Abstract
Bone marrow (BM) stromal cells are required for normal hematopoiesis. A number of soluble factors secreted by these cells that mediate hematopoiesis have been characterized. However, the mechanism of hematopoiesis cannot be explained solely by these known factors, and the existence of other, still unknown stromal factors has been postulated. We showed that hepatocyte growth factor (HGF ) is one such cytokine produced by human BM stromal cells. BM stromal cells were shown to constitutively produce HGF and also to express the c-MET/HGF receptor. The production of HGF was enhanced by addition of heparin and phorbol ester. Dexamethasone and tumor growth factor-β (TGF-β) inhibited the production of HGF. Interleukin-1α (IL-1α) tumor necrosis factor-α (TNF-α), and N6,2′-o-dibutyryl-adenosine-3′:5′-cyclic monophosphate (dbc-AMP) showed no obvious influence on HGF production. Western blot analysis of HGF derived from BM stromal cells showed two bands at 85 and 28 kD corresponding to native and variant HGF, respectively. Addition of recombinant HGF significantly promoted the formation of burst-forming unit-erythroid (BFU-E) and colony-forming unit-granulocyte erythroid macrophage (CFU-GEM) by BM mononuclear cells in the presence of erythropoietin and granulocyte-macrophage colony-stimulating factor (GM-CSF ), but the formation of CFU-GM was not modified. However, HGF had no effects on colony formation by purified CD34+ cells. Within BM mononuclear cells, c-MET was expressed on a proportion of cells (CD34−, CD33+, CD13+, CD14+, and CD15+), but was not found on CD34+ cells. We conclude that HGF is constitutively produced by BM stromal cells and that it enhances hematopoiesis. In addition, expression of c-MET on the stromal cells suggests the presence of an autocrine mechanism, operating through HGF, among stromal cells.
BONE MARROW (BM) stroma provides the microenvironment required for long-term hematopoiesis, and this necessary support is exerted through a direct interaction between stromal cells and hematopoietic cells mediated by adhesion molecules, and through cytokines released from the BM stroma.1 Such constitutively produced cytokines have been identified in humans: granulocyte-macrophage colony-stimulating factor (GM-CSF ), macrophage-CSF (M-CSF ), granulocyte-CSF (G-CSF ), interleukin-1β (IL-1β), IL-7, IL-11, stem cell factor, transforming growth factor-β (TGF-β), and tumor necrosis factor (TNF ).2-6 However, the existence of other, still unknown stroma-derived cytokines is suspected.
Hepatocyte growth factor (HGF ), initially identified as a potent mitogen for mature hepatocytes, is a kringle-containing polypeptide growth factor possessing structural homology with plasminogen.7-11 HGF is a mesenchyme-derived pleiotropic factor that regulates cell growth, cell motility, and morphogenesis in various types of cells,12-16 and it is a natural ligand for the c-MET proto-oncogene product of heterodimeric tyrosine kinase. HGF is considered a humoral mediator of epithelial-mesenchymal interactions responsible for organogenesis and morphogenesis of various tissues and organs, regeneration of organs, and growth, invasion, and metastasis of tumor cells.17 In the hematopoietic system, HGF augments the growth of hematopoietic progenitor cells.18-21 Although studies on the interaction of HGF with hematopoietic cells are scarce, it is possible that HGF plays an important role in hematopoiesis.
In this study, we analyzed the production of HGF by BM stromal cells and identified HGF as a constitutively produced stroma-derived cytokine. We also analyzed the regulation of HGF production and the effect of HGF on the growth of hematopoietic progenitor cells.
MATERIALS AND METHODS
Stromal cells.BM cells were obtained from healthy volunteers after provision of informed consent. Mononuclear cells were separated by Ficoll-Paque (Pharmacia, Piscataway, NJ) gradient centrifugation. The collected mononuclear cells were resuspended in α-minimal essential medium (α-MEM) containing 10% fetal calf serum, 10% horse serum, and 10−6 mol/L hydrocortisone and cultured in 10-cm plastic dishes at 37°C under 5% CO2 . After 4 to 6 weeks of culture, the confluent layer of stromal cells was detached by treatment with pronase and seeded into dishes; the culture was then continued without hydrocortisone.
Measurement of stromal cell–associated HGF.Stromal cells were seeded into 48-well plates at a density of 1 × 105/mL and cultured for 48 hours. After replacement of the medium with 250 μL fresh medium with or without agents, a further culture was performed for 48 hours. HGF in the conditioned medium was determined by enzyme-linked immunosorbent assay (ELISA).22-24 Various recombinant cytokines were added to stromal cells as follows: IL-1α (1 ng/mL), IL-6 (100 ng/mL), TNF-α (100 ng/mL), and TNF-β (10 ng/mL). Heparin, N6,2′-o-dibutyryl-adenosine-3′:5′-cyclic monophosphate (dbc-AMP), and phorbol-12-myristate-13-acetate (TPA) were also used at a final concentration of 2 mg/mL, 1 mol/L, and 100 mmol/L, respectively. IL-1α, TNF-α, and TNF-β were purchased from Genzyme Corp (Boston, MA) and IL-6 was provided by Kirin Brewery (Tokyo, Japan).
Western blot analysis.The purification of proteins and the Western blot analysis have been described previously.22 Briefly, the culture supernatant was applied to a Hi-Trap heparin column (Pharmacia) and the absorbed proteins were eluted and concentrated by lyophilization. The samples were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis using a 4% to 20% gradient gel under nonreducing conditions. The proteins, electrophoretically transferred onto nylon membranes, were incubated with biotinylated anti–human HGF IgG and then with peroxidase-conjugated streptavidin.
Cell preparation and flow cytometry analysis.A single-cell suspension of BM stromal cells was obtained by treating a monolayer of stromal cells with collagenase. BM mononuclear cells were isolated from heparinized BM blood by Ficoll-Paque gradient centrifugation. CD34+ cells were isolated from BM mononuclear cells using an immunomagnetic cell-separation system (Isolex-50; Baxter, Deerfield, IL)25 using anti-CD34 monoclonal antibody (MoAb) (9C5) and sheep anti–mouse IgG1 antibody-coated immunomagnetic beads (Dynal Corp, Oslo, Norway). To determine the purity of the isolated CD34+ cells, the cells were stained with another anti-CD34 MoAb (HPCA-2), which recognizes a different epitope on the CD34 molecule. The purity of CD34+ cells was more than 98%. For flow cytometry analysis, cells were incubated with human γ-globulin for 10 minutes to prevent nonspecific binding of MoAbs to Fc receptors. Cells were incubated with mouse anti–human c-MET MoAb DO-24 (UBI, Lake Placid, NY), recognizing extracellular epitopes of the HGF receptor β-chain. After washing twice with buffer, the cells were stained with fluorescein isothiocyanate–labeled rabbit anti–mouse Ig F(ab′ )2 fragments (Dako, Glostrup, Denmark). For two-color analysis, after incubation with mouse serum for absorption of the free second antibody, phycoerythrin-conjugated MoAbs were added.
Reverse transcriptase–polymerase chain reaction analysis.Total RNA was extracted from BM stromal cells by the acid guanidinium thiocyanate-phenol-chloroform method. First-strand cDNA was synthesized in 20 μL reaction mixture containing 1 μg total RNA, 1 mmol/L of each dNTP, 200 U Mo-MuLV reverse transcriptase, and 100 pmol random hexamer oligonucleotides. For detection of c-MET gene transcription, 1 μL cDNA was used for polymerase chain reaction (PCR) amplification with the sense (3438 to 3458: 5′-CAGGCAGTGCAGCATGTAGTG-3′ )11 and antisense (4099 to 4078: 5′-TAAGGTGGGGCTCCTCTTGTCA-3′ )11 primers. These primers seem to be complementary to sequences contained in the different exons, since amplification of genomic DNA was not observed using these primers. The samples, in 10 μL reaction mixture, underwent 30 cycles of amplification at 95°C for 1 minute, 60°C for 2 minutes, and 72°C for 2 minutes, followed by 7 minutes at 72°C in the last cycle. Five microliters of PCR products were electrophoresed in a 2% agarose gel in the presence of ethidium bromide.
Colony assay.Plastic-adherent cell-depleted mononuclear cells derived from the BM cells and CD34+ cells were plated on 35-mm plastic dishes at a density of 1 × 105/mL and 1 × 104/mL, respectively, in α-MEM containing 20% fetal calf serum, 1.2% methylcellulose, 1% bovine serum albumin, 50 μmol/L 2-mercaptoethanol, 3 U/mL erythropoietin, and 4 ng/mL GM-CSF, with or without recombinant human HGF (rhHGF ) (20 or 100 ng/mL). The culture was performed for 14 days in a humidified atmosphere at 37°C under 5% CO2 . Determination of colony types and colony numbers was made by observation with an inverted microscope. Erythropoietin and GM-CSF were provided by Kirin.
RESULTS
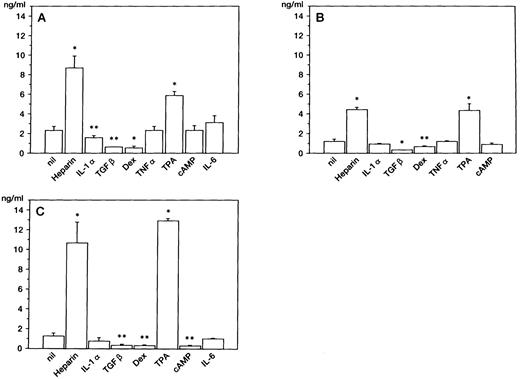
Production of HGF in BM stromal cells.After a 48-hour culture, HGF concentration in the supernatant was measured by ELISA. The constitutive production of HGF was observed (1 to 3 ng/mL) without stimulation; production of HGF was enhanced approximately 4- to 10-fold by addition of heparin, a potent stimulator of HGF production in skin fibroblasts23 (Fig 1). Culture with TPA also enhanced HGF production. On the other hand, TGF-β and dexamethasone inhibited the release of HGF by three stromal cell samples, and dbc-AMP inhibited it by one sample (Fig 1C). Addition of TNF-α or IL-6 had no significant effect on HGF production. HGF production in stromal cells was not enhanced by stimulation with IL-1α, which stimulates HGF synthesis in skin fibroblasts at this concentration.26 In one sample (Fig 1A), IL-1α slightly decreased the release of HGF.
HGF release by BM stromal cells. HGF from stromal cells obtained from 3 different individuals (A, B, and C) was measured by ELISA after 48-hour cultures. Error bars indicate standard deviation of triplicate cultures. *P < .01, **P < .05, v lanes null by Student's t-test.
HGF release by BM stromal cells. HGF from stromal cells obtained from 3 different individuals (A, B, and C) was measured by ELISA after 48-hour cultures. Error bars indicate standard deviation of triplicate cultures. *P < .01, **P < .05, v lanes null by Student's t-test.
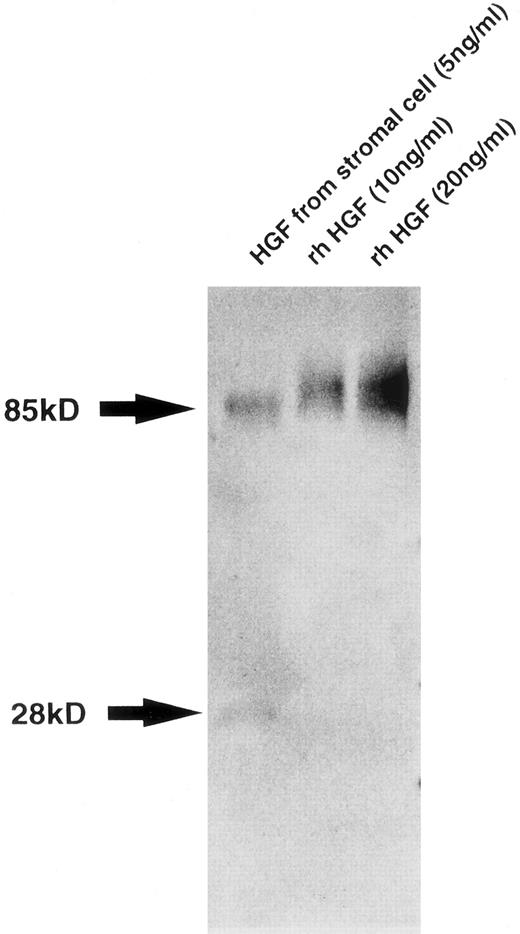
Characterization of HGF produced by BM stromal cells.To confirm the production of intact HGF with the multiple functions characteristic of this cytokine, we characterized HGF secreted into the supernatant of BM stromal cells. Western blot analysis showed two bands of 85 and 28 kD, which correspond to the native and variant HGF, respectively, with the presence of larger amounts of the native type than of the variant type (Fig 2). Whereas the native HGF is known to be translated from full-length 6-kb mRNA, the variant type derived from 1.3-kb mRNA, lacking kringle domains 3 and 4 and the β-chain, has motogenic activity (enhancement of cell motility) alone.27 28 We also examined the biologic activity of HGF from stromal cells. Both addition of supernatant from the stromal cell culture and addition of rhHGF induced the scattering of Mardin-Darby canine kidney epithelial cells and stimulated DNA synthesis in rat hepatocytes in primary culture. These are typical activities of HGF, and were almost completely inhibited by addition of anti–HGF MoAb (data not shown), indicating that human BM stromal cells secrete biologically active HGF.
Western blot analysis of HGF from stromal cells. Molecular weight is shown by arrows at left. Bands of 85 and 28 kD correspond to native and variant HGF, respectively.
Western blot analysis of HGF from stromal cells. Molecular weight is shown by arrows at left. Bands of 85 and 28 kD correspond to native and variant HGF, respectively.
Expression of HGF receptor (c-MET) on BM stromal cells.Expression of c-MET on the surface of BM stromal cells was demonstrated by anti–c-MET MoAb using FACS analysis (Fig 3A). No cells expressing CD45, CD34, CD2, CD19, CD33, CD14, or glycophorin A were identified in this cell population, indicating that there was no contamination of the stromal cells by hematopoietic cells (data not shown). In addition, we performed RT-PCR analysis to detect expression of the c-MET gene in BM stromal cells. Amplification of the c-MET gene was observed after 30 cycles of amplification (Fig 3B). Thus, it was concluded that BM stromal cells express c-MET.
(A) c-MET/HGF receptor expression on cell surface of BM stromal cells. Stromal cells were stained with anti–c-MET MoAb (DO-24). (B) RT-PCR analysis of c-MET gene expression. Lane 1, size marker; lane 2, BM stromal cells; lane 3, HL60 (promyelocytic leukemia cells), negative control; lane 4, Hep G2 (hepatocarcinoma cells), positive control. To clarify cDNA quality, PCR products of the β-actin gene are presented below each lane.
(A) c-MET/HGF receptor expression on cell surface of BM stromal cells. Stromal cells were stained with anti–c-MET MoAb (DO-24). (B) RT-PCR analysis of c-MET gene expression. Lane 1, size marker; lane 2, BM stromal cells; lane 3, HL60 (promyelocytic leukemia cells), negative control; lane 4, Hep G2 (hepatocarcinoma cells), positive control. To clarify cDNA quality, PCR products of the β-actin gene are presented below each lane.
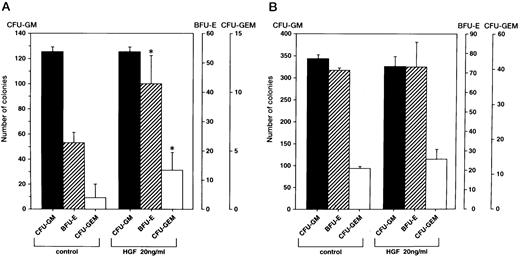
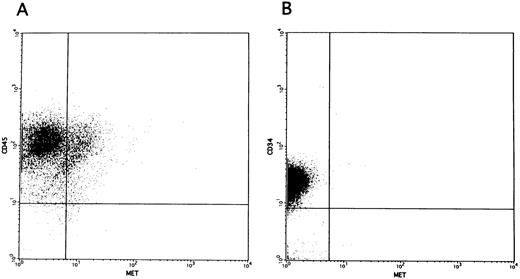
Promotion of colony formation by HGF.To clarify the role played by HGF constitutively secreted by BM stromal cells in the hematopoietic system, we analyzed the influence of rhHGF on colony formation by BM-derived mononuclear cells. No enhancement of colony formation was observed with addition of rhHGF alone (data not shown), but addition of 20 ng/mL rhHGF enhanced the formation of BFU-E and CFU-GEM in the presence of GM-CSF and erythropoietin (Fig 4A). However, the formation of CFU-GM was not modified by the presence of rhHGF, and addition of rhHGF did not cause any enhancement of colony formation by purified CD34+ cells even in the presence of GM-CSF and erythropoietin (Fig 4B). By flow cytometry analysis, a proportion of BM mononuclear cells (CD34−, CD33+, CD13+, CD14+, and CD15+) expressed c-MET, but c-MET expression was not observed on CD34+ cells (Fig 5). This finding was confirmed by RT-PCR analysis; ie, transcription of the c-MET gene was observed in mononuclear cells but not in purified CD34+ cells (Fig 6).
Effect of rhHGF on colony formation. Colony formation by BM mononuclear cells (A) and purified CD34+ cells (B) grown with or without rhHGF in combination with GM-CSF (2 ng/mL) and erythropoietin (3 U/mL). Among 3 repeated experiments, representative data are shown. Error bars indicate standard deviation of triplicate cultures. *P < .05, culture with v without rhHGF by Student's t-test.
Effect of rhHGF on colony formation. Colony formation by BM mononuclear cells (A) and purified CD34+ cells (B) grown with or without rhHGF in combination with GM-CSF (2 ng/mL) and erythropoietin (3 U/mL). Among 3 repeated experiments, representative data are shown. Error bars indicate standard deviation of triplicate cultures. *P < .05, culture with v without rhHGF by Student's t-test.
c-MET expression on the cell surface of unfractionated BM mononuclear cells (A) and purified CD34+ cells (B).
c-MET expression on the cell surface of unfractionated BM mononuclear cells (A) and purified CD34+ cells (B).
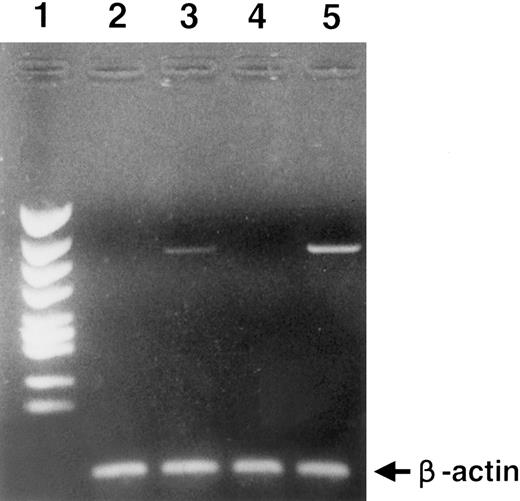
RT-PCR analysis of c-MET gene expression. Lane 1, size marker; lane 2, CD34+ cells; lane 3, BM mononuclear cells; lane 4, MOLT4 (T-cell leukemia cells), negative control; lane 5, HUT78 (T-cell leukemia cells), positive control. To clarify cDNA quality, PCR products of the β-actin gene are presented below each lane.
RT-PCR analysis of c-MET gene expression. Lane 1, size marker; lane 2, CD34+ cells; lane 3, BM mononuclear cells; lane 4, MOLT4 (T-cell leukemia cells), negative control; lane 5, HUT78 (T-cell leukemia cells), positive control. To clarify cDNA quality, PCR products of the β-actin gene are presented below each lane.
DISCUSSION
In this study, we showed that human BM stromal cells constitutively produced a significant amount of biologically active HGF, which had both motogenic and mitogenic activity. In addition, HGF enhanced the formation of BFU-E and CFU-GEM by BM mononuclear cells in the presence of GM-CSF and erythropoietin, concurring with the results of a previous study.21 Thus, we conclude that HGF is one of the cytokines constitutively produced by BM stroma and that it functions in human hematopoiesis. However, CD34+ cells did not express c-MET and rhHGF did not enhance colony formation by CD34+ cells, in contrast to the previous report by Galimi et al.21 They observed that HGF enhanced the formation of BFU-E and CFU-GEM by CD34+ cells. Because their findings were similar to our observation using BM mononuclear cells and the pecentage of CD34+ cells in their experiments was less than 50%, this discrepancy may result from differences in the purity of CD34+ cells. c-MET was not demonstrated on purified CD34+ cells in our analysis, and this finding was confirmed by RT-PCR analysis of the c-MET gene. Thus, HGF seems to induce the cytokine production by CD34− mononuclear cells without the accompaniment of proliferation of these cells (data not shown), which then promote colony formation of BFU-E and CFU-GEM. Since hematopoietic cells gain c-MET on their cell surface in a window of differentiation, where these cells lose CD34, it is also possible that HGF may affect the differentiation and/or proliferation of these CD34− cells. Further study to clarify the cells expressing c-MET and to identify cytokines produced by CD34−/c-MET–positive cells is warranted.
Although c-MET was not expressed on CD34+ cells, in leukemia cells, widespread expression of the c-MET gene has been observed (our unpublished observation, March 1995, manuscript in preparation) and HGF has been shown to induce motility in the J-111 cell line (monocytic leukemia), to promote the proliferation of CEM (T-ALL) cells, and to suppress the growth of IM-9 (myeloma) cells.29,30 Recently, HGF synthesized by inflamed vascular endothelium was shown to induce both the migration and adhesion of T cells to the endothelium.31 Thus, we postulate that HGF plays significant roles in multilineages in both direct and indirect ways.
Since RT-PCR and flow cytometry analyses demonstrated expression of c-MET on stromal cells, it is possible that sustaining the BM stroma itself is another function of HGF produced by the stromal cells. However, addition of rhHGF or anti–HGF MoAb showed no effects on proliferation of stromal cells (data not shown). The BM stroma consists of fibroblasts, adipocytes, vascular endothelial cells, and macrophages. Further studies are required to determine the type of cells that produce HGF. HGF may also be a cytokine that regulates the production of other cytokines released from stromal cells. Indeed, in a murine BM long-term culture system, addition of HGF to the culture significantly enhanced CFU cell counts, whereas there was no enhancement of colony formation without BM stroma.19
In BM stroma, HGF production was enhanced by TPA, indicating that HGF synthesis is, at least in part, regulated by protein kinase C. In addition, heparin showed a potent enhancement of HGF synthesis. This effect has been observed previously in embryonic lung fibroblasts, skin fibroblasts, umbilical vein endothelial cells, and HL60 promyelocytic leukemia cells. Heparin stimulated HGF production in skin fibroblasts by influencing posttranscriptional processes, and simultaneous addition of heparin and TPA caused further marked enhancement of HGF production in a synergistic manner.23 Because heparin is released from injured tissues and HGF exerts potent activity in organ regeneration, it would seem that heparin accelerates organ regeneration through its unique action as an inducer of HGF production. In some stress conditions such as hypoxia and bacterial infection, production of erythropoietin and of G-CSF, IL-1β, and TNF-α is induced, leading to enhancement of erythropoiesis and myelopoiesis, respectively. In an analogous way, injuries to the tissues, including the BM stroma itself, may enhance HGF production and promote hematopoiesis. The synthesis of various cytokines by stromal cells is induced by IL-1 and TNF-α,32-35 and HGF production in skin fibroblasts is fivefold to sixfold enhanced by addition of 1 ng/mL IL-1.26 However, enhancement of HGF production by these cytokines was not observed in BM stroma. A possible explanation for the absence of responsiveness of BM stroma to IL-1 and TNF-α is that stromal cells are stimulated to synthesize HGF only with IL-1 and/or TNF-α constitutively produced by the stroma. Dexamethasone and TGF-β suppressed the production of HGF.36 This suppressive effect is also observed in skin and lung fibroblasts.26 36
In conclusion, BM stromal cells constitutively produce HGF, and HGF promotes the growth of undifferentiated hematopoietic cells and erythroid progenitor cells in an indirect way. In addition to its functions in normal hematopoiesis, HGF may play an important role in leukemogenesis, such as by promoting cell growth, enhancing adhesion to stromal cells, and enhancing infiltration to tissues. This hypothesis is now being investigated in our laboratory.
ACKNOWLEDGMENT
We are indebted to M. Fujiwara and S. Hamano for secretarial assistance.
Supported by the Ministry of Education, Science, and Culture of Japan, the Osaka Community Foundation, and the Osaka Cancer Society.
Address reprint requests to Junichi Hara, MD, Department of Pediatrics, Osaka University School of Medicine, 2-2 Yamadaoka Suita, Osaka, 565, Japan.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal