Abstract
Integrin-mediated interaction of hematopoietic progenitor cells with bone marrow stromal extracellular matrix components is important in hematopoiesis. Focal adhesion kinase (pp125FAK) plays a central role in signal transduction through integrin receptors. We studied matrix-integrin interaction and subsequent signaling in human growth factor-dependent cell line, TF-1. Adherence of unstimulated TF-1 cells to fibronectin-coated wells was blocked by antiintegrin β1 and combination of anti-α4 with anti-α5 antibodies, indicating α4β1 and α5β1 integrin mediated adherence. Steel factor (SLF) increased TF-1 adhesion to fibronectin dose-dependently and 10−7 mol/L wortmannin suppressed SLF-induced adhesion. Immunoprecipitation and immunoblotting with antiphosphotyrosine antibody showed that adherence of TF-1 cells to fibronectin without cytokine caused tyrosine phosphorylation of several proteins identified as pp125FAK and paxillin. SLF induced spreading of adherent TF-1 cells and enhanced tyrosine phosphorylation of pp125FAK and paxillin in a dose-dependent manner. Treatment with SLF without plating on fibronectin did not induce tyrosine phosphorylation of pp125FAK. Wortmannin, at 10−7 mol/L, completely abolished SLF-induced enhancement of pp125FAK tyrosine phosphorylation, while c-kit autophosphorylation was not affected. This suggests that increase of pp125FAK tyrosine phosphorylation was mediated through a wortmannin sensitive pathway, rather than by direct action on c-kit tyrosine kinase. Treatment of adherent TF-1 cells with RGDS peptide plus anti-α4 antibody also inhibited SLF-induced enhancement of pp125FAK tyrosine phosphorylation without detachment of TF-1 cells. These data suggest that SLF enhances integrin-fibronectin-dependent tyrosine phosphorylation of pp125FAK through activation of integrin (“inside-out” signaling) and following integrin occupancy. This establishes a novel linkage between c-kit/SLF pathway and integrin fibronectin signaling.
NORMAL HEMATOPOIESIS of adult humans is regulated under the control of the bone marrow microenvironment. The bone marrow microenvironment is a complex network consisting of several different types of cellular components.1 These cellular components produce and secrete a number of growth regulatory proteins and extracellular matrix proteins.2 Direct contact between hematopoietic cells and the bone marrow microenvironmental cellular components and extracellular matrix proteins are believed to play an important role in positive and negative regulation of hematopoiesis.3 These adhesive interactions involve cytoadhesion molecules present on hematopoietic cells. Normal CD34 positive cells express α4β1 and α5β1 integrin receptors and expression of these integrins is modulated during maturation of progenitor cells.4,5 It is widely accepted that β1 integrin-mediated cell adhesion is crucially involved in the homing and anchoring of hematopoietic cells to bone marrow microenvironment to allow proper cell localization.4,6 7
Integrins are composed of heterodimers of transmembrane glycoproteins consisting of α and β subunits.8 Integrin receptor engagement to the ligand and clustering of integrin receptors on the cell surface leads to the formation of focal adhesions. Focal adhesions are specialized structures of a cell where integrins link to cytoskeletal protein complexes and actin stress fibers providing the anchorage site for the actin cytoskeleton.9 Recently, integrins have been shown to act not only as an adhesion molecule, but to transmit a variety of signals into the cells that is termed “outside-in signaling.”8,10 In several cell types, clustering of β1 or β3 integrins induces tyrosine phosphorylation of a novel cytoplasmic tyrosine kinase termed pp125 focal adhesion kinase (pp125FAK),11-15 which colocalizes with other focal adhesion components such as tensin, vinculin, and talin.16 Because integrin receptors lack an intrinsic kinase domain, pp125FAK is believed to play a central role in integrin-mediated signaling.17,18 The physiological relevance of integrin-mediated signaling in hematopoiesis remains to be elucidated. It has been shown that direct adhesion to fibronectin fragment through α4β1 integrin inhibits hematopoietic progenitor proliferation.19 α5β1 integrin fibronectin binding was shown to induce apoptosis in the growth factor-dependent human hematopoietic cell line, MO7e.20 Recently, integrin-mediated signaling has been reported to activate the Ras pathway of signal transduction in NIH3T3 fibroblasts. When pp125FAK becomes tyrosine phosphorylated and activated, association of pp125FAK with Grb2 by SH2 domain-mediated binding leads to downstream signaling pathways, such as activation of mitogen-activated protein (MAP) kinase.21-23
In this study, we identify pp125FAK and paxillin as tyrosine phosphorylated proteins in a human growth factor-dependent hematopoietic cell line, TF-1, when TF-1 cells adhere to fibronectin-coated wells through the α4β1 and α5β1 integrin receptor. Furthermore, treatment with Steel factor (SLF), the ligand for c-kit receptor, enhances the tyrosine phosphorylation of pp125FAK and paxillin. These results suggest novel linkage between SLF, c-kit signaling, and integrin matrix-mediated signaling in TF-1 cells.
MATERIALS AND METHODS
Antibodies, cytokines, and chemicals.Human fibronectin was purchased from Collaborative Biomedical (Bedford, MA). RGDS and RGES peptides were purchased from Life Technologies (Gaitherburg, MD). Antiintegrin β1 chain monoclonal antibodies (MoAb) (K-20 and Lia 1/2), as well as antiintegrin α4 chain (HP2/1), antiintegrin α5 chain (SAM-1), antiintegrin α6 chain (G0H3), and antiintegrin αv chain (69.6.5) antibodies were purchased from Immunotech (Westbrook, ME). Rabbit anti-pp125FAK antiserum generated against the C-terminal 150-amino-acid residues of mouse pp125FAK was kindly provided by Dr S.K. Hanks (Vanderbilt University, Nashville, TN).11 Antiphosphotyrosine (anti-p-Tyr) MoAb (4G10) and anti-pp125FAK MoAb (2A7) were purchased from Upstate Biotechnology Inc (Lake Placid, NY). Antipaxillin MoAb and antipan MAP kinase MoAb, which recognizes both p42 and p44 MAP kinases were obtained from Transduction Laboratories (Lexington, KY). Mouse antikit MoAb, YB5.B8 was kindly provided by Dr L.K. Ashman (University of Adelaide, South Australia, Australia).24 Fluorescein isothiocyanate (FITC)-labeled goat antimouse IgG was from Southern Biotechnology (Birmingham, AL). Wortmannin was purchased from Sigma (St Louis, MO). Highly purified rhu SLF, rhu granulocyte-macrophage colony-stimulating factor (GM-CSF) and rhu interleukin (IL)-3 were gifts from Immunex Corp (Seattle, WA).
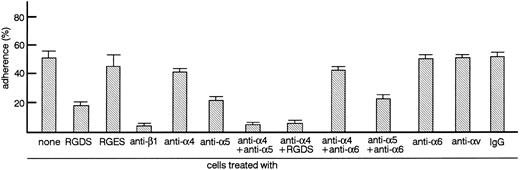
TF-1 cells adhere to fibronectin via integrin. Radiolabeled TF-1 cells suspended in RPMI 1640 medium containing 1% BSA (RPMI-BSA medium) or RPMI-BSA medium containing RGDS peptide (1 mg/mL), RGES peptide (1 mg/mL), antiintegrin antibodies (50 μg/mL each), or control isotype-matched IgG (50 μg/mL) were plated onto tissue culture plates (96-well, 1 × 105 cells/well) coated with fibronectin (20 μg/mL). Cells were incubated for 20 minutes at 37°C. Cell adhesion assay was done as described in Materials and Methods. The values represent the mean ± standard error (SE) of triplicate assays for one representative experiment of three independent experiments.
TF-1 cells adhere to fibronectin via integrin. Radiolabeled TF-1 cells suspended in RPMI 1640 medium containing 1% BSA (RPMI-BSA medium) or RPMI-BSA medium containing RGDS peptide (1 mg/mL), RGES peptide (1 mg/mL), antiintegrin antibodies (50 μg/mL each), or control isotype-matched IgG (50 μg/mL) were plated onto tissue culture plates (96-well, 1 × 105 cells/well) coated with fibronectin (20 μg/mL). Cells were incubated for 20 minutes at 37°C. Cell adhesion assay was done as described in Materials and Methods. The values represent the mean ± standard error (SE) of triplicate assays for one representative experiment of three independent experiments.
Cells and cell culture.The erythroleukemia-derived growth factor-dependent human cell line TF-1 was cultured in RPMI-1640 medium supplemented with 15% fetal calf serum (FCS) (Hyclone Laboratories, Logan, UT) and 100 U/mL GM-CSF. The biological characteristics of this cell line have previously been described.25
Flow cytometric analysis.Cells (4 × 105 cells) were incubated with 20 μL of antibody at 4°C for 30 minutes and then washed twice in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA). Cells were incubated with 20 μL of FITC-labeled goat antimouse IgG and washed twice in the same condition. Cells were analyzed by FACScan (Becton Dickinson, Franklin Lakes, NJ).
Adhesion assay.Human fibronectin and BSA (Sigma) were diluted in PBS and 100 μL of the protein solution (20 μg/mL fibronectin) was distributed in 96-well tissue culture flat-bottom plates (Corning, Corning, NY). After an overnight incubation at 4°C, the coated wells were washed twice with PBS and blocked by adding 100 μL of PBS with 1% BSA at 37°C for 1 hour. The wells were then washed twice with PBS. Cells were labeled with 51Cr (Amersham Corp, Arlington Heights, IL) (100 μCi/5 × 106 cells) for 1 hour at 37°C with appropriate growth medium, washed twice, and resuspended at 1 × 106 cells/mL in RPMI 1640 medium containing 1% BSA. A total of 100 μL of the cell suspension was added to each of the protein-coated wells, centrifuged 600 rpm for 1 minute to allow attachment of cells to the bottom of the wells, and incubated 20 minutes at 37°C. Unattached cells were removed by washing twice with prewarmed RPMI 1640 containing 1% BSA. Adherent cells were solubilized with 1% sodium dodecyl sulfate (SDS) and radioactivity was quantitated in a gamma counter.
Immunoblotting and immunoprecipitation.Immunoblotting and immunoprecipitation was performed as described previously.26 Briefly, cells were lysed in 20 mmol/L Tris HCl, pH 7.4, 150 mmol/L NaCl, 10% glycerol, 1% Nonidet P-40, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), 0.15 U/mL aprotinin, 10 μg/mL leupeptin, 100 mmol/L sodium fluoride 2 mmol/L sodium orthovanadate (Sigma), 5 mmol/L EDTA. Immunoprecipitations were performed by incubating cell lysates with appropriate antibody for 2 hours at 4°C. Immunoprecipitates were collected by protein A Sepharose beads (Pharmacia Biotech, Uppsala, Sweden) and were washed four times gently with washing buffer (100 mmol/L Tris-HCl pH 8.0, 150 mmol/L NaCl, 0.5% Tween 20, 100 mmol/L sodium fluoride, and 1 mmol/L sodium orthovanadate). Proteins were separated by SDS-PAGE and transfered onto Immobilon P membranes (Millipore Corp, Bedford, MA). Immunoblots were blocked in TBST (Tris-buffered saline with Tween 20) (50 mmol/L Tris HCl pH 7.4, and 150 mmol/L NaCl, 0.05% Tween 20) containing 1% BSA for 1 hour. The blots were incubated with the appropriate primary antibody in TBST containing 1% BSA for 2 hours at room temperature. Detection of the primary antibody was done with secondary antibody conjugated to horseradish peroxidase (Amersham) at 1:2,000 dilution and the blots later developed by chemiluminescent reaction and exposure to radiographic film.
RESULTS
TF-1 cells adhere to fibronectin-coated well via integrin.We analyzed the surface expression of integrin receptors on the surface of TF-1 cells by flow cytometry. TF-1 cells expressed integrin subunits including β1, α4, and α5 (data not shown). Adherence of TF-1 cells to fibronectin-coated wells was then examined. As shown in Fig 1, up to 60% of TF-1 cells adhered to fibronectin-coated wells without stimulation. Nonspecific adherence to BSA-coated wells was always less than 5%. To test whether cell adhesion to fibronectin was mediated by integrin receptors, cells were treated with RGDS peptide, which is recognized by the α5β1 fibronectin receptor, with antiintegrin β1 antibody or with antiintegrin α chain antibodies. Anti-β1 integrin antibody completely blocked cell adhesion (P < .001), while anti-α5 integrin antibody decreased cell adhesion significantly (P < .001). At the concentrations used, neither anti-α6 nor anti-αv chain antibodies had a significant effect on adhesion. RGDS peptide significantly inhibited cell adhesion (P < .001), whereas treatment with control RGES peptide had no significant effect (Fig 1). Although anti-α4 integrin antibody alone had no significant effect on adhesion, a combination of anti-α4 antibody with either anti-α5 antibody or RGDS peptide resulted in more efficient inhibition compared with anti-α5 antibody or RGDS alone (Fig 1). Combination of anti-α6 antibody with anti-α4 antibody or anti-α5 antibody did not enhance inhibition further (Fig 1). The combined results with antibodies and peptides suggest that TF-1 cells adhere to fibronectin-coated wells through α4β1 and α5β1 integrin receptors.
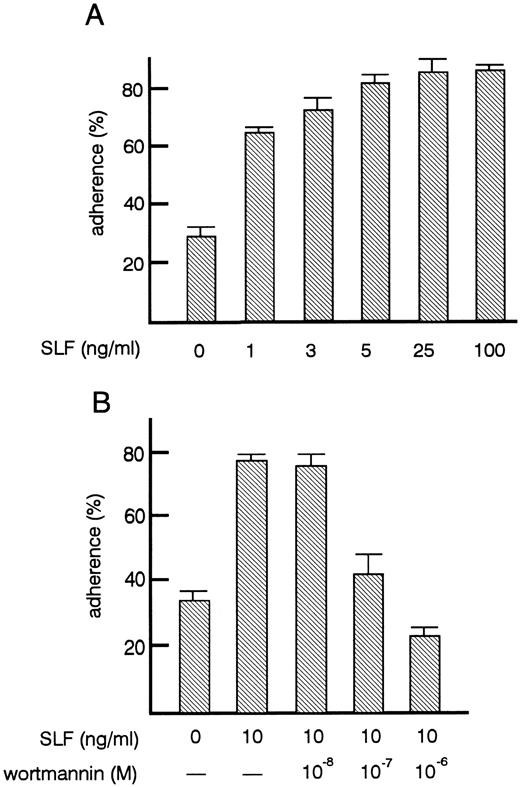
Effects of SLF on TF-1 cell adhesion to fibronectin.As shown previously by others,27 treatment of TF-1 cells with SLF increased their adhesion to fibronectin up to 80% (Fig 2A). After factor starvation, GM-CSF or IL-3 treatment also increased adhesion of TF-1 cells (data not shown) as reported by others.27 The increase of adhesion induced by SLF was dose-dependent. The minimum amount of SLF tested that was required to induce significant increase in adhesion was 1 ng/mL (P < .001). Maximum adhesion was achieved with 5 ng/mL of SLF (Fig 2B). Flow cytometric analysis showed that SLF treatment did not induce significant changes in the expression of α4β1 and α5β1 integrin (data not shown) suggesting that SLF did not affect the expression of integrin molecules, but may have changed the avidity of integrin to fibronectin. It has been reported that in mouse mast cells, phosphatidylinositol 3-kinase (PI3-kinase) activation is important for SLF-mediated adhesion to fibronectin and that the PI3-kinase inhibitor, wortmannin, at a 10−7 mol/L concentration, inhibits SLF-mediated adhesion of mast cells.28 We, therefore, examined the effect of wortmannin on SLF-induced adhesion to fibronectin of TF-1 cells. We preincubated cells with 10−8 to 10−6 mol/L wortmannin for 30 minutes at 37°C. At this range of concentration, wortmannin did not affect cell viability. Wortmannin treatment dose-dependently inhibited SLF-induced adhesion. The dose-dependent curve required for inhibition of adhesion (Fig 2B) was similar to that reported by others for mouse mast cells.28
Effects of SLF on the adherence of TF-1 cells to fibronectin. (A) Dose-dependent increase of adhesion induced by SLF. TF-1 cells were plated on the fibronectin-coated wells in the presence of increasing amounts of SLF and were incubated for 30 minutes at 37°C. Cell adhesion assay was done as described in Materials and Methods. The values represent the mean ± SE of triplicate assays for one representative experiment of three independent experiments. (B) Inhibition of SLF-induced adhesion to fibronectin by wortmannin. TF-1 cells were preincubated with increasing concentrations of wortmannin for 30 minutes at 37°C, the cells were then plated on the fibronectin-coated wells in the presence of SLF (10 ng/mL). Cell adhesion assay was done as described in Materials and Methods. The values represent the mean ± SE of triplicate assays for one experiment.
Effects of SLF on the adherence of TF-1 cells to fibronectin. (A) Dose-dependent increase of adhesion induced by SLF. TF-1 cells were plated on the fibronectin-coated wells in the presence of increasing amounts of SLF and were incubated for 30 minutes at 37°C. Cell adhesion assay was done as described in Materials and Methods. The values represent the mean ± SE of triplicate assays for one representative experiment of three independent experiments. (B) Inhibition of SLF-induced adhesion to fibronectin by wortmannin. TF-1 cells were preincubated with increasing concentrations of wortmannin for 30 minutes at 37°C, the cells were then plated on the fibronectin-coated wells in the presence of SLF (10 ng/mL). Cell adhesion assay was done as described in Materials and Methods. The values represent the mean ± SE of triplicate assays for one experiment.
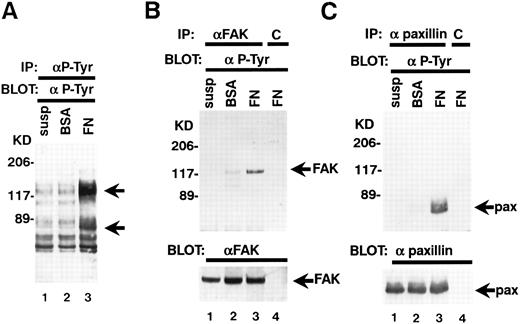
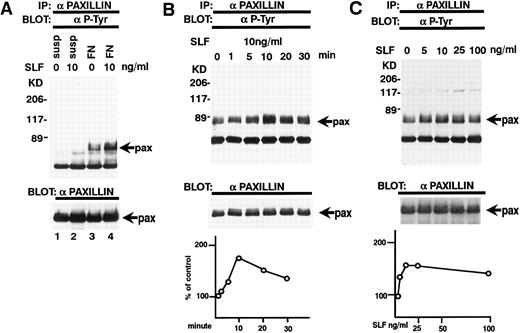
Adherence of TF-1 cells to fibronectin increases protein tyrosine phosphorylation.TF-1 cells were plated on fibronectin-coated wells for 15 minutes. The wells were then washed twice to remove the nonadherent fraction of TF-1 cells. We used adherent fractions of TF-1 cells for further protein analysis. Although there is 30% to 60% variation in TF-1 cell adherence to fibronectin, we confirmed that this variation did not affect the results of protein analysis. Adherent TF-1 cells were lysed and immunoprecipitated with anti-p-Tyr MoAb. The immunoprecipitates were analyzed by SDS-PAGE followed by immunoblotting with anti-p-Tyr MoAb. Cells plated on BSA-coated wells did not show elevation of tyrosine phosphorylation (Fig 3A, lane 2). Cell adhesion to fibronectin resulted in elevated tyrosine phosphorylation of proteins mainly with a molecular mass of 120 to 130 kD and 70 kD (Fig 3A, lane 3). To investigate the possibility that pp125FAK and paxillin were among these phosphoproteins, cell lysates were immunoprecipitated with MoAbs against pp125FAK and paxillin and they were analyzed by immunoblotting with an anti-p-Tyr MoAb. When immunoprecipitated from suspended cells, pp125FAK and paxillin did not exhibit any tyrosine phosphorylation (Fig 3B, upper panel, lane 1, Fig 3C, upper panel, lane 1). For cells plated on BSA-coated wells, to which a small fraction of the cells adhered probably in a nonspecific manner, only marginal phosphorylation was seen (Fig 3B, upper panel, lane 2, Fig 3C, upper panel, lane 2). A large increase in tyrosine phosphorylation was seen in cells adhered to fibronectin-coated wells (Fig 3B, upper panel, lane 3, Fig 3C, upper panel, lane 3). These differences were not attributable to the amounts of pp125FAK and paxillin, as seen when the same blots were stripped and reprobed with anti-pp125FAK polyclonal antibody and antipaxillin MoAb (Fig 3B and C, lower panels, lanes 1 to 3).
TF-1 cell adhesion to fibronectin induces tyrosine phosphorylation of pp125FAK and paxillin. (A) TF-1 cells in suspension (lane 1), plated on BSA-coated wells (lane 2), or plated on fibronectin-coated wells (lane 3) were incubated 15 minutes at 37°C. Cells were lysed and subjected to immunoprecipitation with anti-p-Tyr MoAb. Immunoprecipitates were subjected to immunoblotting with anti-p-Tyr MoAb. (B) TF-1 cells in suspension culture (lane 1), plated on BSA-coated wells (lane 2), or plated on fibronectin-coated wells (lane 3, 4) were incubated 15 minutes at 37°C. Cells were lysed and subjected to immunoprecipitation with anti-pp125FAK MoAb (lanes 1 to 3) or control isotype matched IgG (lane 4). Immunoprecipitates were subjected to immunoblotting with anti-p-Tyr MoAb (upper panel) and anti-pp125FAK polyclonal antibody (lower panel). (C) TF-1 cells in suspension culture (lane 1), plated on BSA-coated wells (lane 2) or plated on fibronectin-coated wells (lanes 3 and 4) were incubated for 15 minutes at 37°C. Cells were lysed and subjected to immunoprecipitation with antipaxillin MoAb (lanes 1 to 3) or control isotype matched IgG (lane 4). Immunoprecipitates were subjected to immunoblotting with anti-p-Tyr MoAb (upper panel) and antipaxillin MoAb (lower panel). Molecular mass markers are in kD. Similar results were obtained in three independent experiments.
TF-1 cell adhesion to fibronectin induces tyrosine phosphorylation of pp125FAK and paxillin. (A) TF-1 cells in suspension (lane 1), plated on BSA-coated wells (lane 2), or plated on fibronectin-coated wells (lane 3) were incubated 15 minutes at 37°C. Cells were lysed and subjected to immunoprecipitation with anti-p-Tyr MoAb. Immunoprecipitates were subjected to immunoblotting with anti-p-Tyr MoAb. (B) TF-1 cells in suspension culture (lane 1), plated on BSA-coated wells (lane 2), or plated on fibronectin-coated wells (lane 3, 4) were incubated 15 minutes at 37°C. Cells were lysed and subjected to immunoprecipitation with anti-pp125FAK MoAb (lanes 1 to 3) or control isotype matched IgG (lane 4). Immunoprecipitates were subjected to immunoblotting with anti-p-Tyr MoAb (upper panel) and anti-pp125FAK polyclonal antibody (lower panel). (C) TF-1 cells in suspension culture (lane 1), plated on BSA-coated wells (lane 2) or plated on fibronectin-coated wells (lanes 3 and 4) were incubated for 15 minutes at 37°C. Cells were lysed and subjected to immunoprecipitation with antipaxillin MoAb (lanes 1 to 3) or control isotype matched IgG (lane 4). Immunoprecipitates were subjected to immunoblotting with anti-p-Tyr MoAb (upper panel) and antipaxillin MoAb (lower panel). Molecular mass markers are in kD. Similar results were obtained in three independent experiments.
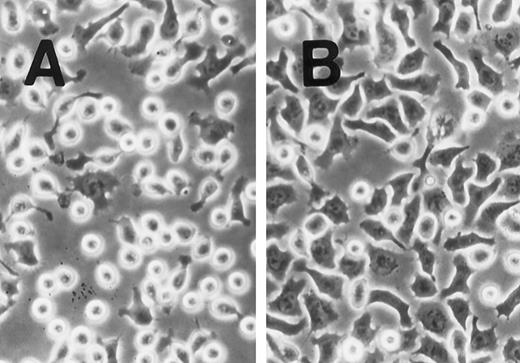
SLF induces cell spreading of adherent TF-1 on fibronectin-coated wells. TF-1 cells were plated on fibronectin-coated wells and incubated for 15 minutes at 37°C. Wells were washed twice with RPMI-BSA medium. The medium without SLF (A), or with SLF (10 ng/mL) (B) was added to the wells and the wells were further incubated for 10 minutes before being photographed at 400 × magnification.
SLF induces cell spreading of adherent TF-1 on fibronectin-coated wells. TF-1 cells were plated on fibronectin-coated wells and incubated for 15 minutes at 37°C. Wells were washed twice with RPMI-BSA medium. The medium without SLF (A), or with SLF (10 ng/mL) (B) was added to the wells and the wells were further incubated for 10 minutes before being photographed at 400 × magnification.
Effects of SLF on pp125FAK tyrosine phosphorylation of pp125FAK. (A) TF-1 cells were plated on fibronectin-coated wells and incubated for 15 minutes at 37°C. Wells were washed twice with RPMI-BSA medium. The medium with SLF (10 ng/mL) (lanes 2 and 4) or without SLF (lanes 1 and 3) was added to the wells and the wells were further incubated for 10 minutes. Cells were lysed and subjected to immunoprecipitation with anti-pp125FAK MoAb. The immunoprecipitates were then blotted with anti-p-Tyr MoAb (upper panel). The Western blot transfer membrane that was probed with the anti-p-Tyr MoAb in the upper panel was stripped and probed with the anti-pp125FAK polyclonal antibody (lower panel). (B) Time course of the SLF-induced enhancement of tyrosine phosphorylation of pp125FAK. TF-1 cells were plated on the fibronectin-coated well and incubated for 15 minutes at 37°C. Wells were washed twice with RPMI-BSA medium. The medium with 10 ng/mL of SLF was added to the wells. The wells were incubated for various times. Time of incubation is shown in minutes. Cells were lysed and subjected to immunoprecipitation with anti-pp125FAK MoAb. The immunoprecipitates were then blotted with anti-p-Tyr MoAb (upper panel). The Western blot transfer membrane that was probed with the anti-p-Tyr MoAb in the upper panel was stripped and probed with the anti-pp125FAK polyclonal antibody (middle panel). Lower graph shows the ratio of tyrosine-phosphorylated pp125FAK to pp125FAK protein expression represented by densitometric scanning of transblot bands. Data represent the percentage of control (non-SLF-treated cells) (lower panel). (C) Dose dependence of SLF-induced enhancement of tyrosine phosphorylation of pp125FAK. TF-1 cells were plated on the fibronectin-coated wells and incubated for 15 minutes at 37°C. Wells were washed twice with RPMI-BSA medium. The medium with increasing concentrations of SLF was added to the wells. Cells were lysed and subjected to immunoprecipitation with anti-pp125FAK MoAb. The immunoprecipitates were then blotted with anti-p-Tyr MoAb (upper panel). The Western blot transfer membrane that was probed with the anti-p-Tyr MoAb in the upper panel was stripped and probed with the anti-pp125FAK polyclonal antibody (middle panel). Lower graph shows the ratio of tyrosine-phosphorylated pp125FAK to pp125FAK protein expression represented by densitometric scanning of transblot bands. Data represent the percentage of control (SLF nontreated cells) (lower panel). The background band at 50 kD is the immunoglobulin heavy chain from the immunoprecipitation. Similar results were obtained in three independent experiments.
Effects of SLF on pp125FAK tyrosine phosphorylation of pp125FAK. (A) TF-1 cells were plated on fibronectin-coated wells and incubated for 15 minutes at 37°C. Wells were washed twice with RPMI-BSA medium. The medium with SLF (10 ng/mL) (lanes 2 and 4) or without SLF (lanes 1 and 3) was added to the wells and the wells were further incubated for 10 minutes. Cells were lysed and subjected to immunoprecipitation with anti-pp125FAK MoAb. The immunoprecipitates were then blotted with anti-p-Tyr MoAb (upper panel). The Western blot transfer membrane that was probed with the anti-p-Tyr MoAb in the upper panel was stripped and probed with the anti-pp125FAK polyclonal antibody (lower panel). (B) Time course of the SLF-induced enhancement of tyrosine phosphorylation of pp125FAK. TF-1 cells were plated on the fibronectin-coated well and incubated for 15 minutes at 37°C. Wells were washed twice with RPMI-BSA medium. The medium with 10 ng/mL of SLF was added to the wells. The wells were incubated for various times. Time of incubation is shown in minutes. Cells were lysed and subjected to immunoprecipitation with anti-pp125FAK MoAb. The immunoprecipitates were then blotted with anti-p-Tyr MoAb (upper panel). The Western blot transfer membrane that was probed with the anti-p-Tyr MoAb in the upper panel was stripped and probed with the anti-pp125FAK polyclonal antibody (middle panel). Lower graph shows the ratio of tyrosine-phosphorylated pp125FAK to pp125FAK protein expression represented by densitometric scanning of transblot bands. Data represent the percentage of control (non-SLF-treated cells) (lower panel). (C) Dose dependence of SLF-induced enhancement of tyrosine phosphorylation of pp125FAK. TF-1 cells were plated on the fibronectin-coated wells and incubated for 15 minutes at 37°C. Wells were washed twice with RPMI-BSA medium. The medium with increasing concentrations of SLF was added to the wells. Cells were lysed and subjected to immunoprecipitation with anti-pp125FAK MoAb. The immunoprecipitates were then blotted with anti-p-Tyr MoAb (upper panel). The Western blot transfer membrane that was probed with the anti-p-Tyr MoAb in the upper panel was stripped and probed with the anti-pp125FAK polyclonal antibody (middle panel). Lower graph shows the ratio of tyrosine-phosphorylated pp125FAK to pp125FAK protein expression represented by densitometric scanning of transblot bands. Data represent the percentage of control (SLF nontreated cells) (lower panel). The background band at 50 kD is the immunoglobulin heavy chain from the immunoprecipitation. Similar results were obtained in three independent experiments.
SLF enhances integrin-fibronectin mediated tyrosine phosphorylation of pp125FAK.After washing the wells, medium with or without SLF (10 ng/mL) was added to the adherent TF-1 cells, and the wells were further incubated for 10 minutes. As seen in Fig 4, SLF treatment greatly induced enhanced spreading of adherent TF-1 cells on fibronectin-coated wells. Lysates were examined for protein tyrosine phosphorylation of pp125FAK by using immunoprecipitation with anti-pp125FAK MoAb and immunoblotting with anti-p-Tyr MoAb. SLF treatment alone without plating on fibronectin failed to induce tyrosine phosphorylation of pp125FAK in suspension-cultured TF-1 cells (Fig 5A, lane 2). SLF treatment induced a significant increase in the level of tyrosine phosphorylation of pp125FAK (1.86 ± 0.06 compared with control by densitometric analysis, P < .01, n = 3) (Fig 5A, lanes 3 and 4, upper panel) with no significant alteration in the amount of pp125FAK protein immunoprecipitated with anti-pp125FAK MoAb (Fig 5A, lanes 3 and 4, lower panel). To further characterize the enhancement of pp125FAK tyrosine phosphorylation, we examined the time-dependence of this effect following SLF treatment. There was a progressive increase in pp125FAK tyrosine phosphorylation that reached a maximum at 10 minutes with SLF treatment at 10 ng/mL (Fig 5B, upper panel). It declined rapidly returning to basal levels of pp125FAK tyrosine phosphorylation in 30 minutes. The membrane was stripped and then probed with anti-pp125FAK polyclonal antibody. During the period of SLF treatment, there was no significant alteration in the amount of immunoprecipitated pp125FAK (Fig 5B, middle panel). Incubation of the cells with increasing concentrations of SLF from 1 ng/mL to 25 ng/mL resulted in a progressive increase in pp125FAK tyrosine phosphorylation. The concentration required for maximum tyrosine phosphorylation of pp125FAK was 10 ng/mL of SLF (Fig 5C, upper panel), while c-kit autophosphorylation progressively increased up to a concentration of 100 ng/mL of SLF (data not shown). The increase in phosphotyrosine content with increasing concentrations of SLF occurred with no significant alteration in the amount of pp125FAK protein immunoprecipitated under these conditions (Fig 5C, middle panel).
Effect of SLF on the tyrosine phosphorylation of paxillin. (A) TF-1 cells in suspension (lanes 1 and 2) or plated on fibronectin-coated wells (lanes 3 and 4) were incubated for 15 minutes at 37°C. Wells were washed twice with RPMI-BSA medium. Medium with SLF (10 ng/mL) (lanes 2 and 4) or without SLF (lanes 1 and 3) were added and the wells were further incubated for 10 minutes. Cells were lysed and subjected to immunoprecipitation with antipaxillin MoAb. The immunoprecipitates were then blotted with anti-p-Tyr MoAb (upper panel). The Western blot transfer membrane that was probed with the anti-p-Tyr MoAb in the upper panel was stripped and probed with antipaxillin MoAb (lower panel). (B) Time course of the SLF-induced enhancement of tyrosine phosphorylation of paxillin. TF-1 cells were plated on the fibronectin-coated wells and incubated for 15 minutes at 37°C. Wells were washed twice with RPMI-BSA medium. Medium with SLF (10 ng/mL) was added to the wells. The wells were incubated for various time periods. Time of incubation is shown in minutes. Cells were lysed and subjected to immunoprecipitation with antipaxillin MoAb. The immunoprecipitates were then blotted with anti-p-Tyr MoAb (upper panel). The Western blot transfer membrane that was probed with the anti-p-Tyr MoAb in the upper panel was stripped and probed with antipaxillin MoAb (middle panel). The lower graph shows the ratio of tyrosine-phosphorylated paxillin to paxillin protein expression represented by densitometric scanning of transblot bands. Data represent the percentage of control (non-SLF-treated cells) (lower panel). (C) Dose dependence of SLF-induced enhancement of tyrosine phosphorylation of paxillin. TF-1 cells were plated on the fibronectin-coated wells and incubated for 15 minutes at 37°C. Wells were washed twice with RPMI-BSA. The medium with increasing concentrations of SLF was added to the wells. Cells were lysed and subjected to immunoprecipitation with a MoAb directed against paxillin. The immunoprecipitates were then blotted with MoAb directed against p-Tyr (upper panel). The Western blot transfer membrane that was probed with the anti-p-Tyr MoAb in the upper panel was stripped and probed with antipaxillin MoAb (middle panel). The lower graph shows the ratio of tyrosine-phosphorylated paxillin to paxillin protein expression represented by densitometric scanning of transblot bands. Data represent the percentage of control (SLF nontreated cells) (lower panel). The background band at 50 kD is the immunoglobulin heavy chain from the immunoprecipitation. Similar results were obtained in three independent experiments.
Effect of SLF on the tyrosine phosphorylation of paxillin. (A) TF-1 cells in suspension (lanes 1 and 2) or plated on fibronectin-coated wells (lanes 3 and 4) were incubated for 15 minutes at 37°C. Wells were washed twice with RPMI-BSA medium. Medium with SLF (10 ng/mL) (lanes 2 and 4) or without SLF (lanes 1 and 3) were added and the wells were further incubated for 10 minutes. Cells were lysed and subjected to immunoprecipitation with antipaxillin MoAb. The immunoprecipitates were then blotted with anti-p-Tyr MoAb (upper panel). The Western blot transfer membrane that was probed with the anti-p-Tyr MoAb in the upper panel was stripped and probed with antipaxillin MoAb (lower panel). (B) Time course of the SLF-induced enhancement of tyrosine phosphorylation of paxillin. TF-1 cells were plated on the fibronectin-coated wells and incubated for 15 minutes at 37°C. Wells were washed twice with RPMI-BSA medium. Medium with SLF (10 ng/mL) was added to the wells. The wells were incubated for various time periods. Time of incubation is shown in minutes. Cells were lysed and subjected to immunoprecipitation with antipaxillin MoAb. The immunoprecipitates were then blotted with anti-p-Tyr MoAb (upper panel). The Western blot transfer membrane that was probed with the anti-p-Tyr MoAb in the upper panel was stripped and probed with antipaxillin MoAb (middle panel). The lower graph shows the ratio of tyrosine-phosphorylated paxillin to paxillin protein expression represented by densitometric scanning of transblot bands. Data represent the percentage of control (non-SLF-treated cells) (lower panel). (C) Dose dependence of SLF-induced enhancement of tyrosine phosphorylation of paxillin. TF-1 cells were plated on the fibronectin-coated wells and incubated for 15 minutes at 37°C. Wells were washed twice with RPMI-BSA. The medium with increasing concentrations of SLF was added to the wells. Cells were lysed and subjected to immunoprecipitation with a MoAb directed against paxillin. The immunoprecipitates were then blotted with MoAb directed against p-Tyr (upper panel). The Western blot transfer membrane that was probed with the anti-p-Tyr MoAb in the upper panel was stripped and probed with antipaxillin MoAb (middle panel). The lower graph shows the ratio of tyrosine-phosphorylated paxillin to paxillin protein expression represented by densitometric scanning of transblot bands. Data represent the percentage of control (SLF nontreated cells) (lower panel). The background band at 50 kD is the immunoglobulin heavy chain from the immunoprecipitation. Similar results were obtained in three independent experiments.
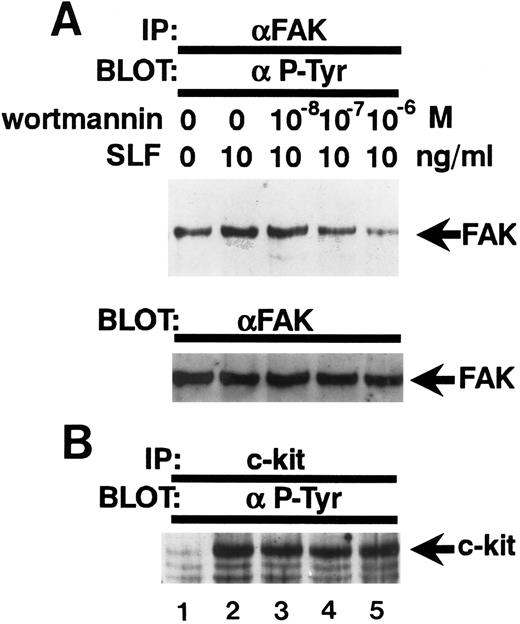
Inhibition of the SLF-induced enhancement of pp125FAK tyrosine phosphorylation by the PI-3 kinase inhibitor, wortmannin. (A) TF-1 cells were pretreated with increasing concentrations of wortmannin as indicated for 15 minutes, then plated on fibronectin-coated wells. After 15 minutes, wells were washed twice and medium containing SLF (10 ng/mL) was added and cells incubated for 10 minutes. Cell extracts were prepared and subjected to immunoprecipitation with anti-pp125FAK MoAb. The immunoprecipitates were then blotted with anti-p-Tyr MoAb (upper panel). The Western blot transfer membrane that was probed with the anti-p-Tyr MoAb in the upper panel was stripped and probed with anti-pp125FAK polyclonal antibody (lower panel). (B) Cell extracts were subjected to immunoprecipitation with antikit MoAb. The immunoprecipitates were then blotted with anti-p-Tyr MoAb. Similar results were obtained in two independent experiments.
Inhibition of the SLF-induced enhancement of pp125FAK tyrosine phosphorylation by the PI-3 kinase inhibitor, wortmannin. (A) TF-1 cells were pretreated with increasing concentrations of wortmannin as indicated for 15 minutes, then plated on fibronectin-coated wells. After 15 minutes, wells were washed twice and medium containing SLF (10 ng/mL) was added and cells incubated for 10 minutes. Cell extracts were prepared and subjected to immunoprecipitation with anti-pp125FAK MoAb. The immunoprecipitates were then blotted with anti-p-Tyr MoAb (upper panel). The Western blot transfer membrane that was probed with the anti-p-Tyr MoAb in the upper panel was stripped and probed with anti-pp125FAK polyclonal antibody (lower panel). (B) Cell extracts were subjected to immunoprecipitation with antikit MoAb. The immunoprecipitates were then blotted with anti-p-Tyr MoAb. Similar results were obtained in two independent experiments.
Tyrosine phosphorylation of paxillin.Paxillin is localized to focal adhesion plaques and is tyrosine-phosphorylated by integrin binding to ligand protein. Paxillin tyrosine phosphorylation has been reported to parallel the activation of the pp125FAK kinase and is believed to be one of the putative substrates of pp125FAK.29 We, therefore, examined whether SLF also stimulated tyrosine phosphorylation of paxillin. Lysates from SLF nontreated or treated suspension-cultured cells and SLF nontreated or treated adherent TF-1 cells on fibronectin-coated wells were immunoprecipitated with a specific antipaxillin MoAb and the immunoprecipitates were analyzed by immunoblotting with anti-p-Tyr MoAb. SLF treatment alone without plating on fibronectin did not induce tyrosine phosphorylation of paxillin in suspension-cultured TF-1 cells (Fig 6A, upper panel, lane 2). On the other hand, a dramatic increase in the antiphosphotyrosine immunoreactivity of paxillin was observed at 10 ng/mL of SLF in the adherent TF-1 cells on fibronectin-coated wells (1.79 ± 0.18 compared with control, by densitometric analysis, P < .01, n = 3) (Fig 6A, upper panel, lanes 3 and 4). Next, we examined the time-dependence of SLF treatment. Similar to pp125FAK tyrosine phosphorylation, tyrosine phosphorylation of paxillin increased progressively and reached a maximum at 10 minutes with SLF treatment at 10 ng/mL (Fig 6B, upper panel). This declined rapidly returning to basal levels of paxillin tyrosine phosphorylation in 30 minutes. The membrane was stripped and then probed with antipaxillin MoAb. During the period of SLF treatment, there was no significant alteration in the amount of immunoprecipitated paxillin (Fig 6B, middle panel). Incubation of the cells with increasing concentrations of SLF from 1 to 100 ng/mL resulted in a progressive increase in paxillin tyrosine phosphorylation. The maximum increase of tyrosine phosphorylation occurred with 10 ng/mL of SLF (Fig 6C, upper panel). The increase in phosphotyrosine content occurred with no significant alteration in the amount of paxillin protein immunoprecipitated under these conditions (Fig 6C, middle panel).
Wortmannin abolishes the enhancement of tyrosine phosphorylation of pp125FAK by SLF.Because wortmannin inhibits SLF-induced adherence of TF-1 cells to fibronectin and to evaluate whether the same mechanism might be involved also in the SLF-induced enhancement of pp125FAK tyrosine phosphorylation of adherent TF-1 cells, we examined the effect of wortmannin on SLF-induced enhancement of pp125FAK tyrosine phosphorylation. TF-1 cells were treated with increasing concentrations of wortmannin at 37°C for 20 minutes, and cells were plated on fibronectin-coated wells. As mentioned previously for the studies shown in Fig 2B, treatment with 10−7 mol/L wortmannin did not inhibit adherence of TF-1 cells without SLF-treatment. After adherence of TF1 cells reached maximum levels, the wells were washed twice and then 10 ng/mL SLF containing medium was added to the wells. Lysates from adherent TF-1 cells were normalized for protein content and were examined for pp125FAK tyrosine phosphorylation by using immunoprecipitation and immunoblotting. As shown in Fig 7A, wortmannin at 10−7 mol/L completely abolished the SLF-induced enhancement of tyrosine phosphorylation of pp125FAK (Fig 7A, upper panel). Blotting with anti-pp125FAK polyclonal antibody showed that the treatment with wortmannin did not affect the amount of protein (Fig 7A, lower panel). Figure 7B shows that the treatment with wortmannin did not affect c-kit tyrosine phosphorylation at this concentration, indicating that increased pp125FAK phosphorylation was most likely mediated through a wortmannin-sensitive pathway rather than through direct action of c-kit tyrosine kinase.
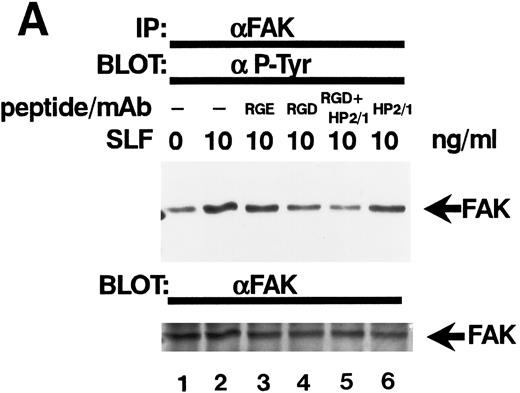
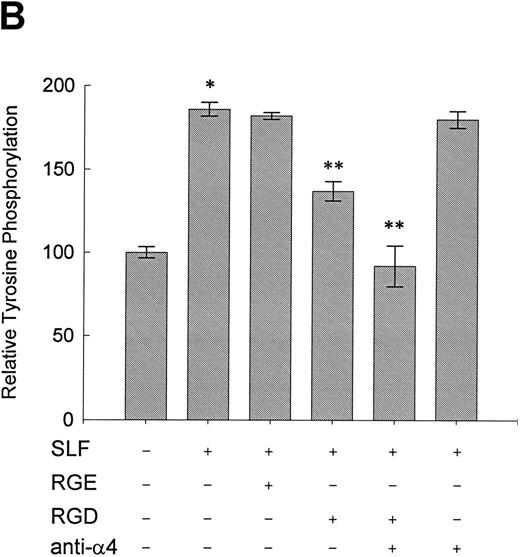
Effect of RGDS peptide on the SLF-induced enhancement of tyrosine phosphorylation of pp125FAK.SLF-induced enhancement of pp125FAK tyrosine phosphorylation was inhibited by wortmannin in a manner similar to that shown in the adhesion assay. To test the possibility that the increase of tyrosine phosphorylation is mediated through the activation of integrin receptors and following increases of integrin occupancy in adherent TF-1 cells, we examined the effect of RGDS peptide on SLF-induced enhancement of pp125FAK tyrosine phosphorylation. TF-1 cells were plated on fibronectin-coated wells and were incubated at 37°C for 15 minutes. After washing the wells twice, medium containing either RGDS and/or anti-α4 antibody (HP2/1) or the control RGES peptide was added with SLF to the wells and the cells further incubated for 10 minutes. RGDS and anti-α4 antibody treatment did not cause detachment of adherent TF-1 cells. Treatment with RGDS peptide (0.5 mg/mL) significantly inhibited the SLF-induced enhancement of pp125FAK tyrosine phosphorylation (45% reduction by densitometric analysis) (Fig 8A, upper panel, lane 4). Control RGES peptide showed no effect (Fig 8A, upper panel, lane 3). Although anti-α4 antibody (HP2/1) alone had no effect, treatment with RGDS plus anti-α4 antibody completely inhibited SLF enhanced tyrosine phosphorylation (Fig 8A, upper panel, lanes 5 and 6). Determination by densitometric analysis of relative tyrosine phosphorylation of FAK normalized by protein amount of FAK in each immunoprecipitate confirmed a significant increase of FAK phosphorylation by SLF (P < .005) and inhibition of this enhancement by RGDS peptide (P < .05) (Fig 8B). These results indicate that SLF-induced enhancement of pp125FAK tyrosine phosphorylation might be mediated through the activation of integrin receptors and following fibronectin binding.
Effect of RGDS peptide on the SLF-induced enhancement of pp125FAK tyrosine phosphorylation. (A) TF-1 cells were plated on the fibronectin-coated wells and incubated for 15 minutes at 37°C. Wells were washed twice with RPMI-BSA medium. Medium without (lane 1) or with SLF (10 ng/mL) (lanes 2 to 6) containing RGES peptide (0.5 mg/mL) (lane 3), RGDS peptide (0.5 ng/mL) (lane 4), RGDS plus anti-α4 MoAb (lane 5), or anti-α4 MoAb (10 μg/mL) (lane 6) was added to the wells. After a 10-minute incubation, cells were lysed and subjected to immunoprecipitation with anti-pp125FAK MoAb. The immunoprecipitates were then blotted with anti-p-Tyr MoAb (upper panel). The Western blot membrane that was probed with the anti-p-Tyr MoAb in the upper panel was stripped and probed with anti-pp125FAK polyclonal antibody (lower panel). This is a representative result from three separate experiments. (B) Relative tyrosine phosphorylation of FAK was determined by densitometry and was normalized by relative FAK amount in these immunoprecipitates. The values represent the mean ± standard deviation (SD) of three separate experiments. *P < .005 versus control, **P < .05 versus SLF.
Effect of RGDS peptide on the SLF-induced enhancement of pp125FAK tyrosine phosphorylation. (A) TF-1 cells were plated on the fibronectin-coated wells and incubated for 15 minutes at 37°C. Wells were washed twice with RPMI-BSA medium. Medium without (lane 1) or with SLF (10 ng/mL) (lanes 2 to 6) containing RGES peptide (0.5 mg/mL) (lane 3), RGDS peptide (0.5 ng/mL) (lane 4), RGDS plus anti-α4 MoAb (lane 5), or anti-α4 MoAb (10 μg/mL) (lane 6) was added to the wells. After a 10-minute incubation, cells were lysed and subjected to immunoprecipitation with anti-pp125FAK MoAb. The immunoprecipitates were then blotted with anti-p-Tyr MoAb (upper panel). The Western blot membrane that was probed with the anti-p-Tyr MoAb in the upper panel was stripped and probed with anti-pp125FAK polyclonal antibody (lower panel). This is a representative result from three separate experiments. (B) Relative tyrosine phosphorylation of FAK was determined by densitometry and was normalized by relative FAK amount in these immunoprecipitates. The values represent the mean ± standard deviation (SD) of three separate experiments. *P < .005 versus control, **P < .05 versus SLF.
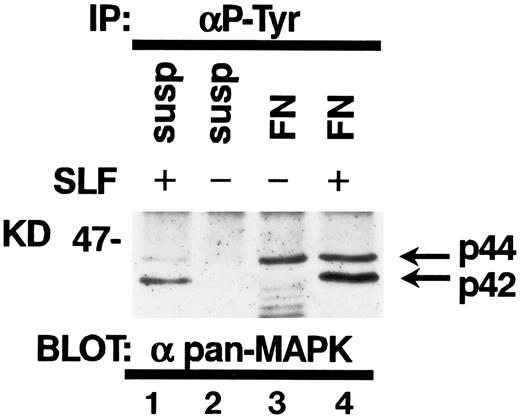
Tyrosine phosphorylation of MAP kinase in TF-1 cells.It has been reported that SLF induces tyrosine phosphorylation and activation of MAP kinase in hematopoietic cells.30 Attachment to extraxcellular matrix has been also reported to activate MAP kinases in nonhematopoietic cell types.23 Therefore, we studied the effect of fibronectin and/or SLF on MAP kinase tyrosine phosphorylation in TF-1 cells. SLF at 10 ng/mL was added to TF-1 cells in suspension or after attachment to fibronectin. After a 10-minute incubation at 37°C, cells were lysed and cell lysates containing equal amounts of protein were immunoprecipitated with anti-p-Tyr. Immunoblotting with antipan MAP kinase MoAb demonstrated that SLF mainly induced tyrosine phosphorylation of p42 MAP kinase (Fig 9). In contrast, attachment to fibronectin preferentially increased tyrosine phosphorylation of p44 MAP kinase (Fig 9). Treatment of fibronectin-attached cells with SLF resulted in increased tyrosine phosphorylation of both p42 and p44 MAP kinase (Fig 9).
Tyrosine phosphorylation of MAP kinases in TF-1 cells. TF-1 cells in suspension culture (lanes 1 and 2) or plated on fibronectin-coated wells (lanes 3 and 4) were incubated for 10 minutes at 37°C. Cells in suspension were subsequently treated with (lane 1) or without (lane 2) SLF (10 ng/mL) for 10 minutes at 37°C, then lysed in lysis buffer. Fibronectin-coated wells were washed and the medium without (lane 3) or with (lane 4) SLF (10 ng/mL) was added to the wells. The wells were further incubated for 10 minutes at 37°C and the cells were then lysed in lysis buffer. Equal amounts of protein were immunoprecipitated with agarose-conjugated-anti-p-Tyr MoAb. Immunoprecipitates were separated by 8% SDS-PAGE and subjected to immunoblotting with antipan MAP kinase MoAb. This is a representative result from three separate experiments.
Tyrosine phosphorylation of MAP kinases in TF-1 cells. TF-1 cells in suspension culture (lanes 1 and 2) or plated on fibronectin-coated wells (lanes 3 and 4) were incubated for 10 minutes at 37°C. Cells in suspension were subsequently treated with (lane 1) or without (lane 2) SLF (10 ng/mL) for 10 minutes at 37°C, then lysed in lysis buffer. Fibronectin-coated wells were washed and the medium without (lane 3) or with (lane 4) SLF (10 ng/mL) was added to the wells. The wells were further incubated for 10 minutes at 37°C and the cells were then lysed in lysis buffer. Equal amounts of protein were immunoprecipitated with agarose-conjugated-anti-p-Tyr MoAb. Immunoprecipitates were separated by 8% SDS-PAGE and subjected to immunoblotting with antipan MAP kinase MoAb. This is a representative result from three separate experiments.
DISCUSSION
In fibroblasts and T lymphocytes, integrin-mediated signals work as costimulatory signals.8 Integrin activation following matrix integrin binding results in an increased tyrosine phosphorylation of pp125FAK.17,18 Activation of pp125FAK has been shown to induce its association with signal-transducing molecules such as pp60src,31 pp59fyn,32 PI3-kinase,33 and the adapter protein Grb2.22 Tyrosine phosphorylation of pp125FAK is considered to play a central role in integrin-mediated signaling.
Evidence is provided here that adherence of TF-1 cells to fibronectin-coated wells through the α4β1 and α5β1 integrin receptors stimulates the phosphorylation of tyrosine residues on pp125FAK. The data also shows that SLF treatment after adherence of TF-1 cells to fibronectin further enhances integrin fibronectin-dependent phosphorylation of pp125FAK in a dose- and time-dependent manner. The significant relative increases ranged from 1.5-fold to 2.2-fold. This increase is comparable with increases of pp125FAK tyrosine phosphorylation, which have been shown for other cell types, such as T lymphocytes induced by T-cell receptor-mediated signals (1.4-fold to 2.5-fold34 ) or Swiss 3T3 cells induced by platelet-derived growth factor (PDGF) (1.56-fold35 or 3.7-fold36 ). Without plating on fibronectin, SLF treatment alone did not induce any tyrosine phosphorylation of pp125FAK, suggesting that SLF-induced enhancement of tyrosine phosphorylation is an indirect action through integrin molecules, rather than a direct action on c-kit receptor tyrosine kinase. c-kit receptor possesses intracellular tyrosine kinase domains, which are activated following binding of a ligand. c-kit ligand binding induces tyrosine phosphorylation of several downstream signaling molecules such as phospholipase C-γ, PI3-kinase, SHP-1, and SHP-2.26,37 38 These molecules have SH-2 domains as a binding site with c-kit receptor tyrosine kinase. The tyrosine phosphorylation of these molecules requires direct association between activated c-kit and these molecules. On the other hand, pp125FAK does not possess an SH-2 domain and, therefore, it is unlikely that pp125FAK associates with c-kit receptor tyrosine kinase and is phosphorylated in an SH-2 domain-dependent manner.
One possible mechanism of SLF-induced enhancement of pp125FAK tyrosine phosphorylation is through integrin occupancy as a result of SLF-mediated activation of integrin receptors. As reported previously, hematopoietic growth factors can enhance integrin-mediated adhesion by inside-out signaling.27,39,40 We demonstrated that SLF stimulates TF-1 cell adhesion at 10 ng/mL and that this SLF-mediated activation of integrin receptors is inhibited by wortmannin at 10−7 mol/L. We also showed that pp125FAK is tyrosine phosphorylated when cells adhere to fibronectin and that SLF further enhances the tyrosine phosphorylation of pp125FAK at 10 ng/mL and that this is inhibited by wortmannin in the same manner as in the adhesion assay. Moreover, treatment of the adherent TF-1 cells with anti-α4 integrin MoAb plus RGDS peptide, which blocks α4β1 and α5β1 integrin fibronectin binding,41 completely inhibits SLF-induced enhancement of pp125FAK tyrosine phosphorylation. These results strongly suggest that SLF-induced enhancement of pp125FAK tyrosine phosphorylation is mediated through integrin occupancy with fibronectin as a result of SLF-induced activation of integrin receptors.
PDGF,36 bombesin, vasopressin, endothelin,42,43 and bradykinin44 were shown to induce pp125FAK tyrosine phosphorylation in Swiss 3T3 cells. In oral squamous cell carcinoma cells, hepatocyte growth factor/scatter factor has been reported to increase pp125FAK tyrosine phosphorylation.45 Of interest, PDGF, to which the c-kit receptor is closely related, has been shown to increase integrin-mediated adherence to fibronectin by inside-out signaling when the PDGF receptor is expressed in mouse mast cells.46 This PDGF-mediated activation of integrin receptors is regulated through both the PI3-kinase pathway and phospholipase C-γ1 pathway. Moreover it has been reported that PDGF-induced actin rearrangement of fibroblasts is inhibited by wortmannin.47 Taken together, it is conceivable that PDGF-mediated tyrosine phosphorylation of pp125FAK might be also mediated through the regulation of integrin activity by inside-out signaling.
Previous studies demonstrated a physical association of tyrosine-phosphorylated FAK with pp59fyn,32 PI3-kinase,33 or Grb2.22 However, we could not detect such associations by a combination of immunoprecipitation and immunoblotting (data not shown), although this procedure is sensitive enough to detect cytokine receptor- or Grb2-associated proteins.26 Because coimmunoprecipitation is largely dependent on the nature of the association and the detection threshold of the method, it is possible that we might have failed to detect weak associations. Alternatively, because previous reports concerning FAK association with Src, Fyn, and Grb2 were shown in cells that grow in an adhesion-dependent manner, FAK may not associate with these molecules upon tyrosine phosphorylation in hematopoietic cells.
Paxillin is a cytoskeletal protein which colocalizes in the structure of focal adhesion and is tyrosine-phosphorylated in parallel with pp125FAK.29 Direct association of paxillin with pp125FAK indicates that paxillin is one of the substrates of pp125FAK.48 It has also been reported that paxillin can bind with pp60c-src 49 and Crk50 implicating the role of paxillin as a signal-transducing molecule. The data presented here show that SLF enhances paxillin tyrosine phosphorylation in a dose- and time-dependent manner when cells bind to fibronectin. SLF-induced enhancement of tyrosine phosphorylation of FAK and paxillin was associated with morphological change by SLF in TF-1 cells (Fig 4). Since paxillin tyrosine phosphorylation is involved in the formation of focal adhesion and the assembly of actin stress fibers,12 these results raise the possibility that SLF might be involved in cytoskeletal regulation and the paxillin-mediated signaling pathway of hematopoietic cells in the bone marrow microenvironment.
Experiments of long-term bone marrow cultures with stromal cells genetically engineered to express soluble or membrane-bound forms of SLF suggest an important role of the membrane-bound forms of SLF for the maintenance of hematopoietic progenitor cells.51 This, in turn, suggests that direct contact with stromal components may be a prerequisite to full exertion of the biological activity of SLF. We demonstrated tyrosine phosphorylation of p44 MAP kinase by attachment to fibronectin (Fig 9). Moreover, SLF preferentially induced tyrosine phosphorylation of p42 MAP kinase in TF-1 cells, and treatment of the cells attached to fibronectin with SLF resulted in both p42 and p44 MAP kinase tyrosine phosphorylation (Fig 9). Since one of the targets for MAP kinases is the transcription factor family, this might suggest that the gene expression in myeloid progenitors in the microenvironment could be regulated by both hematopoietic growth factors and the extracellular matrix. Considering that stem cells in the hematopoietic microenvironment are receiving signals from both the c-kit signaling pathway and the integrin-mediated pathway, it is noteworthy that the c-kit receptor pathway signal modulates an integrin-extracellular matrix protein-dependent signaling pathway.
ACKNOWLEDGMENT
We thank Dr S.K. Hanks, Vanderbilt University, Nashville, TN, for providing anti-pp125FAK polyclonal antibody and Dr L.K. Ashman, University of Adelaide, South Australia, Australia, for providing antikit MoAb. We thank Rebecca Miller for her assistance in the preparation of this manuscript.
Supported by Public Health Service Grants No. R01 HL54037, R37 CA36464, R01 HL49203, HL56416 and a project in P01 HL53586 from the National Cancer Institute and the National Institutes of Health, Bethesda, MD.
H.T. and A.G. contributed equally to these studies and both should be considered as first authors of this report.
Address reprint requests to Hal E. Broxmeyer, PhD, Walther Oncology Center, Indiana University School of Medicine, 975 W Walnut St, Indianapolis, IN 46202-5121.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal