell-conditioned medium alone stimulated augmented IL-6 production by the human osteoblasts (Fig 6).
RT-PCR detection of IL-6 mRNA. RT-PCR was performed (35 cycles) to detect mRNA for β-actin and IL-6 from cells recovered from dual-chambered plates at 96 hours in which IL-6 protein levels were known. Primers were designed to cross intron/exon boundaries. Negative controls included omitting reverse transcriptase (not shown) from the reverse transcription reaction or using H2O. Results from two independent experiments are presented. mRNA from osteoblasts only (HOB), mRNA from osteoblasts and CD34+ cells in coculture (contact), mRNA from osteoblasts recovered from dual-chambered coculture (HOB noncontact), and mRNA from CD34+ cells recovered from dual-chambered coculture (CD34+ non-contact).
RT-PCR detection of IL-6 mRNA. RT-PCR was performed (35 cycles) to detect mRNA for β-actin and IL-6 from cells recovered from dual-chambered plates at 96 hours in which IL-6 protein levels were known. Primers were designed to cross intron/exon boundaries. Negative controls included omitting reverse transcriptase (not shown) from the reverse transcription reaction or using H2O. Results from two independent experiments are presented. mRNA from osteoblasts only (HOB), mRNA from osteoblasts and CD34+ cells in coculture (contact), mRNA from osteoblasts recovered from dual-chambered coculture (HOB noncontact), and mRNA from CD34+ cells recovered from dual-chambered coculture (CD34+ non-contact).
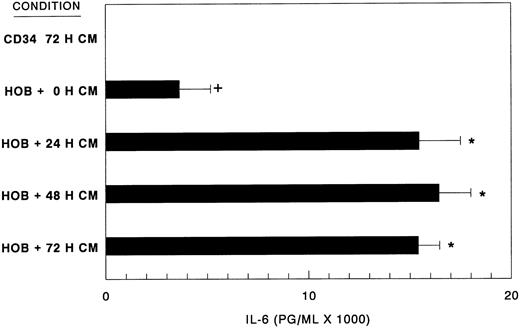
Human CD34+ bone marrow cell-conditioned medium stimulates elevated IL-6 synthesis. Bone marrow CD34+ bone cell-conditioned medium (CM) was prepared as detailed in the Materials and Methods over a 72-hour (0, 24, 48, and 72 hours) period. CM (80% vol/vol) was assayed for IL-6 stimulatory activity on confluent human osteoblast monolayers (HOB) grown in 96-well tissue culture plates at 72 hours by ELISA. Data from a representative of three experiments are presented as the mean ± standard deviation for triplicate determinations. IL-6 levels of CM before HOB exposure were ≤6 pg/mL (CD34 72 H CM). Basal production of IL-6 by HOB cells (HOB + 0 H CM). +Significant difference from CD34+ conditioned medium alone. *Significant difference from HOB basal levels.
Human CD34+ bone marrow cell-conditioned medium stimulates elevated IL-6 synthesis. Bone marrow CD34+ bone cell-conditioned medium (CM) was prepared as detailed in the Materials and Methods over a 72-hour (0, 24, 48, and 72 hours) period. CM (80% vol/vol) was assayed for IL-6 stimulatory activity on confluent human osteoblast monolayers (HOB) grown in 96-well tissue culture plates at 72 hours by ELISA. Data from a representative of three experiments are presented as the mean ± standard deviation for triplicate determinations. IL-6 levels of CM before HOB exposure were ≤6 pg/mL (CD34 72 H CM). Basal production of IL-6 by HOB cells (HOB + 0 H CM). +Significant difference from CD34+ conditioned medium alone. *Significant difference from HOB basal levels.
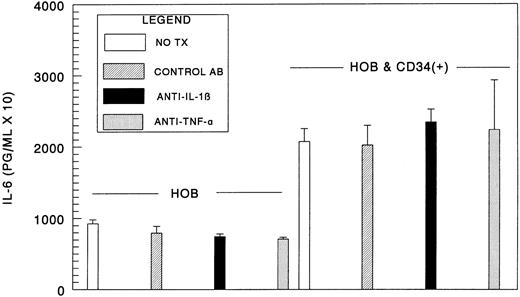
IL-1β and TNF-α production by CD34+ cells is not responsible for increased IL-6 synthesis by human osteoblasts.We next hypothesized that IL-1β or TNF-α was responsible for the soluble activity produced by the CD34+ cells that stimulates IL-6 production by human osteoblasts. To address this issue, conditioned CD34+ cell medium was assayed by ELISA to determine to what level IL-1β and TNF-α were present in the culture supernatants. IL-1β levels were below the level of detection under the conditions of the assay (assay range, 3.9 to 250 pg/mL). Similarly, we found that TNF-α levels were less than 6 pg/mL (assay range, 6 to 1,024 pg/mL). Next, we evaluated whether IL-1β and TNF-α neutralizing antibodies would inhibit the increase in IL-6 levels. For these experiments, daily addition of neutralizing monoclonal antibodies at concentrations at or greater than 10 times the anticipated neutralizing dose reported by the manufacturer were used. We observed no significant reductions in IL-6 levels in the presence of the neutralizing antibodies (Fig 7). Therefore, based on the extremely low levels of IL-1β or TNF-α produced by the hematopoietic cells and the neutralizing antibody investigations, it is unlikely that IL-1β or TNF-α were responsible for increased IL-6 synthesis by human osteoblasts.
IL-1β and TNF-α production by CD34+ cells is not primarily responsible for increased IL-6 synthesis by HOB cells. Bone marrow CD34+ cells (0 to 1.0 × 104) were seeded directly onto confluent human osteoblast monolayers (HOB) in 96-well tissue culture plates containing 1% FBS. For these experiments, daily addition of murine neutralizing monoclonal IgG1 antibodies to human IL-1β, TNF-α, isotype-matched control, or vehicle were added at concentrations approaching 10 times the anticipated neutralizing dose (ND50 ) reported by the manufacturer (10 μg/mL). At 96 hours, conditioned medium was collected and assayed for IL-6 by ELISA. Data from a representative of two experiments are presented as the mean ± standard deviation for triplicate determinations.
IL-1β and TNF-α production by CD34+ cells is not primarily responsible for increased IL-6 synthesis by HOB cells. Bone marrow CD34+ cells (0 to 1.0 × 104) were seeded directly onto confluent human osteoblast monolayers (HOB) in 96-well tissue culture plates containing 1% FBS. For these experiments, daily addition of murine neutralizing monoclonal IgG1 antibodies to human IL-1β, TNF-α, isotype-matched control, or vehicle were added at concentrations approaching 10 times the anticipated neutralizing dose (ND50 ) reported by the manufacturer (10 μg/mL). At 96 hours, conditioned medium was collected and assayed for IL-6 by ELISA. Data from a representative of two experiments are presented as the mean ± standard deviation for triplicate determinations.
DISCUSSION
Because events localized to endosteal surfaces are probably critical for the maintenance of early hematopoietic cells in vivo, we have begun to explore this unique tissue compartment at the cellular and molecular level with several models of endosteal hematopoiesis.24-29 Based on the knowledge that hematopoiesis is facilitated by close associations with cells of the stromal cell microenvironment3,9,19,22,30 31 and that osteoblasts are present on endosteal surfaces, we hypothesized that affiliations between osteoblasts and hematopoietic cells might stimulate production of factors required for normal hematopoiesis. In the present study, we examined the ability of normal human CD34+ bone marrow progenitor cells for their ability to stimulate the production of cytokines by primary human osteoblasts.
We determined that human CD34+ hematopoietic bone marrow progenitors stimulate human osteoblasts to produce IL-6 but not soluble levels of G-CSF, GM-CSF, or TGF-β1 to the limits of the assays. The augmented production of IL-6 over basal levels correlated well with the CD34+ cell number where the most rapid rate in protein accumulation occurred during the initial 24-hour period. Based on RT-PCR and in situ fixation, it seems unlikely that the blood cells themselves were directly responsible for the augmented IL-6 production. However, we determined that CD34+ cells are metabolically engaged in the production of IL-6, because CD34+ cells that are prevented from direct contact with osteoblasts stimulate the secretion of IL-6 to levels roughly comparable to those in which direct contact is permitted. The nature of the molecules/signals produced by CD34+ cells that stimulate osteoblast-derived IL-6 remains to be determined.
Previous studies have shown that osteoblasts produce IL-6,31-38 but its role in mineralized tissue metabolism remains uncertain. Under basal conditions, most human osteoblasts examined produce IL-6 levels that can be enhanced by steroids or proinflammatory mediators, including IL-1 or TNF with marked synergism.34,39-44 For these reasons, we evaluated whether CD34+ cells stimulate IL-6 production by IL-1β– or TNF-α–dependent pathways. We found that the activity produced by CD34+ cells is not likely due to IL-1β or TNF-α. Because IL-6 is believed to uncouple bone resorption from bone formation by activating osteoclastic activity45 while inhibiting osteoblastic activity,46 determining the identity of the CD34+ cell-derived activities may be of great clinical importance.
Like those effects observed on mineralized tissue, IL-6 has multiple activities on hematopoietic cells. Alone, IL-6 has some proliferative activity on early hematopoietic cells,47 but, in combination with IL-3, IL-1, and c-kit ligand, it exerts synergistic activity by recruiting cells out of Go and promoting the transition to G1 .48,49 On other populations, IL-6 facilitates the development of burst-forming unit-erythroid and multipotent colony-forming units (colony-forming unit granulocyte, erythroid, monocyte, megakaryocyte) from hematopoietic progenitors.50 IL-6 is also a critical regulator of B-cell differentiation into plasma cells.51 Furthermore, IL-6 is a potent growth factor for plasmacytoma and myeloma cells in which overproduction of IL-6 is associated with progressive disease.52,53 Although both autocrine and paracrine IL-6 production has been reported, in most cases it appears that cells of the tumor microenvironment are largely responsible for IL-6 synthesis rather than the tumor cells.52-56 For example, Barillé et al54 showed that coculture of XG1 and XG6 myeloma cell lines induces the production of IL-6 by several human osteosarcoma cell lines (MG-63 and SaOS-2), possibly through activation of an NF-κB pathway.57 However, curiously, the production of an osteoblast-specific protein (osteocalcin) was reduced in an autocrine-like manner as anti–IL-6 antibodies reversed the decreases in osteocalcin synthesis.54 That stimulated osteoblast synthesis of IL-6 can be viewed as a generalized feature of osteoblast-hematopoietic metabolism and not restricted to B-cell neoplasms alone is suggested by the demonstration that some human osteosarcomas (HOS cells) secrete IL-6 in response to the adherence of human T cells and/or by crosslinking intercellular adhesion molecule-1 and vascular cell adhesion molecule-1.58 Thus, the observations that (1) osteoblasts stimulate myeloma cell proliferation by producing IL-6 in response to the tumor cells themselves, while at the same time (2) IL-6 inhibits bone formation in an autocrine fashion suggest that the relationships we have observed among normal cells can be co-opted by tumor cells.
Recent experimentation suggests that close associations between hematopoietic cells and stromal cells are important for maintaining hematopoiesis in vivo, although they are probably not an absolute requirement in vitro.59 When hematopoietic cells are in direct contact with stromal cells, cell-to-cell adhesive molecules, stromal cell-associated or extracellular matrix-associated factors, and/or soluble growth factors probably all cooperate in stimulating blood cell proliferation and/or maintenance. The importance of this relationship is illustrated by the observation that, if the two tissues are separated by more than a few millimeters in vitro, a decline in LTC-IC and progenitor cell populations may ensue.13,60 The basis for stromal cell support of hematopoietic cells that are not in intimate contact with the parenchymal cells of the marrow is not clear but may involve high molecular weight proteoglycans.59 Alternatively, these observations suggest that molecules critical for hematopoiesis are either short-lived and/or require high local concentrations for function.13 The nature of those molecules responsible for the enhanced IL-6 production remains to be determined.
In summary, we determined that CD34+ bone marrow cells had no significant effect on osteoblast-secretion of G-CSF, GM-CSF, and TGF-β1 . When CD34+ bone marrow cells were cocultured with osteoblasts, augmented IL-6 synthesis was observed. The accumulation of IL-6 was most rapid during the initial 24-hour period and cell-to-cell contact does not appear to be required for this activity. As important as de novo cytokine synthesis by stromal cells or osteoblasts may be after blood cell binding, it will be difficult to differentiate experimentally between these tissue interactions from activities generated from the engagement of receptor-counterreceptor molecules on the hematopoietic cells themselves. Nevertheless, local resident cells responding to the requirements of hematopoietic cells represent hematopoietic regulation of the bone marrow microenvironment. Our finding that CD34+ hematopoietic cells induce IL-6 production by osteoblasts, not necessarily requiring direct cell-cell contact, suggests that the interaction between these two cell types may be similarly coupled as are those between other members of the stromal cell family and blood cells. To our knowledge, these data represent the first demonstration that normal hematopoietic progenitor CD34+ cells induce the production of hematopoietic growth factors, in this case IL-6, by cells of the bone marrow microenvironment.
ACKNOWLEDGMENT
The authors are indebted to Dr T. Frank and the members of the Surgical Pathology Department for their help in obtaining bone tissues, to Dr M. Baird and M. Tuck for bone marrow, and to Drs J.A. D'Errico and G.H. Sam for helpful discussions.
Supported in part by National Institutes of Health Grants. S.G.E. is supported by a Scholar Award from the Leukemia Society of America.
Address reprint requests to Russell S. Taichman, DMD, DMSc, Department of Periodontics, Prevention, Geriatrics, University of Michigan School of Dentistry, 1011 N University Ave, Ann Arbor, MI 48109-1078.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal