Abstract
Sixty-four cases of mantle cell (centrocytic) non-Hodgkin's lymphomas have been analyzed for their cytomorphologic features, proliferation indices, bcl-1 rearrangements, p53 expression patterns, and DNA content by both interphase cytogenetic and DNA flow cytometric analyses. According to cytomorphology, three subtypes were recognized: a common, a lymphoblastoid, and a pleomorphic variant of mantle cell lymphoma (MCL). Blastoid MCL subtypes were characterized by distinctly elevated mitotic counts (57 and 51/10 HPF v 21/10 high-power fields in common MCL), proliferation indices (58% and 53% v 27% in common types, respectively; P < .001), frequent bcl-1 rearrangements at the major translocation cluster locus (59% v 40%), and overexpression of p53 (21% v 6%). However, the most interesting finding was a striking tendency of blastoid MCL subtypes to harbor chromosome numbers in the tetraploid range (36% of lymphoblastoid and 80% of pleomorphic types v 8% of common variants, P < .001), a feature clearly separating these neoplasms from other types of B-cell non-Hodgkin's lymphoma and possibly being related to cyclin D1 overexpression. Our data indicate that, although characterized by a uniform immunophenotype and common biologic background, MCL shows a broad spectrum of morphologic features ranging from small cell to blastoid types and that the morphologic spectrum is mirrored by distinct biologic features.
MANTLE CELL LYMPHOMA (MCL) is a malignant non-Hodgkin's lymphoma (NHL) of B-cell lineage derived from immature CD5+ virgin B cells of the follicular mantle zone. This tumor was first described by Gerard-Marchant et al1 as centrocytic lymphoma and integrated as an entity in the Kiel classification system. A large number of malignant lymphomas classified as intermediate lymphocytic lymphoma or lymphocytic lymphoma of intermediate differentiation in the modified Rappaport classification were found to correspond to the description of centrocytic lymphoma2 and the term mantle cell lymphoma was proposed as a unifying concept.3
The chromosomal translocation t(11; 14)(q13;q32), resulting in the rearrangement and overexpression of the PRAD1/cyclin D1 gene, has been shown to be highly characteristic of MCL.4-6 Cyclin D1 plays a crucial role in the regulation of the cell cycle,7-9 and the finding of increased cyclin D1 mRNA10,11 and protein12 13 expression in mantle cell lymphoma stresses the importance of the t(11; 14) as a putative initial event in multistep tumorigenesis.
Shortly after the first description of MCL, it had become obvious that this tumor is not always composed of small- to medium-sized cells exclusively.14 Other investigators also looking at larger series of MCL recognized, next to the small-cell variant, the existence of a so-called large-cell or blastic variant of the disease.2,15 16 However, the significance of this finding is still controversial as are the exact criteria and biologic features separating small-cell and blastic variants.
The aim of the study presented here was to more exactly define the cytomorphologic spectrum of MCL in a large series and to correlate immunohistochemical, cytogenetic, interphase cytogenetic, DNA flow cytometric, and molecular genetic findings with the morphologic features of this neoplasm.
MATERIALS AND METHODS
Specimen selection.All cases of MCL had been diagnosed and categorized by routine diagnostics by two of us (G.O. and H.K.M.-H.). Sixty-four cases were selected for the present study. To achieve an equal distribution with comparable data for each subgroup, the number of cases with a morphology of classic MCL was restricted to 35 cases. Thirty-two of the 64 samples had already been included in two earlier studies.13 17 All diagnoses had been performed using formalin-fixed and paraffin-embedded material stained for hematoxylin and eosin, giemsa, periodic acid-Schiff, and Gomori's silver impregnation. In all cases, immunophenotyping was performed on paraffin sections using B-cell (CD20) and T-cell markers (CD3, CD43, and CD45RO), among others. In 44 cases altogether, fresh-frozen material was available enabling the additional testing of CD3, CD4, CD5, CD8, CD10, CD19, CD22, CD23, and CD30 antigens next to Ig light and heavy chains, including IgD.
Mitotic index.Mitotic activity was assessed in all cases by counting mitoses in 10 randomly selected high-power fields (×40) in Giemsa-stained slides.
Proliferation index and p53 expression.Proliferation indexes were estimated by two observers using the MIB1 antibody (Dianova, Hamburg, Germany), detecting the Ki67 antigen on paraffin slides. The expression of p53 was assessed on paraffin sections as well using the DO-1 antibody (Dianova). In 39 cases, the DO-7 antibody (Dako, Hamburg, Germany) was used in addition. Immunohistochemical staining was performed using the peroxidase antiperoxidase method after microwave irradiation. Paraffin sections of 4 to 6 μm were mounted on slides pretreated with Silan and then deparaffinized and rehydrized in decreasing ethanol concentrations. The slides were treated for 30 minutes in a microwave oven (750 W) in a citrate solution (2.1 g citrate/L H2O, pH 6.0) before incubation with the respective antibody.
Southern blot analysis and polymerase chain reaction (PCR).In 39 of the 44 cases with fresh-frozen tissue available, high molecular weight DNA could be extracted from remaining tissues after immunohistochemistry. After digestion with appropriate restriction enzymes (Sst I, HindIII, and Pst I) and electrophoresis on 0.8% agarose gels, Southern blotting was performed on nitrocellulose filters. The filters were hybridized with a 32P random-labeled DNA probe (a 2.1-kb DNA fragment of the major translocation cluster [MTC] region of bcl-1; probe b; kindly provided by C. Croce, Philadelphia, PA).18 In the remaining 25 cases, DNA could only be extracted from formalin-fixed, paraffin-embedded tissues after deparaffinizing procedures. Therefore, a two-step PCR was used to detect bcl-1/JH conjugates in these lymphomas. As described earlier,13 a JH-consensus primer19 and a primer for the bcl-1 MTC region were applied in the first PCR with 30 cycles of denaturation (1 minute at 95°C), annealing (1 minute at 58°C), and extension (1 minute at 72°C). The reaction was performed with 1 μg DNA per sample. One microliter of the PCR product was used in a second PCR with the same JH-consensus primer and an inner primer for the bcl-1 MTC region and similar reaction conditions (1 minute at 95°C, 1 minute at 60°C, and 1 minute at 72°C). Both bcl-1 primers (P2 for chromosome 1120) and primer B according to Williams et al21 were slightly modified as recently described.13 The samples were separated on a 3% agarose gel and amplificates were visualized with ethidium bromide under UV light. The presence of amplifiable DNA extracted from paraffin blocks was checked by PCR with amplification of the betaglobin gene.
In situ hybridization (ISH) studies.Fifty cases of MCL were selected for ISH studies. Cells were disaggregated according to the method of Visakorpi et al.22 Briefly, up to 10 10-μm sections were cut from a representative paraffin block, and the material was dewaxed in xylene, rehydrated in graded alcohols, and digested with 1 mg proteinase K in 2 mL Carlsberg solution (0.1% protease XXIV [Sigma, Deisenhofen, Germany], 0.1 mol/L Tris-buffer, 0.07 mol/L NaCl, pH 7.2) for 60 minutes. The tissue was then disaggregated mechanically by vigorous shaking, rinsed in phosphate-buffered saline (PBS), and centrifuged. The resulting nuclear pellet was resuspended in PBS and dropped onto a glass slide.
After washing twice in 2× SSC, a proteolytic treatment was performed with pepsin (4 mg/mL in 0.2 N HCl) with or without prior treatment with 1 mol/L NaSCN according to the protocol of Hopman et al23 at 37°C. Generally, digestion times had to be evaluated for each case ranging from 0 to 2 minutes. After digestion and rinsing in 2× SSC, dehydration followed.
Biotin- or digoxigenin-labeled centromeric repetitive satellite DNA probes specific for chromosomes 3, 7, 12, 18, X, and Y (in male patients) were purchased from Oncor (Gaithersburg, MD). They were used in double-fluorescence ISH (FISH) experiments at concentrations of 1 to 2 μg/mL DNA probe in approximately 10 μL hybridization mixture containing 60% formamide, 2× SSC, 10% dextrane sulfate, and 0.1% salmon sperm DNA.
Simultaneous denaturation of the slides and the hybridization mixture containing the probe under a coverslip sealed with rubber cement was performed at 80°C for 5 minutes in a tin plate floating in a water bath.
Hybridization was allowed to take place overnight at 37°C. After removing the coverslips, slides were washed three times for 10 minutes in 60% formamide in 2× SSC at 47°C and subsequently in 2× SSC at 42°C. Signal detection was accomplished using appropriate Cy3- and fluorescein isothiocyanate-conjugated antibodies. Visualization of the signals was performed with a Zeiss Axiophot fluorescence microscope (Zeiss, Oberkochen, Germany). Twelve reactive lymph nodes and two tonsil specimens served as controls.
Cytogenetic studies.Classical cytogenetic studies could be performed in 17 cases. Unstimulated 10-mL cell cultures were set up in RPMI 1640 medium containing 1 to 2 × 106 cells/mL culture medium and allowed to grow overnight. Metaphase preparation involved 30 minutes of exposure to colchicin, hypotonic shock in 0.075 mol/L KCl, and fixation in methanol/acetic glacial acid (3:1). Metaphases were stained using a trypsin-Giemsa standard technique and results were evaluated according to the ISCN.24
DNA flow cytometry.Nuclei from 45 of the 50 lymphomas that were studied in ISH experiments and from which enough material was still available were prepared from paraffin blocks as described above, filtered through a nylon gaze (50-μm mesh), and stained with propidium iodide. Fluorescence of 20,000 nuclei was measured in a FACScan flow cytometer. The data were analyzed with the software program Multicycle AV (P. Rabinovitch, University of Washington, Seattle, WA), with background subtraction and correction for sliced nuclei.
Statistical analysis.Differences in the mitotic rate and the number of cells positive for MIB1 among the morphologic subtypes of MCL were analyzed by the application of the t-test for independent samples. The Pearson χ2 test was used to study differences in p53 expression, bcl-1 rearrangements, and ploidy. The Pearson correlation coefficents were applied to study the correlation between proliferative and mitotic indices as well as the relationships of these two parameters and p53 expression, bcl-1 rearrangements, and ploidy. The analysis of variance (ANOVA) was used to estimate the 95% confidence interval of the mean.
All statistical analyses were performed by the statistical software SPSS for Windows, release 6.1.3 (German version; SPSS Inc, Chicago, IL).
RESULTS
Cytomorphologic classification of MCL and growth patterns.Sixty-four lymphomas classified as MCL were analyzed for their cytomorphologic features. Three variants were recognized in the present study. The common type of MCL (35 cases) largely corresponded to lymphomas classified as centrocytic in the Kiel classification.14 This type of MCL is characterized by small to medium-sized cells with a scant cytoplasm and irregular cleaved nuclei with finely dispersed chromatin and inconspicuous nucleoli (Fig 1A). Some size variation was observed among the cases classified as common MCL, with nuclei sometimes having a rounder appearance; however, these cases had no blastic features. In the lymphoblastoid type (15 cases), tumor cells were larger and had an equally monotonous appearance with scant cytoplasm, but showed somewhat rounder nuclear contours and a more vesicular chromatin, so that the tumor cells frequently resembled lymphoblasts (Fig 1B). Sometimes large macrophages were interspersed between the tumor cells. The third variant of MCL recognized, the pleomorphic type (14 cases), in contrast, consisted of large cells or a mixture of medium-sized and large cells (Fig 1C). The tumor cells possessed a small rim of light to slightly basophilic cytoplasm and pleomorphic, sometimes deeply indented or folded nuclei with a more primitive chromatin distribution and several nucleoli. Usually, numerous mitotic figures were encountered in the two latter types. In all variants, cases with a characteristic perifollicular (mantle zone) growth pattern were observed, although lymphoblastoid and pleomorphic lymphomas frequently presented with diffuse infiltrates (Table 1). Immunophenotyping of 44 lymphomas in which frozen tissue blocks were available disclosed the characteristic immunophenotype of MCL (CD22+, CD5+, CD10−, CD23−, IgD+/−) in all cases, of which 26 belonged to the common and 9 cases each to lymphoblastoid and pleomorphic types, respectively. In 20 cases with only paraffin-embedded tissues available for immunohistochemistry, the tumor cells were all positive for CD20, with coexpression of CD43 in 13 tumors.
The cytomorphologic spectrum of MCL. (A) Common variant, (B) lymphoblastoid variant, and (C) pleomorphic variant of MCL. Note the same amount of magnification (×1,000) in (A), (B), and (C).
The cytomorphologic spectrum of MCL. (A) Common variant, (B) lymphoblastoid variant, and (C) pleomorphic variant of MCL. Note the same amount of magnification (×1,000) in (A), (B), and (C).
Bicolor FISH in a pleomorphic variant of MCL. Note four red signals for chromosome 7 and two green signals for chromosome X in two cells (arrows) of a male patient, indicating a tetraploid chromosome clone.
Bicolor FISH in a pleomorphic variant of MCL. Note four red signals for chromosome 7 and two green signals for chromosome X in two cells (arrows) of a male patient, indicating a tetraploid chromosome clone.
Growth Pattern Distribution in Common, Lymphoblastoid, and Pleomorphic Types of MCL
| . | Mantle Zone . | Nodular . | Diffuse . |
|---|---|---|---|
| Common (n = 35) | 9 (26%) | 16 (46%) | 10 (28%) |
| Lymphoblastoid (n = 15) | 3 (20%) | 1 (7%) | 11 (73%) |
| Pleomorphic (n = 14) | 2 (14%) | 1 (7%) | 11 (79%) |
| . | Mantle Zone . | Nodular . | Diffuse . |
|---|---|---|---|
| Common (n = 35) | 9 (26%) | 16 (46%) | 10 (28%) |
| Lymphoblastoid (n = 15) | 3 (20%) | 1 (7%) | 11 (73%) |
| Pleomorphic (n = 14) | 2 (14%) | 1 (7%) | 11 (79%) |
Mitotic activity.The number of mitoses counted in 10 randomly selected high-power fields was considerably different between common and blastoid variants. The mean mitotic indices for lymphoblastoid MCL (mean = 57.33/10 high-power fields [HPF ]) and pleomorphic MCL (mean = 51.07/10 HPF ) were significantly higher than in common variants (mean = 21.26/10 HPF; Table 2).
Proliferation Indexes, Mean Mitotic Activity, p53 Overexpression, Ploidy Status, and bcl-1 Rearrangement in the Different Subtypes of MCL and in Common Versus Blastoid MCL Types
| MCL Subtype . | Proliferation Index (CI) . | Mean Mitotic Activity (CI) . | p53 Overexpressed . | Tetraploid Chromosome Numbers . | No. of Cases bcl-1 Rearranged . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | . | . | . |
| A.Different Subtypes of MCL | ||||||||||
| Common (n = 35) | 27% (±21.1%) | 21.26 (±15.4) | 2 (6%) | 2/26 (8%) | 14 (40%) | |||||
| Range, 5%-60% | Range, 2-71 | |||||||||
| Lymphoblastoid (n = 15) | 58% (±49.9%) | P > .1 | 57.33 (±37.3) | P > .1 | 4 (27%) | P > .1 | 5/14 (36%) | P < .032 | 8 (53%) | P > .1 |
| Range, 25%-80% | Range, 4-111 | |||||||||
| Pleomorphic (n = 14) | 53% (±39.9%) | 51.07 (±30.2) | 2 (14%) | 8/10 (80%) | 9 (64%) B.Common versus blastoid MCL types | |||||
| Range, 20%-90% | Range, 9-126 | |||||||||
| Common (n = 35) | 27% (±21.1%) | 21.26 (±15.4) | 2 (6%) | 2/26 (8%) | 14 (40%) | |||||
| Range, 5%-60% | Range, 2-71 | |||||||||
| Blastoid*(n = 29) | 55% (±48.3%) | P < .001 | 54.31 (±40.73) | P < .001 | 6 (21%) | P = .0713 | 13/24 (54%) | P = .0003 | 17 (59%) | P = .143 |
| Range, 20%-90% | Range, 4-126 | |||||||||
| MCL Subtype . | Proliferation Index (CI) . | Mean Mitotic Activity (CI) . | p53 Overexpressed . | Tetraploid Chromosome Numbers . | No. of Cases bcl-1 Rearranged . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | . | . | . |
| A.Different Subtypes of MCL | ||||||||||
| Common (n = 35) | 27% (±21.1%) | 21.26 (±15.4) | 2 (6%) | 2/26 (8%) | 14 (40%) | |||||
| Range, 5%-60% | Range, 2-71 | |||||||||
| Lymphoblastoid (n = 15) | 58% (±49.9%) | P > .1 | 57.33 (±37.3) | P > .1 | 4 (27%) | P > .1 | 5/14 (36%) | P < .032 | 8 (53%) | P > .1 |
| Range, 25%-80% | Range, 4-111 | |||||||||
| Pleomorphic (n = 14) | 53% (±39.9%) | 51.07 (±30.2) | 2 (14%) | 8/10 (80%) | 9 (64%) B.Common versus blastoid MCL types | |||||
| Range, 20%-90% | Range, 9-126 | |||||||||
| Common (n = 35) | 27% (±21.1%) | 21.26 (±15.4) | 2 (6%) | 2/26 (8%) | 14 (40%) | |||||
| Range, 5%-60% | Range, 2-71 | |||||||||
| Blastoid*(n = 29) | 55% (±48.3%) | P < .001 | 54.31 (±40.73) | P < .001 | 6 (21%) | P = .0713 | 13/24 (54%) | P = .0003 | 17 (59%) | P = .143 |
| Range, 20%-90% | Range, 4-126 | |||||||||
P is the results of the tests for equality.
Abbreviation: CI, 95% confidence interval.
Both lymphoblastoid and pleomorphic types.
Proliferation indexes.The number of cells expressing the Ki67 antigen as detected by the monoclonal antibody MIB-1 in paraffin sections was estimated to be between 5% and 60%, with a mean value of 27% in common MCL types. Blastoid variants, on the other hand, showed mean values of 58% (range, 25% to 80%) in lymphoblastoid and of 53% (range, 20% to 90%) in pleomorphic types. The difference in mean values of proliferative fractions in common versus blastoid MCL proved to be statistically different. There was a positve correlation between the number of mitoses and the proliferation index (r = .684, P < .001).
p53 expression.p53 overexpression was observed in 11 cases. In 3 of these samples, less than 10% of nuclei were stained, and these cases were scored as negative. In the remaining cases, more than 50% up to 90% of nuclei showed distinct staining. Overall, 2 of 35 common MCL (6%) showed overexpression of p53 as opposed to 4 of 15 (27%) lymphoblastoid and 2 of 14 (14%) pleomorphic variants. No differences in staining results were observed in 39 cases, in which both DO-1 and DO-7 were used. However, the difference in p53 expression in blastoid versus nonblastoid cases was not highly significant (P = .0714).
bcl-1 rearrangements.DNA was available for Southern blot analysis of bcl-1 rearrangements at the MTC region in 39 lymphomas. The remainder of 25 cases was studied using a seminested PCR technique equally allowing for the detection of bcl-1 MTC locus rearrangements. The sensitivity of the PCR procedure had been tested by comparing the results from Southern blotting with those obtained by PCR using DNA from deparaffinized tissues of the corresponding cases as described earlier in detail.13 Overall, 31 of the 64 (48%) samples showed bcl-1 locus rearrangements. Eighteen of 39 (46%) cases were rearranged in Southern blotting, whereas in 13 of 25 lymphomas (52%), distinctly amplified bands of slightly varying size were observed after PCR amplification of bcl-1/JH in DNA isolated from paraffin blocks. Although a certain difference in the number of rearranged cases was seen in the different subgroups (14 of 35 [40%] common types in contrast to 8 of 15 [53%] lymphoblastoid and 9 of 14 [64%] pleomorphic variants), this result did not attain statistical difference.
ISH and cytogenetic studies.In control experiments using disaggregated nuclei from 12 reactive lymph nodes and 2 tonsil specimens, up to 80% of cells showed two signals when hybridized to chromosome probes 3, 7, 12, and 18. Cells with three or four signals were observed in 0% to 2% of 200 nuclei evaluated per case. A cutoff value for trisomies was established at a minimum of 5% nuclei for avoiding false-positive results. Likewise, a tumor was regarded as tetraploid if more than 5% of nuclei showed four hybridization signals with at least three different (disomic) chromosome probes (or 2 signals for X or Y in male patients).
Twenty-seven of 50 cases analyzed by bicolor FISH with centromere-specific probes for chromosomes 3, 7, 12, 18, X, and Y (male patients) were shown to harbor chromosomal aneusomies. Trisomy 3 was detected in 6 cases, trisomy 7 in 4, and trisomy 18 in 2. In 3 lymphomas, ISH results suggested the loss of the Y chromosome. However, the most frequent numerical aberration encountered in our cases studied was a signal distribution indicative of a tetraploid chromosome clone in 15 of 50 (30%) lymphomas. Two of 26 (8%) common variants, 5 of 14 (36%) lymphoblastoid MCL and 8 of 10 (80%) blastoid MCL of the pleomorphic type showed between 10% and 34% of nuclei with four distinct signals when hybridized to disomic (3, 7, 18, and X) chromosome probes or up to 78% with 2 signals when hybridized to monosomic (Y and X in male patients) chromosome probes (Fig 2 and Table 3).
ISH Results, G2/M Fractions, and Cytogenetic Results in Tetraploid MCL
| No. . | MCL Subtype . | ISH Results . | No. of Aberrant Metaphases . | |||
|---|---|---|---|---|---|---|
| . | . | (chromosome 7 probe) . | Diploid . | Tetraploid . | ||
| . | . | % of Nuclei With . | G2/M Fraction (%) . | . | . | |
| . | . | 3 Signals . | 4 Signals . | . | . | . |
| 1 | Common | 22 | 20 | 74.9 | ND | |
| 2 | Common | 16 | 11 | ND | ND | |
| 3 | Lymphoblastoid | 15 | 28 | 45 | ND | |
| 4 | Lymphoblastoid | 22 | 22 | 69.5 | ND | |
| 5 | Lymphoblastoid | 22 | 25 | 81 | ND | |
| 6 | Lymphoblastoid | 31 | 24 | 28.7 | 24 | 30 |
| 7 | Lymphoblastoid | 24 | 32 | ND | ND | |
| 8 | Pleomorphic | 23 | 28 | 73.5 | 0 | 30 |
| 9 | Pleomorphic | 10 | 16 | 36.9 | 14 | 9 |
| 10 | Pleomorphic | 5 | 10 | ND | ND | |
| 11 | Pleomorphic | 37 | 34 | 38.3 | ND | |
| 12 | Pleomorphic | 17 | 16 | 68.4 | ND | |
| 13 | Pleomorphic | 26 | 34 | 85.6 | ND | |
| 14 | Pleomorphic | 23 | 19 | 81.2 | ND | |
| 15 | Pleomorphic | 25 | 32 | 74.1 | 0 | 28 |
| No. . | MCL Subtype . | ISH Results . | No. of Aberrant Metaphases . | |||
|---|---|---|---|---|---|---|
| . | . | (chromosome 7 probe) . | Diploid . | Tetraploid . | ||
| . | . | % of Nuclei With . | G2/M Fraction (%) . | . | . | |
| . | . | 3 Signals . | 4 Signals . | . | . | . |
| 1 | Common | 22 | 20 | 74.9 | ND | |
| 2 | Common | 16 | 11 | ND | ND | |
| 3 | Lymphoblastoid | 15 | 28 | 45 | ND | |
| 4 | Lymphoblastoid | 22 | 22 | 69.5 | ND | |
| 5 | Lymphoblastoid | 22 | 25 | 81 | ND | |
| 6 | Lymphoblastoid | 31 | 24 | 28.7 | 24 | 30 |
| 7 | Lymphoblastoid | 24 | 32 | ND | ND | |
| 8 | Pleomorphic | 23 | 28 | 73.5 | 0 | 30 |
| 9 | Pleomorphic | 10 | 16 | 36.9 | 14 | 9 |
| 10 | Pleomorphic | 5 | 10 | ND | ND | |
| 11 | Pleomorphic | 37 | 34 | 38.3 | ND | |
| 12 | Pleomorphic | 17 | 16 | 68.4 | ND | |
| 13 | Pleomorphic | 26 | 34 | 85.6 | ND | |
| 14 | Pleomorphic | 23 | 19 | 81.2 | ND | |
| 15 | Pleomorphic | 25 | 32 | 74.1 | 0 | 28 |
Abbreviation: ND, not done.
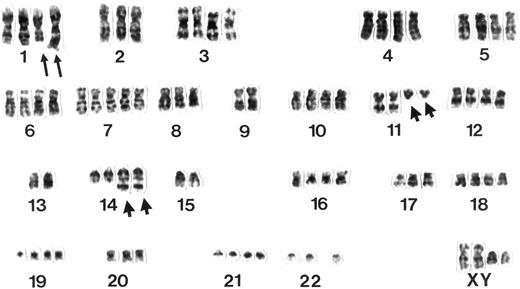
Classical cytogenetic analyses showed clonal chromosome aberrations in 14 of 17 MCL studied. A t(11; 14) (q13;q32) was observed in 13 of 14 (93%) cases. Four of 14 (29%) MCL, all of the pleomorphic subtype, were characterized by chromosome numbers in the tetraploid or hypotetraploid range with two copies of rearranged chromosomes 11 and 14 (Fig 3). In 2 of these cases (Table 2), an additional diploid or near-diploid clone could be identified showing the same structural and numerical chromosome aberrations and the t(11; 14). Concordance of the ploidy status as assessed by interphase and classical cytogenetics in these 14 lymphomas was 100%. The difference in the number of tetraploid chromosome clones in the three subgroups proved to be of high significance in statistical analysis (Table 3).
Example of a G-banded karyotype from a pleomorphic variant of MCL. Note two rearranged chromosomes 11 and 14 displaying a t(11; 14)(q13;q32) and additional structural aberrations in the long arms of two chromosomes 1 next to a polysomy for chromosome 7.
Example of a G-banded karyotype from a pleomorphic variant of MCL. Note two rearranged chromosomes 11 and 14 displaying a t(11; 14)(q13;q32) and additional structural aberrations in the long arms of two chromosomes 1 next to a polysomy for chromosome 7.
DNA flow cytometry.Thirty-two of 45 cases analyzed were euploid, with a mean coefficient of variation of the G0/G1-peak of 6.7 ± 1.4, mean S-phase fractions of 8.2% ± 5.1%, and a mean G2/M fraction of 5.4% ± 3% (range, 0.9% to 12.3%). One case was aneuploid, with a DNA index of 1.27. Twelve cases judged to be tetraploid in ISH had a significantly increased G2/M fraction with a mean value of 63% ± 19% (range, 28.7% to 85.6%). A small octoploid peak was found in 8 of these 12 cases. One lymphoma had evidence of two tetraploid populations, with a DNA index of 1.25 (Fig 4).
DNA flow cytometry of a tetraploid tumor with a small octoploid peak (no. 712). DNA flow cytogram with a double G0/G1 and G2/M peak, indicating the presence of two tetraploid stem lines with a DNA index of 1.25 (no. 258).
DNA flow cytometry of a tetraploid tumor with a small octoploid peak (no. 712). DNA flow cytogram with a double G0/G1 and G2/M peak, indicating the presence of two tetraploid stem lines with a DNA index of 1.25 (no. 258).
Table 2 summarizes the ISH, cytogenetic, and flow cytometric data of the tetraploid cases. There was a 100% fit of DNA flow cytometric, ISH, and cytogenetic studies concerning the classification of euploid/aneuploid or tetraploid.
No positive correlation was found between mitotic and proliferation indices, p53 expression, and ploidy status.
DISCUSSION
MCL was originally described as a low-grade lymphoma and introduced into the Kiel classification system.1,14 According to the classical description, the tumor is composed of a monotonous population of small to medium-sized cells with scant cytoplasm and irregular, often cleaved nuclei and small indistinct nucleoli. However, there are cases in which the tumor cells are larger, with a more vesicular nuclear chromatin texture and higher proliferation indices, variously termed as anaplastic, large-cell, or blastoid variants of MCL.2,14,15,16,25,26 In the REAL classification system,27 constituting a proposal from both European and American hematopathologists, the terms blastoid or lymphoblastoid variants have been favored because of the resemblance of the cells to lymphoblasts. Because blastoid variants of MCL may follow a more aggressive clinical course, a distinction of small cell or common and blastoid MCL may be of importance.15 16 However, there are no exact criteria for the differentiation of MCL variants based on histology. Furthermore, it is not known if there are differences in biologic features characterizing MCL subtypes.
In the present study, we reviewed 64 cases of malignant lymphomas previously characterized as MCL according to their cytomorphologic features and characteristic immunophenotype. After careful morphologic evaluation, three variants of MCL were recognized. The common variant closely corresponds to cases classified as centrocytic lymphoma according to the Kiel classification. In addition, two blastoid variants were discerned. The lymphoblastoid variant, being composed of medium-sized cells with rounder nuclear contours and more vesicular chromatin distribution, may correspond to cases earlier classified as blastic,28 blastoid,2,16 or large-cell MCL/centrocytic lymphoma.25 The second type of blastoid MCL, the pleomorphic variant, is composed of large cells or a mixture of medium to large cells, some of them resembling large centrocytes or centrocytoid centroblasts with irregular and deeply indented nuclei and sometimes prominent nucleoli. In the Kiel classification, some of the cases would most probably have been termed centroblastic lymphoma of centrocytoid subtype.17 29 In all three variants, cases with a perifollicular or mantle zone growth pattern characteristic of MCL were observed and immunophenotyping on frozen sections showed the classical CD5+, CD10−, CD23− immunophenotype of MCL (26 of common, 9 of lymphoblastoid, and 9 of pleomorphic lymphomas).
In keeping with data from the literature,2,16,17,25,26 blastoid variants were characterized by high mitotic rates irrespective of cytomorphologic subclassification. The increased number of mitoses in these cases is paralleled by higher Ki67 indices, both features being of high statistical value in the differentiation of blastoid and nonblastoid and distinctly correlated on statistical analysis. In our series, common variants displayed mean values of 21 mitoses/10 HPF and Ki67 indices of 27% with some variations. Similar data have been reported by Zukerberg et al,26 Lennert and Feller,25 and Kreipe et al30 for centrocytic lymphomas. In contrast, blastoid variants were characterized by elevated mitotic counts (51 and 57/10 HPF ) and higher proliferation indices of 53% (pleomorphic) and 57% (lymphoblastoid), confirming data from our previous study17 and data from the literature.26
Cytogenetic and molecular genetic studies have disclosed an association of the chromosomal translocation t(11; 14) to MCL,4,5 resulting in rearrangements of the bcl-1 locus31,32 and the deregulation and overexpression of the cyclin D1 gene at 11q13.10-12 Up to 73% of MCL have been shown to harbor detectable bcl-1 rearrangements when analyzed with multiple breakpoint probes.33 Most of the breaks have been reported to occur within the MTC region, although literature data suggest strong variations of positivity rates for MTC rearrangements. Thus, de Boer et al34 showed 6 of 20 cases rearranged in contrast to 16 of 33 positive cases reported by Rimokh et al.11 In the present study, we were able to confirm our previous findings17 on the preferential breakage of the bcl-1 locus at the MTC region in blastoid variants of MCL, although the difference is not statistically significant. Using Southern blotting as well as a PCR-based approach in identifying cyclin D1 rearrangements, we found 14 of 35 (40%) common MCL rearranged at the MTC as opposed to 8 of 15 (53%) lymphoblastoid and 9 of 14 (64%) pleomorphic variants. Because bcl-1 rearrangements have been shown to be a highly characteristic genomic change in MCL and have only sporadically been observed in other types of B-cell NHL, this finding indicates that all three variants share a common biologic background and that the t(11; 14) indeed is the crucial step in tumorigenesis of these lymphomas, irrespective of cytomorphologic variation. However, few data exist in the literature concerning bcl-1 rearrangements or cyclin D1 overexpression in blastoid variants of MCL. Bosch et al35 found bcl-1 MTC rearrangements in 2 of 8 (25%) diffuse and in 3 of 3 (100%) blastic cases. Yang et al12 reported on a more intense staining of a polyclonal anti-cyclin D1 antibody in transformed areas in some of their MCL cases. Ott et al13 observed a higher positivity rate of a monoclonal anti-cyclin D1 antibody (DCS-6) in blastoid as compared with classical MCL (85% v 68%, respectively).
Secondary structural and numerical chromosome aberrations, next to characteristic reciprocal translocations in hematopoietic neoplasms, have been shown to provide informations about the clinical course of individual patients or the risk of transformation to a high-grade malignancy. In a recent report on cytogenetic data from 66 patients with follicular lymphoma, Tilly et al36 reported deletions of chromosome 6 at bands q23-q26 and in the short arm of chromosome 17 as indicative of poor prognosis and shorter time to transformation compared with patients without these genomic changes. p53 mutations have also been shown to be associated with the progression of follicular lymphomas.37,38 In the present study on MCL, an overexpression of p53 was detected in only 6% of common types, but in 21% of blastoid variants. Our findings are confirmed by recent reports of Hernandez et al39 and Greiner et al40 indicating that p53 gene mutations are an infrequent phenomenon in MCL and are mainly associated with a subset described by these investigators as aggressive variants of MCL.
However, few data are available concerning secondary chromosome aberrations next to the t(11; 14) in MCL. Deletions in the long arm of chromosome 6, translocations or deletions involving various bands in 1p, and trisomies for chromosomes 3, 7, and 18 have been reported.41 In 50 MCL cases from our series analyzed by interphase cytogenetics, few aneusomies for single chromosomes were detected. However, the most striking and consistent genomic change in MCL was the detection of tetraploid chromosome clones in 15 of 50 (30%) cases. Interestingly, only 8% of common, as opposed to 36% of lymphoblastoid and even 80% of pleomorphic variants, harbored tetraploid chromosome sets. The finding of tetraploidizations in NHL of B-cell lineage is a rare phenomenon.42 In our own cytogenetic material (data not shown), we identified tetraploid chromosome (sub)clones in only 2% of germinal center and 9% of diffuse large B-cell lymphomas. Polyploidization of the chromosome set, on the other hand, is a frequent finding in peripheral T-cell lymphomas.43
MCL, therefore, on the genetic level, is not only characterized by a highly specific oncogene rearrangement, but also by a pronounced tendency to the evolution of tetraploid chromosome clones as compared with other types of B-cell NHL. This finding is in good agreement with recent data reported from Daniel et al,44 who observed near triploid or near tetraploid chromosome clones in 5 cases of leukemic MCL with unusually large cells associated with the t(11; 14)(q13;q32). Little doubt exists that the t(11; 14)-induced overexpression of the cyclin D1 gene is the crucial event in lymphomagenesis of this particular neoplasm. An overexpression of cyclin D1, which is not normally expressed by lymphoid cells, has been shown to shorten the cell cycle and to increase G0 and G1 transition considerably.45 Cyclin D1 is expressed at high levels during mid/late G1 phase of the cell cycle,9,46 allowing tumor cells with inappropriate expression of this cell-cycle regulating protein to pass the G1 restriction point (START) and to enter S phase.47 Because tetraploidization of the chromosome set is a rare phenomenon in other lymphoid neoplasms of B-cell lineage and because lymphoid cells in general do not express cyclin D1 but rather cyclins D2 and D3, it is tempting to speculate that, in the light of its cell cycle regulating capacities, the inappropriate expression of cyclin D1 in MCL is the reason for this particular karyotypic alteration. Tumor cells characterized by a t(11; 14) would then undergo two subsequent S phases without intervening mitotic disjunction of the sister chromatids. Alternatively, karyokinesis might be impaired. The finding of tetraploid subclones, next to a pseudo- or near-diploid stemline suggested by the lower percentage of cells with four signals in ISH in some of our samples as well as the simultaneous demonstration of diploid and tetraploid chromosome clones in classical cytogenetics in two cases, possibly indicates a spectrum of transition to tetraploidy, the cytomorphologic equivalent of which is the formation of large and pleomorphic tumor cells as compared with classical MCL. DNA flow cytometric analysis of 45 cases showed tetraploid chromosome clones in 12 tumors, but demonstrated small octoploid peaks in only 8 cases. However, this finding indicates a discrepancy between the low proliferation rate of the tetraploid cells as shown by flow cytometry and the high Ki67 index in these tumors.
No explanation exists so far as to why only a proportion of MCL develop tetraploid chromosome clones, although the majority of them are obviously characterized by the t(11; 14). It is also not clear at present if there is a correlation to the biologic and, hence, clinical behavior of these cases. An overexpression of cyclin D1 mRNA/protein has been observed also in cases of hairy cell leukemia (HCL).48-50 However, no gene rearrangements at the bcl-1 locus could be verified in these studies. The investigators postulated a different mechanism of cyclin D1 mRNA expression in HCL as compared with MCL. Therefore, it seems less probable that tetraploidization takes place in HCL in a similar way as in MCL; however, this question cannot be solved but in a separate study.
In conclusion, our study denotes that, despite an identical immunophenotype, MCL shows a broad cytomorphologic spectrum ranging from small-cell (common) to blastoid variants. Although a characteristic t(11; 14)/bcl-1 rearrangement is found in all morphologic variants, the distribution of the molecular breakpoints may vary within these subsets; blastoid variants do show a unique tendency to tetraploidization, a feature possibly related to the characteristic inappropriate expression of cyclin D1 in this neoplasm.
ACKNOWLEDGMENT
The expert technical assistance of H. Brückner, C. Gärtner, and K. Heintz is gratefully acknowledged. We also thank Dr U. Mäder for his help with the statistical analysis.
Supported by the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 172, Teilprojekt C-8.
Address reprint requests to German Ott, MD, Pathologisches Institut der Universität, Josef-Schneider-Strasse 2, D-97080 Würzburg, Germany.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal