Abstract
Factor X deficiency results in a rare but serious bleeding disorder that might be treated by expressing a normal factor X gene in patients. We generated an amphotropic retroviral vector with the human FX cDNA and delivered it to rat hepatocytes in vivo during liver regeneration. The human α1-antitrypsin promoter was chosen to direct expression because it was the most efficient of several tested in yielding expression of α1-antitrypsin protein from a retroviral vector in hepatocytes in vivo. We achieved expression of factor X in four rats at levels sufficient to maintain hemostasis in humans (10% to 43% of normal). The factor X was determined to be functional by using a chromogenic substrate assay after immunoprecipitation with human specific antibodies. Expression of factor X remained stable for more than 10 months in two rats. It is likely that expression will be maintained for the life of the animals, because retroviral vectors integrate into the chromosome and hepatocytes are long-lived. The high and stable levels of expression achieved using this liver-specific promoter overcomes one of the two major obstacles to successful human gene therapy for hemophilia.
HEMOPHILIAS RESULT in spontaneous and injury-induced bleeding due to a deficiency of a coagulation factor.1 Expression of a normal gene by some tissue of these patients could ameliorate the clinical manifestations of hemophilia by achieving continuous production of the appropriate coagulation factor. Liver is the optimal target organ to express coagulation factors because it is the major site of their synthesis and has direct contact with the blood. Gene therapy for hemophilia has been attempted by delivering plasmid DNA or viral vectors to various cell types using ex vivo or in vivo methods.2-5 Delivery of adenoviral vectors or plasmid DNA to the livers of mice, rats, or dogs has resulted in therapeutic levels of factor VIII6,7 (FVIII) or factor IX8-12 (FIX), but levels decreased rapidly in normal animals. Retroviral vectors have resulted in long-term expression of FIX from hepatocytes13 or myoblasts14 15 in vivo, but the levels were subtherapeutic.
We are investigating the use of retroviral vector-mediated hepatic gene therapy to treat blood protein deficiencies. Recently, we determined that the human α1-antitrypsin (hAAT) promoter led to higher expression of hAAT protein from a retroviral vector than from several other promoters tested. These included the long terminal repeat (LTR), albumin, apolipoprotein AI, phosphoenolpyruvate carboxykinase, or the large subunit of RNA polymerase II promoters.16-18 We hypothesized that therapeutic levels of a coagulation factor might be achieved by using a retroviral vector containing the hAAT promoter. Coagulation factor X (FX) was chosen as the protein to study for developing gene therapy for hemophilia. Activated FX plays a pivotal role in coagulation by converting prothrombin to thrombin. Because the incidence of FX deficiency is low at 1 per 500,000, concentrated human FX (hFX) preparations have not been developed.1 Patients with severe hFX deficiency might be more appropriate for initial trials of gene therapy than patients with hemophilia A or B, for which safe preparations of purified factors are available. In addition, the half-life of FX in humans is longer (40 hours) than the half-life of FIX (20 hours) or FVIII (10 hours),1 which could facilitate achieving therapeutic levels at low biosynthetic rates. We therefore generated a high-titer retroviral vector with the hFX cDNA and delivered it to regenerating rat livers in vivo using a technique that selectively modifies hepatocytes.16 This retroviral vector resulted in expression of stable and therapeutic levels of functional hFX in vivo. This high-level long-term expression of therapeutic levels of a coagulation factor in animals overcomes one of the primary obstacles to successful application of gene therapy in humans.
MATERIALS AND METHODS
Construction of retroviral vectors.The previously described retroviral vector RS839119 was digested with Bgl II and ligated with annealed oligonucleotides A (5′ GATCTGCGGCCGC 3′) and B (5′GATCGCGGCCGCA 3′) to generate RS8391-BN, which has a unique Bgl II site upstream of a unique Not I site. A 1.5-kb hFX cDNA20 was obtained from Dr Earl Davie (University of Washington, Seattle, WA). It was modified to contain 35 nt of 5′ untranslated and 53 nt of 3′ untranslated sequence and Not I sites and was cloned into the Not I site of RS8391-BN to generate LTR-FX. The hAAT promoter (−347 to +56) was cloned into the Bgl II site of LTR-FX as described17 to create hFX-514. hAAT-540B contains the 600-bp apolipoprotein E enhancer, the 402-bp hAAT promoter, the 1.3-kb hAAT cDNA, and the internal ribosome entry site (IRES)-mutant dihydrofolate reductase (*DHFR) cassette. It was previously designated ApoE(−)hAAT-LTR.18 The nomenclature was changed to avoid confusion between the hAAT promoter and the hAAT cDNA.
Generation of the retroviral packaging cell lines.The amphotropic GP+envAM12,21 NIH 3T3 murine fibroblast,22 and Hepa1A23 cells were maintained as described previously17 in medium containing 10 μg/mL vitamin K (Sigma Chemical, St Louis, MO). CaPO2-mediated transfection and selection of GP+envAM12 cells with 250 nmol/L methotrexate (Sigma Chemical) were performed as described previously.16 To screen colonies, 1 mL of conditioned medium was used to infect NIH 3T3 cells. The supernatant from infected NIH 3T3 cells was tested for hFX by enzyme-linked immunosorbent assay (ELISA) after a 4-day collection.
In vivo transduction of rat hepatocytes.Adult male Lewis rats (Sprague Dawley, Indianapolis, IN) weighing ∼200 g received standard institutional care. Twelve milliliters of conditioned medium was collected from each of 24 confluent 15-cm plates of packaging cells grown at 32°C24 and concentrated by ultracentrifugation.16 The pellets were suspended in medium with 8 μg/mL polybrene and injected into the portal vein of a rat that received a 70% hepatectomy 24 hours previously.18 Retroorbital blood was anticoagulated with a 1/10 vol of 3.8% trisodium citrate.
FX ELISA.Mouse monoclonal antibodies no. 1 and 1066 were obtained from Dr J. Miletich (Washington University, St Louis, MO). ELISA25 was performed using antibody no. 1 as the first antibody and horseradish peroxidase (HRP)-coupled antibody no. 1066 as the second antibody. Standards were created by diluting a pool of normal human plasma (George King Biomedicals, Overland Park, KS) that was assumed to have an hFX concentration of 8 μg/mL.1 All samples obtained at 5 months or earlier were analyzed in the same ELISA. Samples obtained at later time points were analyzed in a separate assay using different aliquots from the same set of standards.
ELISA for anti-hFX or anti-hAAT antibodies.ELISA plates were coated with 100 μL containing 1 μg/mL of hFX or hAAT and then blocked with 100 mmol/L NaCl, 50 mmol/L Tris[hydroxymethyl]amino-methane-HCl (pH 7.5) with 5% dry milk (Schnuck's Grocery, St Louis, MO) (TBS-milk). Plasma samples were diluted at ≥1:100 in TBS-milk and incubated on the plates for 2 hours at 25°C and washed with TBS-0.05% Tween 20. An affinity-purified goat antirat IgG antibody (Organon Teknika Corp, Durham, NC) coupled to HRP was added at a 1:1,000 dilution, the washed plate was developed with 3,3′,5,5′-tetramethylbenzidine dihydrochloride, and the optical density (OD) was read at 450 nm.25 Samples were considered positive if the OD was at least twice as high as that observed in the 1:100 dilution of the pretreatment sample for that rat.
Immunoprecipitation/chromogenic substrate assay.Fifty microliters containing 250 μg of 0.87-μm diameter polystyrene beads (IDC Spheres, Portland, OR) coupled to 5 μg of antibody no. 1 was incubated with 5 or 20 μL of rat plasma or 2.5 to 40 μL of human plasma for 90 minutes at 25°C. The beads were washed twice with TBS and resuspended in 100 μL of HBS (10 mmol/L HEPES, pH 7.4, 100 mmol/L NaCl, 1 mg/mL polyethylene glycol [8,000 gm/mol], and 1 mg/mL bovine serum albumin) containing 5 mmol/L CaCl2 and 50 nmol/L Russsell's viper venom (Sigma Chemical). After 30 minutes at 37°C, 100 μL of HBS with 5 mmol/L CaCl2 and 200 μmol/L spectrozyme Xa (American Diagnostica, Greenwich, CT) was added. After 15 minutes at 25°C, 40 μL of 1 mol/L acetic acid was added, and the samples were centrifuged for 1 minute at 12,000 rpm. The OD of the supernatant was read at 405 nm. The activity in rat plasma was determined by comparison with the human plasma standard curve. Blanks did not receive any plasma and were subtracted from all samples.
DNA and RNA analysis.DNA and RNA were isolated and analyzed as described previously.16 A small liver biopsy sample was homogenized in a guanididium-HCl solution. Half of the sample was used to prepare DNA by proteinase K digestion, and the other half was used to prepare RNA by acid phenol extraction. For DNA analysis, a 30-cycle multiplex polymerase chain reaction (PCR) technique was employed, using one primer set that amplified proviral (IRES) DNA and one primer set that amplified rat genomic (liver fatty acid binding protein [LFABP]) DNA, as described previously.16 Amplified DNA was electrophoresed through a 2% agarose/1× Tris-ammonium acetate gel, transferred to an Optitran nitrocellulose membrane, and hybridized with a 217-bp HindIII/Kpn I fragment of the IRES labeled by random primer extension. After quantitation, the membrane was stripped and reprobed with a radiolabeled 620-bp LFABP probe. Radioactivity was quantitated on a Betascope 630 two-dimensional Beta counter (Betagen, Waltham, MA).
Some preparations of RNA were hybrid selected with an oligo-(dT)-sepharose column (Collaborative Research Inc, Bedford, MA). RNA was electrophoresed on a 1% agarose gel in the presence of formaldehyde and transferred to an Optitran nitrocellulose membrane (Schleicher and Schuell, Keene, NH). Membranes were hybridized with the 1.5-kb hFX cDNA probe labeled to a specific activity of 3 × 109 cpm/μg DNA by random primer extension. For both Southern and Northern blots, the final wash was performed at 60°C in 0.1× SSC.
RESULTS
Production of a retroviral packaging cell line.The retroviral vector designated as hFX-514 is shown in Fig 1. hFX-514 was used to create an amphotropic packaging cell line with a titer of 7 × 105 ± 1.8 colony-forming units (cfu)/mL on NIH 3T3 cells. Pools of NIH 3T3 and Hepa1A cells with one copy of the retrovirus per cell produced 246 and 276 ng of hFX/106 cells/24 hours, respectively. More than 90% of the transcripts from packaging cells, transduced NIH 3T3 cells, or transduced Hepa1A cells were 5.1 kb and thus initiated from the LTR promoter (data not shown).
Retroviral vector hFX-514. hFX-514 contains the hAAT promoter, hFX cDNA, and 1.2-kb IRES-mutant *DHFR cassette. The size of the mRNAs that initiate from the LTR or the hAAT promoter is indicated.
Retroviral vector hFX-514. hFX-514 contains the hAAT promoter, hFX cDNA, and 1.2-kb IRES-mutant *DHFR cassette. The size of the mRNAs that initiate from the LTR or the hAAT promoter is indicated.
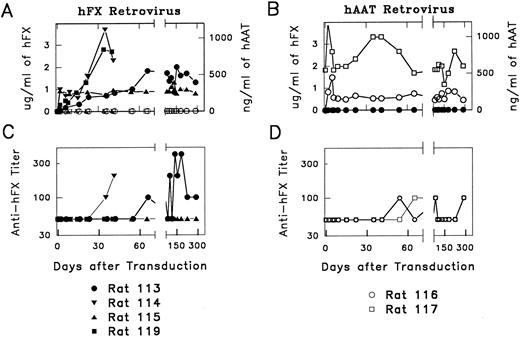
In vivo expression of functional hFX.hFX-514 was delivered to regenerating rat hepatocytes in vivo. As controls, some rats were transduced with hAAT-540B, which contains the hAAT cDNA. Plasma obtained at various times after transduction was tested for hFX or hAAT by immunoassay. hFX levels in hFX-514–transduced rats increased gradually over the first several weeks after transduction for reasons that are unclear. At 4 weeks or later after transduction, all hFX-transduced rats had hFX levels of 0.8 to 3.5 μg/mL, whereas hAAT levels were zero, as expected (Fig 2A). Two hFX-514–transduced rats have exhibited stable expression of hFX for 10 months to date. Two other rats maintained hFX expression for 6 weeks, but died during liver biopsy at that time. Because there was no evidence of bleeding or thrombosis at autopsy and we have previously observed a mortality rate of ∼25% at liver biopsy after transduction with other retroviral vectors, it is unlikely that the deaths were related to the fact that these animals exhibited the highest level of hFX expression. hAAT-540B–transduced rats had no detectable hFX, but exhibited stable expression of hAAT at 0.2 to 0.6 μg/mL (Fig 2B). hAAT levels reached their peak at 1 week after transduction, which is consistent with previous studies.
Expression of retroviral vectors hFX-514 or hAAT-540B and production of anti-hFX antibodies in vivo in rats. (A) hFX and hAAT levels in hFX-514–transduced rats. Rats were treated with a 70% partial hepatectomy. Twenty-four hours later, 4 mL containing ∼12 × 106 cfu of hFX-514 was injected into the portal vein. Plasma was analyzed for hFX (solid symbols) and hAAT (open symbols) by ELISA. (B) hFX and hAAT levels in hAAT-540B–transduced rats . Rats were transduced with ∼2 × 106 cfu of hAAT-540B. Plasma was analyzed for hFX (solid symbols) and hAAT (open symbols). (C) Titers of anti-hFX antibodies in hFX-514–transduced rats. Plasma was initially analyzed for anti-hFX antibodies at a 1:100 dilution as described in the Materials and Methods. If the result was negative, these samples were reported as having a titer of 1:50 or less. Samples that were positive at 1:100 were diluted serially and are reported as the highest dilution at which the OD in the assay was at least twice as high as the OD observed in the same assay using a 1:100 dilution of the pretreatment plasma for that rat. Rats no. 115 and 119 had no detectable anti-hFX antibodies. Rats no. 113 and 114 had anti-hFX antibody titers of 1:100 to 1:400 at 3 weeks or later after transduction. (D) Titers of anti-hFX antibodies in hAAT-540B–transduced rats. Plasma from 540B-transduced rats was analyzed for anti-hFX antibodies as described in (C). Both rats were occasionally weakly positive in this assay.
Expression of retroviral vectors hFX-514 or hAAT-540B and production of anti-hFX antibodies in vivo in rats. (A) hFX and hAAT levels in hFX-514–transduced rats. Rats were treated with a 70% partial hepatectomy. Twenty-four hours later, 4 mL containing ∼12 × 106 cfu of hFX-514 was injected into the portal vein. Plasma was analyzed for hFX (solid symbols) and hAAT (open symbols) by ELISA. (B) hFX and hAAT levels in hAAT-540B–transduced rats . Rats were transduced with ∼2 × 106 cfu of hAAT-540B. Plasma was analyzed for hFX (solid symbols) and hAAT (open symbols). (C) Titers of anti-hFX antibodies in hFX-514–transduced rats. Plasma was initially analyzed for anti-hFX antibodies at a 1:100 dilution as described in the Materials and Methods. If the result was negative, these samples were reported as having a titer of 1:50 or less. Samples that were positive at 1:100 were diluted serially and are reported as the highest dilution at which the OD in the assay was at least twice as high as the OD observed in the same assay using a 1:100 dilution of the pretreatment plasma for that rat. Rats no. 115 and 119 had no detectable anti-hFX antibodies. Rats no. 113 and 114 had anti-hFX antibody titers of 1:100 to 1:400 at 3 weeks or later after transduction. (D) Titers of anti-hFX antibodies in hAAT-540B–transduced rats. Plasma from 540B-transduced rats was analyzed for anti-hFX antibodies as described in (C). Both rats were occasionally weakly positive in this assay.
The hFX levels achieved in the hFX-514–transduced rats would be sufficient to correct the clinical manifestations of hFX deficiency if it were functional. However, the presence of rat FX in normal rats complicates a functional assay using rat plasma. We therefore immunoprecipitated hFX with a human specific antibody before performing an assay measuring proteolysis of an FX chromogenic substrate. The hFX-514–transduced rat plasma had 10% to 54% as much FX activity as did normal human plasma (Table 1). The hAAT-540B–transduced samples had no detectable FX activity, demonstrating the specificity of the assay for human FX. The functional activity correlated well with antigen levels for individual rats, suggesting that the majority of the hFX in hFX-514–transduced rats was functional.
Summary of Results in Rats
| Rat No. . | hFX Antigen . | Functional hFX† . | Percentage of hFX That Functions‡ . | Transduction Efficiencyρ . | Normalized Expression . |
|---|---|---|---|---|---|
| . | (μg/mL)* . | . | . | . | (μg hFX/mL/1% transduction)¶ . |
| 113 | 1.1 ± 0.5 (14) | 17.25 ± 0.75 | 123 ± 15 | 0.3 ± 0.1 | 3.67 ± 1.29 |
| 114 | 2.3 ± 0.1 (29) | 54 ± 10.7 | 174 ± 35 | 1.5 ± 0.5 | 1.53 ± 0.52 |
| 115 | 0.88 ± 0.06 (11) | 9.5 ± 0.5 | 79 ± 7 | 1 ± 0.5 | 0.88 ± 0.44 |
| 119 | 2.71 ± 0.1 (34) | 27 ± 3.2 | 108 ± 13 | 1.1 ± 0.5 | 2.46 ± 1.23 |
| Rat No. . | hFX Antigen . | Functional hFX† . | Percentage of hFX That Functions‡ . | Transduction Efficiencyρ . | Normalized Expression . |
|---|---|---|---|---|---|
| . | (μg/mL)* . | . | . | . | (μg hFX/mL/1% transduction)¶ . |
| 113 | 1.1 ± 0.5 (14) | 17.25 ± 0.75 | 123 ± 15 | 0.3 ± 0.1 | 3.67 ± 1.29 |
| 114 | 2.3 ± 0.1 (29) | 54 ± 10.7 | 174 ± 35 | 1.5 ± 0.5 | 1.53 ± 0.52 |
| 115 | 0.88 ± 0.06 (11) | 9.5 ± 0.5 | 79 ± 7 | 1 ± 0.5 | 0.88 ± 0.44 |
| 119 | 2.71 ± 0.1 (34) | 27 ± 3.2 | 108 ± 13 | 1.1 ± 0.5 | 2.46 ± 1.23 |
Plasma was obtained at 6 weeks after transduction. For all columns, the result ± the standard deviation is shown.
The hFX antigenic activity in micrograms per milliliter is shown, with the percentage of normal human levels shown in parentheses.
The hFX functional activity was determined and is reported as the percentage of normal human activity.
The percentage of hFX that functions was determined by dividing the hFX functional activity by the hFX antigen.
ρ Transduction efficiency was determined by using the PCR-based assay shown in Fig 3A. The percentage of liver cells that were transduced was calculated by averaging the results obtained from three separate assays.
¶ Normalized expression was obtained by dividing the hFX antigen levels by the transduction efficiency.
Test for anti-hFX antibodies.Rat plasma was tested for anti-hFX antibodies. Initial experiments showed that all pretransduction plasma samples had a low activity in the anti-hFX antibody assay when tested at a 1:100 dilution, whereas most samples had detectable activity above background when assayed at a 1:50 dilution. We therefore tested all posttransduction samples at a 1:100 dilution and performed additional serial dilutions if the initial value was positive. Two hFX-514–transduced rats (rats no. 115 and 119) had no detectable anti-hFX antibodies for 6 weeks or 10 months after transduction, as shown in Fig 2C. Rat no. 114 had anti-hFX antibodies that appeared at 22 days after transduction and were present at a titer of ≤1:400 at the time of his demise at 42 days. Rat no. 113 had a low-titer antibody that appeared at day 36 and fluctuated slightly over time, but never exceeded 1:400. The antibody had no apparent effect on the serum level of hFX for rat no. 113, which remained stable. At all time points for up to 10 months after transduction, both of the hAAT-540B–transduced rats were negative or were positive at a dilution of 1:100 in the anti-hFX antibody assay, as shown in Fig 2D. We conclude that significant titers of anti-hFX antibodies did not develop in the hFX-514–transduced rats. Neither hFX-h514– nor hAAT-540B–transduced rats had any detectable antibody against hAAT protein at any time point when plasma was tested at a 1:100 dilution (data not shown).
DNA and RNA analysis of livers from transduced rats.The expression of hFX in plasma depends on both the copy number and expression per copy of the retroviral vector. PCR-based analysis of rat liver DNA (Fig 3A) showed that 0.3% to 1.5% of cells from hFX-514–transduced rats contained the retrovirus. This value is slightly lower than the fraction of cells that were transduced in previous studies after in vivo delivery of a similar number of retroviral particles.16-18 The expression of hFX was normalized for the transduction efficiency, as shown in Table 1. hFX-514–transduced rats expressed an average of 2.13 μg of hFX/mL/1% transduction efficiency.
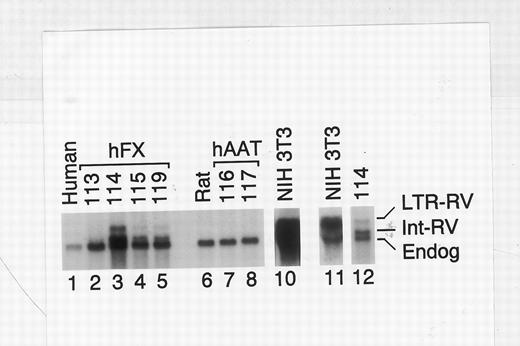
DNA and RNA analysis of retroviral vector-transduced rat livers. A rat liver biopsy was performed at 6 weeks after retroviral transduction, and DNA and RNA were isolated. (A) DNA analysis. PCR was performed using primers specific for retrovirus (IRES) and rat genomic DNA (LFABP).16 Southern blot was performed using an IRES probe followed by stripping and hybridization with an LFABP probe. Standards (lanes 1 through 6) contain DNA from NIH 3T3 cells with 1 copy of the retrovirus per cell diluted with DNA from a nontransduced rat liver. The number above each lane represents the percentage of DNA that was derived from the singly transduced NIH 3T3 cells. Lane 7 shows DNA from a nontransduced rat, whereas lane 8 shows a sample that had no DNA added. Lanes 9 through 12 show DNA from hFX-514–transduced rats. The animal number is listed above each lane. Lanes 13 and 14 show DNA derived from hAAT-540B–transduced rats. (B) Northern blot analysis. Northern blot analysis was performed using 1 μg of poly-A–selected RNA (human liver sample), 5 μg of poly-A–selected RNA (all rat liver samples), or 10 μg of total cellular RNA (NIH 3T3 cells). The blot was hybridized with an hFX probe, and a 14-hour (lanes 1 through 10) or a 4-hour (lanes 11 and 12) autoradiogram was obtained. Lane 1 shows RNA from a normal human liver. The position of the endogenous 2.2-kb hFX mRNA is marked on the right by “Endog.” Lanes 2 through 5 show RNA from hFX-514–transduced rats, with the animal number listed above each lane. The position of the 5.1-kb LTR-initiated retroviral transcript (LTR-RV) and the 3.7-kb internal promoter-initiated retroviral transcript (Int-RV) are shown. Lane 6 (Rat) shows nontransduced rat liver RNA. Lanes 7 and 8 show RNA from hAAT-540B–transduced rats. Lane 10 shows RNA from hFX-514–transduced NIH 3T3 cells. Lanes 11 and 12 show a shorter exposure of the samples in lanes 10 and 3, respectively.
DNA and RNA analysis of retroviral vector-transduced rat livers. A rat liver biopsy was performed at 6 weeks after retroviral transduction, and DNA and RNA were isolated. (A) DNA analysis. PCR was performed using primers specific for retrovirus (IRES) and rat genomic DNA (LFABP).16 Southern blot was performed using an IRES probe followed by stripping and hybridization with an LFABP probe. Standards (lanes 1 through 6) contain DNA from NIH 3T3 cells with 1 copy of the retrovirus per cell diluted with DNA from a nontransduced rat liver. The number above each lane represents the percentage of DNA that was derived from the singly transduced NIH 3T3 cells. Lane 7 shows DNA from a nontransduced rat, whereas lane 8 shows a sample that had no DNA added. Lanes 9 through 12 show DNA from hFX-514–transduced rats. The animal number is listed above each lane. Lanes 13 and 14 show DNA derived from hAAT-540B–transduced rats. (B) Northern blot analysis. Northern blot analysis was performed using 1 μg of poly-A–selected RNA (human liver sample), 5 μg of poly-A–selected RNA (all rat liver samples), or 10 μg of total cellular RNA (NIH 3T3 cells). The blot was hybridized with an hFX probe, and a 14-hour (lanes 1 through 10) or a 4-hour (lanes 11 and 12) autoradiogram was obtained. Lane 1 shows RNA from a normal human liver. The position of the endogenous 2.2-kb hFX mRNA is marked on the right by “Endog.” Lanes 2 through 5 show RNA from hFX-514–transduced rats, with the animal number listed above each lane. The position of the 5.1-kb LTR-initiated retroviral transcript (LTR-RV) and the 3.7-kb internal promoter-initiated retroviral transcript (Int-RV) are shown. Lane 6 (Rat) shows nontransduced rat liver RNA. Lanes 7 and 8 show RNA from hAAT-540B–transduced rats. Lane 10 shows RNA from hFX-514–transduced NIH 3T3 cells. Lanes 11 and 12 show a shorter exposure of the samples in lanes 10 and 3, respectively.
To determine which retroviral promoter was used in vivo, RNA was analyzed by Northern blot using a human FX probe (Fig 3B). Normal human liver contained a 2.2-kb band that is similar to the reported size of the human FX mRNA.26 hFX-514–transduced NIH 3T3 have a major band at 5.1 kb that represents LTR-initiated transcripts and a minor band at ∼2.2 kb that probably represents a splicing event between the 5′ splice site of the retroviral vector and a 3′ splice site in the hFX coding sequence. The smaller RNA does not represent endogenous FX, because the same band was present when RNA from hFX-514–transduced NIH 3T3 cells were probed with an IRES-specific probe and was absent from RNA from nontransduced NIH 3T3 cells that were probed with an hFX-specific probe (data not shown).
All rat samples had a 2.2-kb RNA that probably represents the endogenous rat FX mRNA and can be used to estimate the loading efficiency for each sample. Samples from hFX-514–transduced rats had additional bands that were absent in control rats. The 5.1-kb band that comigrates with the major band in hFX-514–transduced NIH 3T3 cells represents LTR-initiated transcripts. The 3.7-kb band is the expected size of the hAAT promoter-initiated transcript. Because hAAT-initiated transcripts were more abundant than the LTR-initiated transcripts, the hAAT promoter is probably the major promoter used in vivo. Retroviral mRNA levels for individual rats did not correlate perfectly with the protein levels that were observed on the day of liver biopsy, although they did correlate well with the calculated DNA copy number for each rat. This discrepancy may be due to the fact that retroviral vector transduction is not uniform throughout the liver (Y.Z. Lin and K.P.P., unpublished data), and a relatively small sample of the liver was obtained for isolation of RNA and DNA.
DISCUSSION
This study shows that 0.8 to 2 μg/mL of functional hFX (10% to 25% of levels contained in human plasma) can be achieved for more than 10 months in rat plasma by using retroviral vector-mediated hepatic gene therapy. These levels would correct most or all of the bleeding manifestations in human patients with hFX deficiency, because patients with greater than 10% of normal activity have few hemorrhages.1 We believe that the hAAT promoter is responsible for the high-level expression observed, because we previously showed that it is much stronger than several other promoters from a retroviral vector in vivo. hFX expression will probably be maintained, because stable expression of hAAT protein has been observed in rats for 2 years after transduction with similar retroviral vectors.5 Although the number of rats analyzed in this study was small, four additional Lewis rats have exhibited stable expression of hFX for up to 7 months after transduction.
Previous studies attempting gene therapy have failed to achieve stable expression of therapeutic levels of coagulation factors. In vivo transduction of hepatocytes of FIX-deficient beagles with a canine FIX-expressing retroviral vector resulted in stable FIX levels of ∼5 ng/mL (0.1% of normal). The low expression was probably partially due to the use of the LTR promoter, which is weak in hepatocytes in vivo,16 although a low transduction efficiency or differences in expression of FIX as compared with FX may have played a role. Similarly, ex vivo transduction of primary myoblasts with a retroviral vector containing a muscle-specific enhancer upstream of the CMV14 or β-actin15 promoter resulted in stable but subtherapeutic FIX levels of ∼10 ng/mL. In contrast, adenoviral vectors have resulted in 0.3 to 1 μg/mL of FVIII (150% to 500% of normal)6,7 or 0.35 to 100 μg/mL of FIX (7% to 2,000% of normal)8-11 in plasma of animals, whereas plasmid DNA resulted in up to 35% of normal FIX levels.12 Although therapeutic, expression has been transient due to the immunologic rejection of adenoviral-transduced cells and the inability of plasmid vectors to integrate into the chromosome. Immunosuppressant therapy slowed but did not prevent the inexorable loss of expression from adenoviral vectors in most animals.8 27
Absence of significant levels of anti-hFX antibodies in Lewis rats.Patients with hemophilia could develop antibodies directed against a coagulation factor protein that is expressed de novo in their blood. This might limit the efficacy of the gene therapy procedure or lead to immune-complex disease. In this study, two hFX-514–transduced Lewis rats had no detectable anti-hFX antibodies. Two other Lewis rats had low-titer antibodies that did not increase over time and had no effect on the serum level of hFX. We conclude that anti-hFX antibody production was not a significant problem in the Lewis rats. In contrast, all of the 10 Sprague-Dawley rats that received the same retroviral vector developed anti-hFX IgG antibodies whose titer was ≥1:6,400 in the assay described here (data not shown). Furthermore, although the expression of hFX was high at 1 to 4 μg/mL at 1 week after transduction, it decreased to undetectable levels by 1 month after transduction. Intriguingly, retroviral vector DNA was absent from the Sprague-Dawley rats at 2 months after transduction, suggesting that retroviral-transduced cells were rejected by an as yet undefined immunologic mechanism. This shows that the genetic background can affect the immunologic response to novel proteins. Further experiments are in progress to study the development of anti-hFX antibodies in Sprague-Dawley rats.
Implications for human gene therapy.Retroviral vector-mediated hepatic gene therapy has been limited in humans because of subtherapeutic protein expression and the surgical risks associated with the genetic modification of hepatocytes. The first problem, that of low-level expression, has been overcome in the current study by using a strong liver-specific promoter. The second major obstacle is that in vivo and ex vivo approaches require a major surgical procedure to induce hepatocyte replication or to harvest hepatocytes, respectively.5
Although partial hepatectomy effectively facilitated retroviral vector transduction in this study, implementation of retroviral vector-mediated gene therapy for the treatment of hemophilia will require a safer method for retroviral vector transfer to hepatocytes. A major focus of this laboratory is to identify safer methods for the in vivo delivery of retroviral vectors to the liver. We have recently determined that portal branch occlusion is nearly as effective as partial hepatectomy at facilitating retroviral vector transduction in vivo and has a lower mortality.28 Portal branch occlusion could be performed in larger animals by minimally invasive techniques and has been used safely to treat human patients with liver cancer. Portal branch occlusion might ultimately be used in humans to facilitate retroviral vector transduction in vivo for the treatment of genetic diseases. Alternatively, it was recently reported that an adenoviral vector that encodes a toxic protein could facilitate retroviral vector transduction of the liver.29 We are currently exploring the possibility that therapeutic levels of hFX can be achieved after induction of hepatocyte replication by one of these nonsurgical methods without significant toxicity. If this approach is successful and safe in large animals, in vivo retroviral vector-mediated gene therapy would be a very attractive method for permanently correcting the clinical manifestations of patients with severe hemophilia due to hFX deficiency. Methods that are successful for hFX deficiency should be efficacious for hemophilia B, because FIX and FX are highly homologous proteins.
ACKNOWLEDGMENT
We thank Drs J. Miletich and A. Rudolph for antibodies and assistance in FX assays, Dr E. Davie for the hFX cDNA, and Dr P. Majerus for helpful comments on the manuscript.
Supported by grants from the National Institutes of Health (R29-DK44593) and the March of Dimes awarded to K.P.P. M.-T.L. was supported by a fellowship from the American Liver Foundation.
Address reprint requests to Katherine Parker Ponder, MD, Box 8125, 660 S Euclid Ave, St Louis, MO 63110.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal