Abstract
NNKY5-5, an IgG monoclonal antibody directed against the von Willebrand factor-binding domain of glycoprotein (GP) Ibα, induced weak but irreversible aggregation (or association) of platelets in citrate-anticoagulated platelet-rich plasma. This phenomenon was defined as small aggregate formation (SAF ). Platelets in hirudin-anticoagulated plasma or washed platelets showed little response to NNKY5-5 alone, but the antibody potentiated aggregation induced by low concentrations of adenosine diphosphate or platelet-activating factor. NNKY5-5 did not induce granule release or intracellular Ca2+ mobilization. However, NNKY5-5 caused tyrosine phosphorylation of a 64-kD protein and activation of a tyrosine kinase, p72syk. An anti-FcγII receptor antibody had no effect on SAF, suggesting that NNKY5-5 activated platelets by interacting with glycoprotein Ib. Fab′ fragments of NNKY5-5 did not induce SAF, but potentiated aggregation induced by other agonists. The Fab′ fragment of NNKY5-5 induced the activation of p72syk, suggesting that such activation was independent of the FcγII receptor. Cross-linking of the receptor-bound Fab′ fragment of NNKY5-5 with a secondary antibody induced SAF. GRGDS peptide, chelation of extracellular Ca2+, and an anti-GPIIb/IIIa antibody inhibited NNKY5-5-induced SAF, but had no effect on 64-kD protein tyrosine phosphorylation or p72syk activations. Various inhibitors, including aspirin and protein kinase C, had no effect on SAF, protein tyrosine phosphorylation, or p72syk activation. In contrast, tyrphostin 47, a potent tyrosine kinase inhibitor, inhibited NNKY5-5–induced SAF as well as tyrosine phosphorylation and p72syk activation. Our findings suggest that binding of NNKY5-5 to GPIb potentiates platelet aggregation by facilitating the interaction between fibrinogen and GPIIb/IIIa through a mechanism associated with p72syk activation and tyrosine phosphorylation of a 64-kD protein.
PLATELETS PROVIDE an excellent model for investigation of the mechanisms of signal transduction. Agonist-induced activation of platelets leads to a variety of responses, including shape change, aggregation, and granule secretion. The process of signal transduction involves the breakdown of phospholipids, arachidonic acid metabolism, calcium mobilization, and the phosphorylation of specific proteins, events mediated by the activation of various kinases.1 Recently, an increasing body of evidence has suggested that tyrosine phosphorylation of proteins plays an important role in receptor-mediated transmembrane signaling during platelet activation.2-4 In particular, tyrosine phosphorylation catalyzed by tyrosine kinases appears to influence platelet membrane glycoproteins. Recent studies have shown that when glycoprotein (GP) IIb/IIIa is activated by a specific monoclonal antibody (MoAb), tyrosine phosphorylation of certain proteins occurs5 and the tyrosine kinase p72syk is activated.6 Several tyrosine kinases (fyn, lyn, and yes) are physically associated with GPIV, and the interaction between GPIV and these kinases is believed to activate signaling pathways through tyrosine phosphorylation.7 Platelet activation mediated via the FcγII receptor (FcγRII) induces the tyrosine phosphoryration of a number of proteins, including FcγRII itself, suggesting that such phosphorylation may be involved in this signaling pathway.8 In addition, p72syk shows noncovalent binding to the FcγRII on cross-linking of this receptor.9 These observations suggest that activation of tyrosine kinases and tyrosine phosphorylation are involved in the early stage of platelet aggregation after the binding of ligands to receptors on the platelet membrane.
The GPIb complex is a heterodimer containing both GPIb and GPIX. GPIb consists of disulfide-linked heavy (Ib α) and light (Ib β) chains. GPIX and GPV show noncovalent binding to GPIb.10,11 Platelet adhesion to the subendothelium of damaged vessels is the initial hemostatic response to vascular injury, and the interaction between von Willebrand factor (vWF ) and GPIb is the primary event in this response. GPIb also plays a key role in initiating platelet activation during shear-induced platelet aggregation.12 Recent reports have provided evidence for the involvement of tyrosine phosphorylation and tyrosine kinases in vWF and GPIb-mediated platelet activation.13,14 Although the molecular mechanism of the interaction between GPIb and vWF has been investigated extensively, the intracellular signal transduction pathway remains largely unknown. In the presence of ristocetin, a macrolide antibiotic, vWF multimers promote thromboxane A2 synthesis, and protein kinase C activation in platelets, as well as an increase in the intracellular Ca2+ concentration ([Ca2+]i).15 Asialo vWF has the ability to bind to GPIb on resting platelets in the absence of ristocetin, and has been shown to promote thromboxane A2 synthesis, granule release, and fibrinogen binding to GPIIb/IIIa.16,17 However, whether thromboxane A2 production or granule release constitute initial signaling events or are simply the consequences of aggregation remains to be determined. In addition, granule release and thromboxane A2 production appear to have little to do with shear-induced platelet activation.18
NNKY5-5 is a murine MoAb directed against GPIb that completely inhibits ristocetin-induced platelet agglutination.19 In the present study, we determined the effects of NNKY5-5 on platelet function, and assessed the mechanism of NNKY5-5–induced platelet activation in relation to protein tyrosine phosphorylation. The purpose of this study was to investigate the mechanisms underlying GPIb-mediated platelet activation.
MATERIALS AND METHODS
Materials.Adenosine diphosphate (ADP), aspirin, EGTA, prostaglandin E1 , prostaglandin I2 , thrombin, ristocetin, enolase, leupeptin, phenylmethylsulfonyl fluoride (PMSF ), and sodium orthovanadate were purchased from Sigma Chemical Co (St Louis, MO). Luciferin-luciferase was obtained from Chrono-log Co (Havertown, PA), fura 2-AM from Dojindo Lab. (Kumamoto, Japan), immobilized pepsin from Pierce (Rockford, IL), tyrphostin 47 and H-7 from Funakoshi (Tokyo, Japan), and Gly-Arg-Gly-Asp-Ser (GRGDS) from the Peptide Institute (Osaka, Japan). Peroxidase-conjugated or unconjugated goat anti-mouse IgG was purchased from Cappel (West Chester, PA). Anti-FcγII receptor (IV.3) and anti-phosphotyrosine (PY-20) MoAbs were purchased from Medarex Inc (West Lebanon, NH) and ICN Biomedicals Inc. (Costa Mesa, CA), respectively, while MoAbs for p72syk and p60c-src were obtained from Wako Chemicals (Tokyo, Japan). An anti-GPIb MoAb (SZ-2) was purchased from Immunotech (Marseille, France). The other anti-GPIb MoAbs (TM-60 and WGA-III) were kind gifts from Dr Kenjirou Tanoue (Tokyo Metropolitan Institute of Clinical Investigation, Tokyo, Japan) and Dr Makoto Handa (Keiou Medical University, Tokyo, Japan).
Platelet preparation.Whole blood from healthy donors was collected into tubes containing 110 mmol/L trisodium citrate (1:9) or hirudin and centrifuged at 200g for 10 minutes at room temperature to obtained platelet-rich plasma (PRP). To obtain washed platelets, 1 μmol/L prostaglandin E1 was added to PRP, and the mixture was centrifuged at 800g for 15 minutes. Platelets were washed twice in a platelet-washing buffer (10 mmol/L Tris-HCl, 150 mmol/L NaCl, 1 mmol/L EDTA, and 5 mmol/L glucose, pH 7.4), and were resuspended in HEPES-Tyrode's buffer (138 mmol/L NaCl, 2.8 mmol/L KCl, 1 mmol/L CaCl2 , 0.5 mmol/L MgCl2 , 0.5 mmol/L NaH2PO4 , 12 mmol/L NaHCO3 , 10 mmol/L glucose, and 10 mmol/L HEPES, pH 7.4). In some experiments, platelets were separated from plasma by gel filtration on a sepharose-2B column equilibrated with HEPES-Tyrode's buffer.20
NNKY5-5.NNKY5-5 (IgG2b), a MoAb directed against GPIb, was produced by immunizing BALB/c mice as described previously.19 An attempt was made to produce F(ab′)2 fragments of NNKY5-5 using pepsin digestion,21 but only Fab′ fragments and the Fc portion were obtained. As described in a previous report,21 mouse IgG2b is generally degraded to Fab′, but not F(ab′)2 by pepsin digestion. We confirmed the Fab′ fragment of pepsin-digested NNKY5-5 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with and/or without reduction by 2-mercaptoethanol. Thus, the effects of the intact antibody and its Fab′ fragment, but not those of the F(ab′)2 fragment, were evaluated in this study.
Assessment of platelet aggregation and adenosine triphosphate (ATP) production.PRP and washed platelets were adjusted to a final concentration of 3 × 108/mL and preincubated with luciferin-luciferase. Platelet aggregation and ATP production were measured using a lumi-aggregometer (Payton, Scarborough, Canada). Various inhibitors were added to the PRP at 10 minutes before NNKY5-5, to determine their effects on aggregation induced by this MoAb.
Assessment of platelet aggregation by light scatter.Platelet aggregation was also measured by the particle counting technique using light scatter.22 Briefly, a diode laser light beam (width: 40 μm; wavelength: 675 nm) was passed through PRP in a cylindrical glass cuvette maintained at 37°C with constant stirring. The scattering of light by particles in a limited volume (33 × 65 × 65 μm) was measured with an optical system that minimized repeated light scatter. The signals were digitized with an analogue-to-digital converter and processed by a computer; to quantitate the number and size of the platelet aggregates. We have previously confirmed that the light scatter intensity from 0.2 to 2.0 V/min represent small aggregates consisting of less than 100 platelets.22 23 Our instrument was particularly sensitive to small aggregates and thus was able to evaluate weak platelet activation.
Measurement of the platelet Ca2+ concentration ([Ca2+]i) with fura-2.PRP was incubated with 5 μmol/L fura-2 AM for 15 minutes at 37°C . The pelleted platelets were washed twice with Ca2+-free HEPES buffer containing 1 μmol/L prostaglandin E1 and were then suspended in Ca2+-free HEPES buffer at a final concentration of 1 × 108/mL. Fura 2 fluorescence was measured in the presence of 1 mmol/L Ca2+ using a calcium-ion analyzer (FS-100; Kowa Co, Tokyo, Japan) at excitation wavelengths of 340 and 380 nm. Thrombin as low as 0.01 U/mL induced detectable changes in [Ca2+]i, and the average increase in [Ca2+]i induced by 0.1 U/mL thrombin was 780 ± 126 nmol/L, as assessed with this method.
Identification of phosphotyrosine-containing platelet proteins by immunoblotting.After platelet activation, the reaction was terminated by the addition of Laemmli SDS reducing buffer24 containing 100 mmol/L Na3VO4 , 10 mmol/L EDTA, and 1 mmol/L phenylmethanesulfonyl fluoride, followed by boiling for 3 minutes. Platelet proteins were separated by 8% SDS-PAGE, and electroblotted onto clear Blot Membrane P (Atto, Tokyo, Japan). The membranes were incubated with 1% bovine serum albumin in phosphate-buffered saline, washed, and then incubated with PY-20, an MoAb specific for phosphotyrosine residues. Antibody binding was detected using peroxidase-conjugated goat anti-mouse IgG and visualized with enhanced chemiluminescence detection reagents (Amersham Co, Buckinghamshire, UK).
Immunoprecipitation kinase assay.Platelets were incubated with NNKY5-5, and incubation was terminated at the specified times by addition of an equal volume of ice-cold lysis buffer (2% Triton X-100, 100 mmol/L Tris/HCl, pH 7.5, 50 mmol/L NaCl, 5 mmol/L EDTA, 2 mmol/L vanadate, 1 mmol/L PMSF, and 100 μg/mL leupeptin).25 Then the lysate was sonicated and centrifiged at 16,000g for 5 minutes. The supernatant was precleared twice with sepharose beads, mixed with anti-p72syk or anti-p60c-src antibody bound to protein A sepharose or CNBr-activated sepharose, and stirred for 2 hours at 4. Next, the sepharose beads were washed three times with lysis buffer and divided into two portions. One portion was used directly for immunoblotting, as described below, and the other was processed further for the in vitro kinase assay.26 These beads were washed once with low-salt buffer (100 mmol/L NaCl, 5 mmol/L MnCl2 , 10 mmol/L Tris [pH 7.4]), and were incubated with 25 μL of kinase reaction buffer (20 mmol/L Tris, pH 7.5, 10 mmol/L MnCl2 ) in the presence or absence of 10 μg of acid-treated enolase. The reaction was initiated by the addition of 10 μCi of [γ-32P] ATP and 2 μmol/L ATP. After 10 minutes at 20, the reaction was stopped by the addition of Laemmli buffer and boiling for 3 minutes. For immunoblotting, proteins were separated under reducing conditions by 8% or 12% SDS-PAGE and electrically transferred onto a Clear Blot Membrane P (Atto). The membrane was treated with 1 mol/L KOH for 60 minutes, dried, and quantified with a BAS-2000 Phosphor imager (Fuji Film, Tokyo, Japan).
RESULTS
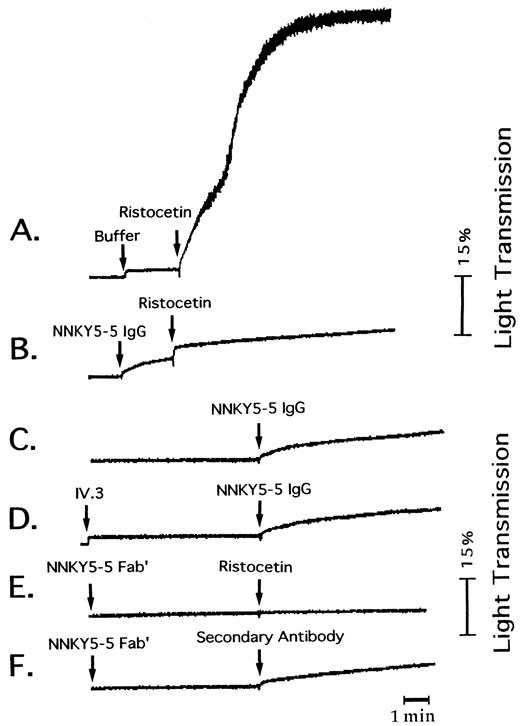
Characterization of NNKY5-5.A previous study19 showed that NNKY5-5 is an IgG2b antibody. There was very little binding of NNKY5-5 to Bernard Soulier syndrome platelets. The molecular weight of the platelet-membrane antigen recognized by NNKY5-5 was determined to be 150 kD under reducing conditions. We also analyzed the amino acid sequence of the NNKY5-5–immunoprecipitated protein by microsequencing. Glycocalicine was applied to NNKY5-5–immobilized Sepharose and protein was eluted with 2 mol/L guanidine HCl in 0.1 mol/L sodium acetate buffer (pH 4.0)/1.5 mol/L NaCl. After dialysis, the eluted protein was immobilized on a polyvinylidene difluoride (PVDF) membrane (ProSpin; Applied Biosystems Inc, Foster City, CA). Then the membrane was pyridylethylated and directly applied to an amino acid sequence analyzer (model 473A, Applied Biosystems Inc). The sequence obtained was NH2 -H-P-I-C-E-V-S-K-V-A, which corresponded to the reported N-terminal sequence of GPIb,27 confirming the binding of NNKY5-5 to this glycoprotein. As shown in Fig 1A and B, NNKY5-5 completely inhibited ristocetin (1.5 mg/mL)-induced platelet agglutination, but had no effect on collagen- or thrombin-induced aggregation.
Effect of NNKY5-5 on platelet aggregation. Platelet aggregation was measured from the changes of light transmission using PRP anticoagulated with citrate and maintained at 37°C with constant stirring. (A) Effect of ristocetin (1.5 mg/mL). (B) NNKY5-5 (10 μg/mL) was added 2 minutes before ristocetin. (C) and (D) NNKY5-5 was added to PRP in the absence (C) or the presence (D) of MoAb IV.3 (10 μg/mL). The traces shown are representative ones from at least 4 experiments. (E) and (F ) NNKY5-5 Fab′ (10 μg/mL) was added 5 minutes before ristocetin (E) or of goat anti-mouse antibody (F ).
Effect of NNKY5-5 on platelet aggregation. Platelet aggregation was measured from the changes of light transmission using PRP anticoagulated with citrate and maintained at 37°C with constant stirring. (A) Effect of ristocetin (1.5 mg/mL). (B) NNKY5-5 (10 μg/mL) was added 2 minutes before ristocetin. (C) and (D) NNKY5-5 was added to PRP in the absence (C) or the presence (D) of MoAb IV.3 (10 μg/mL). The traces shown are representative ones from at least 4 experiments. (E) and (F ) NNKY5-5 Fab′ (10 μg/mL) was added 5 minutes before ristocetin (E) or of goat anti-mouse antibody (F ).
Assessment of platelet aggregation by light transmission.NNKY5-5 (10 μg/mL) induced irreversible aggregation of citrate-anticoagulated PRP, with the change in light transmission ranging from 10% to 15%. The aggregation curve showed a gentle slope, a minimal lag time, and no fluctuation (Fig 1C). We defined this phenomenon as SAF. Although the extent of the change in light transmission differed slightly among individuals, all the platelet samples obtained from 10 healthy donors responded to NNKY5-5. A wide variety of antiplatelet MoAbs induce platelet aggregation, with the majority activating platelets through FcγRII-dependent mechanisms.28 Therefore, we evaluated the effect of IV.3, an anti-FcγRII MoAb, on SAF induced by NNKY5-5 as well as the effect of pepsin-digested fragments of NNKY5-5 that did not include the Fc portion. IV.3 failed to inhibit SAF induced by NNKY5-5 (Fig 1D). We were not able to obtain F(ab′)2 fragments of NNKY5-5, but Fab′ fragments caused no detectable SAF (Fig 1E). Use of a secondary antibody to cross-link the receptor-bound NNKY5-5 Fab also induced SAF (Fig 1F ). These findings suggested that NNKY5-5–induced SAF was independent of FcγRII activation and that divalent binding of the antibody was required. The other anti-GPIb MoAbs (TM-60,29 SZ-2,30 and WGA-III) did not induce any platelet aggregation (data not shown).
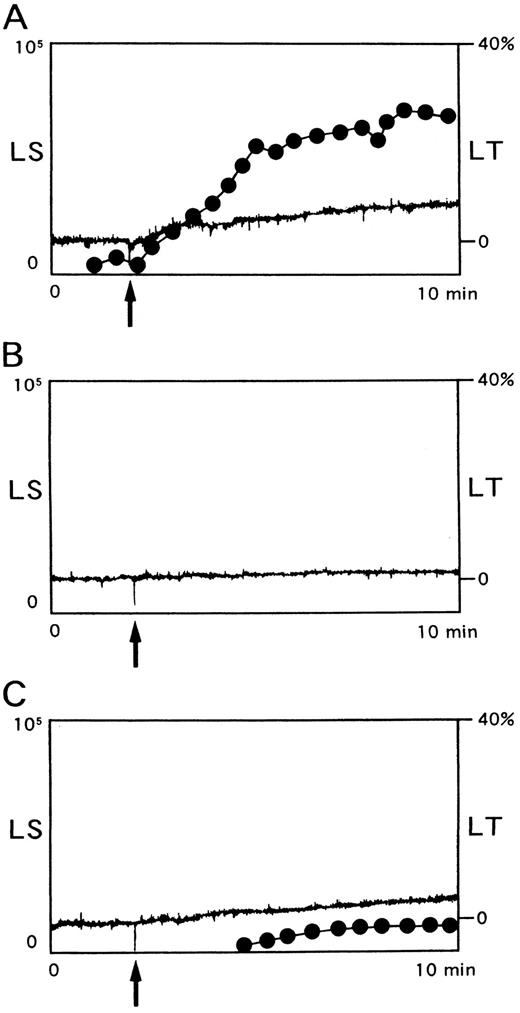
Assessment of platelet aggregation by light scatter.Since NNKY5-5 induced only slight changes in light transmission, it was possible that this MoAb induced shape change but not aggregation. Therefore, aggregation was also assessed by a newly developed light scatter method, which could detect the formation of small aggregates as well as large ones and allowed quantification of the number and size of the aggregates in a suspension22. NNKY5-5 (10 μg/mL) caused the irreversible formation of aggregates consisting of 6 to 100 platelets in a time-dependent manner (Fig 2A). Larger aggregates of more than 100 platelets were not detected. Prostaglandin I2 (0.2 μmol/L) completely suppressed the formation of small aggregates (data not shown). GRGDS (400 μmol/L), which blocks the interaction between GPIIb/IIIa and fibrinogen, as well as NNKY1-32 (10 μg/mL), an anti-GPIIb/IIIa MoAb, which interferes with fibrinogen binding,31 potently inhibited NNKY5-5–induced SAF (Fig 2B). When blood samples were anticoagulated with 50 U/mL hirudin instead of citrate, ADP-induced platelet aggregation was similar to that obtained with citrate-anticoagulated samples. In contrast, the aggregation induced by NNKY5-5 was greatly reduced (Fig 2C). In addition, washed platelets only showed a minimal response to NNKY5-5 (data not shown).
Platelet aggregation induced by NNKY5-5 as assessed by aggregometry using light scatter. Platelet aggregation was measured using PRP anticoagulated with citrate (A and B) or 50 U/mL hirudin (C). (A) NNKY5-5 (10 μg/mL) was added to PRP (arrow) and the changes in light transmission (LT, solid line) and light scatter (LS, broken line) were monitored simultaneously. (B) GRGDS (400 μmol/L) was added 10 minutes before the addition of 10 μg/mL NNKY5-5 (arrow). (C) Hirudin was used to maintain a physiological concentration of Ca2+ and NNKY5-5 (10 μg/mL) was added at the time indicated by the arrow. Light scatter intensity of 0.2 to 2V, which represents platelet aggregates of small size, is shown in arbitary units. The data are representative of at least four experiments.
Platelet aggregation induced by NNKY5-5 as assessed by aggregometry using light scatter. Platelet aggregation was measured using PRP anticoagulated with citrate (A and B) or 50 U/mL hirudin (C). (A) NNKY5-5 (10 μg/mL) was added to PRP (arrow) and the changes in light transmission (LT, solid line) and light scatter (LS, broken line) were monitored simultaneously. (B) GRGDS (400 μmol/L) was added 10 minutes before the addition of 10 μg/mL NNKY5-5 (arrow). (C) Hirudin was used to maintain a physiological concentration of Ca2+ and NNKY5-5 (10 μg/mL) was added at the time indicated by the arrow. Light scatter intensity of 0.2 to 2V, which represents platelet aggregates of small size, is shown in arbitary units. The data are representative of at least four experiments.
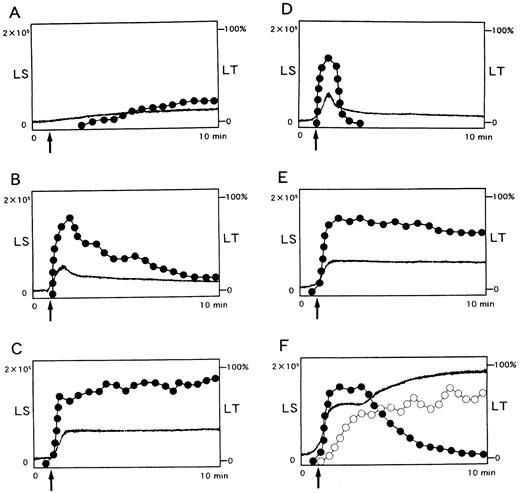
Potentiation of platelet aggregation induced by other agonists.The reduced or absent ability of NNKY5-5 to elicit platelet aggregation in the presence of a physiological concentration of Ca2+ was reminiscent of the effects of epinephrine, which is not an aggregating agent in its own right but potentiates platelet aggregation induced by other agonists.32 Therefore, we evaluated the effect of NNKY5-5 on platelet aggregation induced by low concentrations of ADP or platelet-activating factor (PAF ). When platelets were activated with 0.5 μmol/L ADP or 10 nmol/L PAF, there was transient formation of small aggregates consisting of less than 100 cells, producing a brief increase in light transmission (primary aggregation, Fig 3B and D). When platelets were preincubated with 10 μg/mL NNKY5-5 for 2 minutes, the number of aggregates increased and the aggregates persisted for up to 10 minutes after stimulation with ADP or PAF (Fig 3C and E). Platelet aggregation induced by 0.2 mol/L U-46619, a thromboxane A2 agonist, was also potentiated by NNKY5-5 in a similar manner (data not shown). Panel F shows changes in LS (light scatter) and LT (light transmission) induced by 5 μmol/L ADP, which induced full aggregation. ADP first induced the formation of small aggregates (closed dots), followed by an increase in aggregation of larger size (open dots) with concomitant decrease in small aggregate number.
Potentiation by NNKY5-5 of platelet aggregation induced by other agonists. Light scatter (LS) was divided into two portions by intensity. Changes in LS between 0.2 to 2V, which represents aggregates consisting less than 150 cells, are shown as closed dots, and LS that represents larger aggregates shown as open dots. Changes in light transmission (LT) is shown as solid lines. (A) NNKY5-5 alone (10 μg/mL) induced moderate LS of small size and a change in LT of only around 10%. ADP (0.5 μmol/L) or PAF (10 nmol/L) induced only primary aggregation, which gradually disappeared (B and D, respectively). Addition of NNKY5-5 (10 μg/mL) 2 minutes before ADP or PAF enhanced platelet aggregation and prolonged its duration (C and E, respectively). (F ) shows a typical pattern of ADP-induced platelet aggregation, detected by LS, which can quantitatively measure the size and number of platelet aggregates. It shows changes in LS and LT induced by 5 μmol/L ADP, which induced full aggregation. ADP first induced the formation of small aggregates (closed dots), followed by an increase in larger aggregates (open dots) with a concomitant decrease in small aggregates. LS is shown in arbitary units. Representative data from at least four experiments are shown.
Potentiation by NNKY5-5 of platelet aggregation induced by other agonists. Light scatter (LS) was divided into two portions by intensity. Changes in LS between 0.2 to 2V, which represents aggregates consisting less than 150 cells, are shown as closed dots, and LS that represents larger aggregates shown as open dots. Changes in light transmission (LT) is shown as solid lines. (A) NNKY5-5 alone (10 μg/mL) induced moderate LS of small size and a change in LT of only around 10%. ADP (0.5 μmol/L) or PAF (10 nmol/L) induced only primary aggregation, which gradually disappeared (B and D, respectively). Addition of NNKY5-5 (10 μg/mL) 2 minutes before ADP or PAF enhanced platelet aggregation and prolonged its duration (C and E, respectively). (F ) shows a typical pattern of ADP-induced platelet aggregation, detected by LS, which can quantitatively measure the size and number of platelet aggregates. It shows changes in LS and LT induced by 5 μmol/L ADP, which induced full aggregation. ADP first induced the formation of small aggregates (closed dots), followed by an increase in larger aggregates (open dots) with a concomitant decrease in small aggregates. LS is shown in arbitary units. Representative data from at least four experiments are shown.
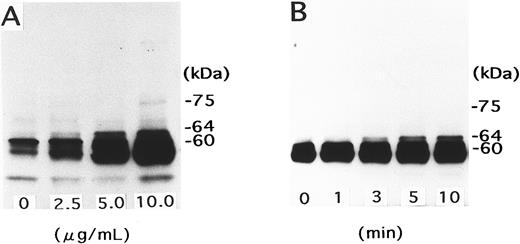
Signal transduction pathway of NNKY5-5–induced platelet activation.At concentrations up to 30 μg/mL, NNKY5-5 induced no ATP production or change in [Ca2+]i, as assessed using fura 2-loaded platelets (data not shown). Resting platelets contain a major 60-kD protein band that shows heavy tyrosine phosphorylation and this protein is considered to be p60c-src.33 NNKY5-5 induced tyrosine phosphorylation of a 64-kD protein in a concentration-dependent manner. This band could be detected as early as 1 minute after the addition of NNKY5-5, and its density increased in a time-dependent manner (Fig 4). Addition of various inhibitors at 10 minutes before NNKY5-5 was also evaluated with respect to the effect on platelet aggregation and tyrosine phosphorylation of the 64-kD protein. Platelet aggregation was measured with PRP, because it was difficult to quantitate aggregate formation using washed platelets. However, tyrosine phosphorylation of the 64-kD protein was evaluated using washed platelets, because the presence of serum proteins circumvented the detections of platelet tyrosine phosphorylations. Aspirin or H-7, a protein kinase C inhibitor, failed to inhibit tyrosine phosphorylation of the 64-kD protein and platelet aggregation. GRGDS (a peptide that inhibits the binding of fibrinogen to GPIIb/IIIa) and EGTA (a chelator of extracellular Ca2+) inhibited aggregation induced by NNKY5-5, but had no effect on phosphorylation of the 64-kD protein. Prostaglandin I2 , a stimulator of adenylate cyclase, inhibited both NNKY5-5–induced protein phosphorylation and platelet aggregation. In the presence of tyrphostin 47 or herbimycin, which are potent tyrosine kinase inhibitors,34 35 no protein phosphorylation or platelet aggregation was observed. Table 1 summarizes the results obtained with various inhibitors. These findings suggested that NNKY5-5 induced phosphorylation of the 64-kD protein independent of thromboxane A2 , protein kinase C, or platelet aggregation, but that tyrosine kinase activation (which was susceptible to tyrosine kinase inhibitors) and elevation of the intracellular cAMP level played an essential role in NNKY5-5–induced platelet activation.
Tyrosine phosphorylation of a 64-kD protein induced by NNKY5-5. (A) Gel-filtered platelets were stimulated for 10 minutes with NNKY5-5 at the indicated concentrations. The reaction was terminated with Laemmli sample buffer, and the lysates were subjected to SDS-PAGE followed by Western blotting with an anti-phosphotyrosine antibody. (B) Platelets were stimulated with 10 μg/mL NNKY5-5 for the indicated periods, and changes of protein tyrosine phosphorylation were evaluated using whole cell lysates, as described in (A).
Tyrosine phosphorylation of a 64-kD protein induced by NNKY5-5. (A) Gel-filtered platelets were stimulated for 10 minutes with NNKY5-5 at the indicated concentrations. The reaction was terminated with Laemmli sample buffer, and the lysates were subjected to SDS-PAGE followed by Western blotting with an anti-phosphotyrosine antibody. (B) Platelets were stimulated with 10 μg/mL NNKY5-5 for the indicated periods, and changes of protein tyrosine phosphorylation were evaluated using whole cell lysates, as described in (A).
Effects of Various Inhibitors on Aggregation and Tyrosine Phosphorylation of the 64-kD Protein Induced by NNKY5-5
| Agent . | Aggregation . | 64-kD Protein Phosphorylation . |
|---|---|---|
| Aspirin (500 μmol/L) | + | + |
| H-7 (100 μmol/L) | + | + |
| EGTA (3 mmol/L) | − | + |
| GRGDS (400 μmol/L) | − | + |
| Prostaglandin I2 (0.2 μmol/L) | − | − |
| Tyrphostin (100 μmol/L) | − | − |
| Herbimycin (25 μmol/L) | − | − |
| Agent . | Aggregation . | 64-kD Protein Phosphorylation . |
|---|---|---|
| Aspirin (500 μmol/L) | + | + |
| H-7 (100 μmol/L) | + | + |
| EGTA (3 mmol/L) | − | + |
| GRGDS (400 μmol/L) | − | + |
| Prostaglandin I2 (0.2 μmol/L) | − | − |
| Tyrphostin (100 μmol/L) | − | − |
| Herbimycin (25 μmol/L) | − | − |
Inhibitors were added to PRP 10 minutes before stimulation with 10 μg/mL NNKY5-5 and protein phosphorylation was assessed using gel-filtered platelets. The data were compiled from two experiments. Inhibition was judged to be negative when its magnitude was less than 30% on densitometry. PGI2 , tyrphostin, and herbmycin all caused almost complete inhibition of protein phosphorylation.
−, inhibited; +, not inhibited.
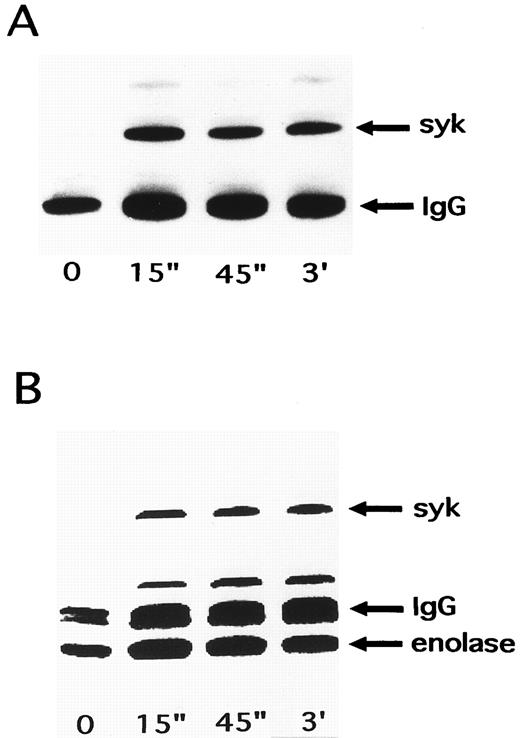
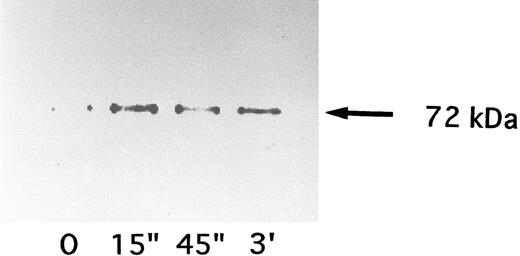
Activation of p72syk by NNKY5-5.Since these findings suggested the involvement of at least one tyrosine kinase in NNKY5-5-induced platelet activation, the effects of NNKY5-5 were investigated on two tyrosine kinases (p60c-src and p72syk) that were reported to show an increase of activity during platelet activation induced by other agonists.26,36 p72syk was rapidly activated by optimal concentrations of thrombin, with its activity increasing approximately 10-fold. p60c-src is known to be activated with a slower time course and lower magnitude.26 p60c-src showed no change of activity in response to NNKY5-5 (data not shown). In contrast, NNKY5-5 increased the autophosphorylation of p72syk from 15 seconds after stimulation (Fig 5A). This increase of p72syk activity was also confirmed by the in vitro kinase assay, which showed a rapid increase in enzyme activity (Fig 5B). In contrast to thrombin activation, the increase of p72syk activity induced by NNKY5-5 was of a lower magnitude, being 2.2-fold to fivefold. The band just above IgG has not been identified, but may be a protein that associates with p72syk after platelet activation induced by NNKY5-5. Activation of p72syk by NNKY5-5 was found to be independent of the extracellular Ca2+ concentration or platelet aggregation. p72syk activation was also caused by Fab′ fragments of NNKY5-5 (Fig 6).
Time course of changes in p72syk activity induced by NNKY5-5. Platelets were activated with 10 μg/mL NNKY5-5 and the reaction was terminated by adding lysis buffer after the indicated periods. Platelet proteins associated with p72syk were immunoprecipitated with an anti-p72syk antibody. The sample was then split into portions for an in vitro tyrosine kinase assay and for Western blotting with an anti-phosphotyrosine antibody to detect the level of autophosphorylation of p72syk. (A) Western blot. (B) In vitro kinase assay. The arrowheads represent the bands presumably derived from the heavy chain of the anti-p72syk antibody.
Time course of changes in p72syk activity induced by NNKY5-5. Platelets were activated with 10 μg/mL NNKY5-5 and the reaction was terminated by adding lysis buffer after the indicated periods. Platelet proteins associated with p72syk were immunoprecipitated with an anti-p72syk antibody. The sample was then split into portions for an in vitro tyrosine kinase assay and for Western blotting with an anti-phosphotyrosine antibody to detect the level of autophosphorylation of p72syk. (A) Western blot. (B) In vitro kinase assay. The arrowheads represent the bands presumably derived from the heavy chain of the anti-p72syk antibody.
Time course of changes in p72syk activity induced by Fab′ fragments of NNKY5-5. Platelets were activated with 10 μg/mL of the antibody and the reaction was terminated by adding lysis buffer after the indicated periods. The p72syk was immunoprecipitated and then the reaction was initiated by the addition of 32P-ATP.
Time course of changes in p72syk activity induced by Fab′ fragments of NNKY5-5. Platelets were activated with 10 μg/mL of the antibody and the reaction was terminated by adding lysis buffer after the indicated periods. The p72syk was immunoprecipitated and then the reaction was initiated by the addition of 32P-ATP.
DISCUSSION
A number of MoAbs directed against GPIb inhibit ristocetin-dependent binding of vWF to platelets and ristocetin-induced platelet agglutination,29,30,37 confirming that vWF interacts with GPIb. Some of these antibodies also inhibit collagen-induced30,37 or thrombin-induced38 aggregation, leading to the suggestion that GPIb also acts as a receptor for collagen and thrombin. In the present study, we showed that NNKY5-5, an anti-GPIb MoAb, not only completely inhibited ristocetin-induced agglutination but also induced the formation of small aggregates in citrate anticoagulated PRP. We used the term SAF for this formation of small aggregates, since it is currently unclear whether this phenomenon is the same as ordinary platelet aggregation. To our knowledge, this is the first report of an anti-GPIb MoAb with platelet-activating properties. For example, Kelton and Horsewood39 observed no activation when platelets were stimulated with various MoAbs directed against the GPIb-IX complex. We also evaluated the effects of three other anti-GPIb MoAbs, and found that none of them had an action similar to NNKY5-5.
SAF was slight, amounting to a 10% to 15% change in light transmission at most. A new platelet aggregometry, based on particle counting using light scatter, showed that SAF consisted of small aggregates formed by less than 100 cells. No SAF was observed when platelets were treated with prostaglandin I2 , suggesting that the platelet response to NNKY5-5 represented aggregation and not agglutination. However, SAF was greatly reduced when hirudin was used as the anticoagulant instead of citrate, suggesting that NNKY5-5 could not elicit this response in the presence of physiological levels of Ca2+. Washed platelets also showed a minimal response to NNKY5-5. Thus, NNKY5-5–induced SAF resembled that induced by epinephrine in several respects. Epinephrine is considered to be a potentiator of platelet activation but not a true aggregating agent, since it does not produce aggregation in the presence of a physiological concentration of Ca2+.32 At low extracellular Ca2+ concentrations, however, platelets become hypersensitive to epinephrine and form aggregates through a presently unidentified mechanism. Thus, we assessed whether NNKY5-5 acted as a potentiator of platelet aggregation induced by other agonists. NNKY5-5 increased the number of aggregates and the duration of aggregation induced by low concentrations of ADP, PAF, and U-46619 (a thromboxane A2 agonist), suggesting for the potentiating effect of NNKY5-5.
The majority of antiplatelet antibodies that cause platelet activation are FcγRII-dependent. However, IV.3, an anti-FcγRII MoAb that inhibits Fc receptor-dependent platelet activation, had no effect on SAF induced by NNKY5-5, suggesting that NNKY5-5 induced platelet activation is independent of FcγRII. We were not able to produce F(ab′)2 fragments of NNKY5-5, probably because it is an IgG2b antibody. Although the Fab′ fragment of NNKY5-5 failed to induce SAF, it potentiated platelet aggregation induced by other agonists. These findings suggest that binding of the Fab′ fragment of NNKY5-5 to GPIb elicited certain intracellular signals that lead to the potentiating effect. In addition, cross-linking the receptor-bound Fab′ fragment of NNKY5-5 with a secondary antibody induced SAF. Thus, it is likely that NNKY5-5 activates platelets by binding to the α chain of GPIb, independent of FcγRII involvement.
Which intracellular signal transduction pathway is responsible for NNKY5-5–induced platelet potentiation? NNKY5-5 induced neither granule secretion nor [Ca2+]i elevation. However, tyrosine phosphorylation of a 64 kD protein consistently occurred after stimulation with NNKY5-5 and the intensity of the 64-kD band showed a concentration- and time-dependent increase. In contrast, other tyrosine phosphorylated proteins, which appear with platelet activation by thrombin or collagen,2-4 were not observed. The F(ab′)2 fragments of P256, an anti-GPIIb/IIIa MoAb, are reported to cause tyrosine phosphorylation of a 64-kD protein, even when fibrinogen binding does not cause GPIIb/IIIa activation.40 P256 induces weak platelet aggregation, with no activation of phospholipase C, increase in [Ca2+]i, or release of granules.41 42 These characteristics are quite similar to those of NNKY5-5–induced platelet activation, suggesting that they share a common mechanism of platelet activation. To further characterize the mechanism, the effects of various inhibitors were assessed on platelet aggregation and tyrosine phosphorylation of the 64-kD protein induced by NNKY5-5. Aspirin and H-7 had no inhibitory effect on aggregation and protein phosphorylation induced by NNKY5-5, suggesting that platelet activation was independent of both the cyclooxygenase pathway and protein kinase C activation. GRGDS (a peptide which blocks fibrinogen binding to GPIIb/IIIa) at a concentration of 400 μmol/L and EGTA at 1 mmol/L inhibited NNKY5-5–induced SAF, although phosphorylation of the 64-kD protein was still observed, suggesting that it was not a consequence of the binding of fibrinogen to GPIIb/IIIa. In contrast, tyrphostin and herbimycin (protein tyrosine kinase inhibitors) potently suppressed both phosphorylation of the 64-kD protein and platelet aggregation. Taken together, these findings suggest that the binding of NNKY5-5 to GPIb induced tyrosine phosphorylation of the 64-kD protein catalyzed by a tyrosine kinase, and that the phosphorylated protein promoted the activation of GPIIb/IIIa with resultant SAF.
There is increasing evidence for an important role of tyrosine kinases in the regulation of platelet function.43 Although it is not yet clear which tyrosine kinase is responsible for phosphorylating particular proteins, three of these kinases (p72syk, p60c-src, and p125FAK) show a change in activity with platelet stimulation. The activity of p72syk rapidly increases by 10-fold with thrombin activation of platelets, reaching a maximum at 10 seconds.36 Platelet activation also elevates p60c-src activity, albeit to a lesser extent and with a slower time course.35 In contrast, p125FAK is only activated after the interaction between GPIIb/IIIa and fibrinogen has occurred.44 These findings suggest that tyrosine kinases are actively engaged in the regulation of platelet function from the initial phase of activation to the late stage of aggregation. In the present study, we found that NNKY5-5 elicited a rapid increase in the activity of p72syk but not p60c-src, whereas p125FAK would not be responsible for phosphorylation of the 64-kD protein because it is only activated after platelet aggregation. The Fab′ fragment of NNKY5-5, which itself did not cause platelet aggregation but potentiated aggregation induced by various agonists, also activated p72syk. Thus, it is possible that activation of p72syk is involved in the generation of an intracellular activation signal and tyrosine phosphorylation of the 64-kD protein during NNKY5-5–induced platelet activation, although we have no direct evidence to support this hypothesis.
In the present study, we found that NNKY5-5 induced platelet aggregates of small size in citrated-PRP. In the presence of vWF, botrocetin at concentrations below 0.5 μg/mL also induced the formation of small aggregates (data not shown) similar to those induced by NNKY5-5. The persistent formation of small aggregates is not unique to platelet activation mediated by GPIb, since it is also observed with epinephrine-induced aggregation in the presence of inhibitors of thromboxane A2 production or cGMP-elevating agents.45 However, unlike epinephrine, NNKY5-5 did not promote the formation of large platelet aggregates. Several factors appear to influence MoAb-mediated platelet activation, such the number and density of the platelet epitopes and the affinity of the antibody for its epitope.46-50 The mobility of glycoproteins within the platelet membrane51 is also believed to be important. There are about 25,000 copies of GPIb-IX per platelet.39 However, GPIb binds to a submembranous cytoskeleton composed of short actin filaments and actin-binding protein,52 which may reduces its mobility, and poor mobility of GPIb-IX may have contributed to the weak activation induced by NNKY5-5 in the present study.
GRGDS and NNKY1-32, an anti-GPIIb/IIIa MoAb which potently blocks the interaction between GPIIb/IIIa and fibrinogen,31 inhibited SAF induced by NNKY5-5, suggesting that activation of GPIIb/IIIa plays an essential role in this process. Taken together, these findings suggest that the binding of NNKY5-5 to GPIb initiates intracellular signals that activate GPIIb/IIIa. There is increasing evidence that activation of GPIb is linked to GPIIb/IIIa activation and subsequent fibrinogen binding. For example, the interaction of asialo vWF with GPIb induces the binding of fibrinogen to GPIIb/IIIa.53 Bothrombin (purified from snake venom) interacts with GPIb but not with the thrombin receptor, and induces platelet aggregation by activating GPIIb/IIIa.54 Similar to NNKY5-5, it induces neither [Ca2+]i elevation nor granule release. Whether p72syk activation is involved in these processes, as with NNKY5-5–induced platelet activation, remains to be determined. It is also of interest that aurin tricarboxylic acid, which interferes with the interaction between GPIb and vWF, potentiates platelet aggregation induced by other agonists.55 This finding along with those of NNKY5-5 suggest that inhibitors of the interaction between vWF and GPIb can also be partial activators of platelets. Thus, NNKY5-5 appears to provide a useful tool for investigating the mechanism of GPIb-dependent platelet activation. Analysis of the epitope on the α chain of GPIb recognized by NNKY5-5 is now underway.
In conclusion, our findings suggest that the binding of NNKY5-5 to GPIb activates p72syk with subsequent phosphorylation of a 64-kD protein and that the intracellular activation signal mediated by tyrosine phosphorylation leads to the potentiation of platelet function.
ACKNOWLEDGMENT
The authors thank Dr Kojiro Yasunaga (Ooi Clinic, Kansai Electric Co, Fukui, Japan) for his helpful suggestions, Dr Kenjiro Tanoue for his kind donation of TM-60 (an anti-GPIb MoAb), and Dr Makoto Handa for his kind donation of WGA-III (an anti-GPIb MoAb).
Supported in part by a Research Grant for Advanced Medical Care from the Ministry of Health and Welfare of Japan.
Address reprint requests to Shosaku Nomura, MD, The First Department of Internal Medicine, Kansai Medical University, 10-15 Fumizono-cho, Moriguchi Osaka 570, Japan.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal