Abstract
The human promonocytic cell line U937 is highly sensitive to the lytic effect of the autonomous parvovirus H-1. Rare cell variants that resisted H-1 virus infection could be isolated, of which four (RU1, RU2, RU3, and RU4) were further characterized. In contrast to parental cells, the RU clones sustained an abortive H-1 virus infection. Three of the clones showed a significant decrease in the accumulation levels of the c-Myc oncoprotein and in their capacity for forming tumors in immunodeficient mice. Surprisingly, all RU clones resisted the suppressing effect of 12-O-tetradecanoylphorbol-13-acetate (TPA) on c-myc oncogene expression and cell proliferation. In contrast, RU clones exhibited the TPA-induced changes in membrane surface antigens and nonspecific esterase activities that are characteristic of monocytic differentiation. Studies of the activation steady-state of RU cells demonstrated the constitutive production of significant amounts of nitric oxide (NO) and superoxide anion (O−2⋅ ). Inhibitors of NO and O−2⋅ . production sensitized all RU cells to the killing effect of parvovirus H-1 and increased the production of infectious viral particles. These data argue for the participation of active oxygen species in macrophage defence mechanisms against parvovirus infection. Moreover, the use of parvovirus H-1 as a selective agent in a cell-colony formation assay allowed us to show that expression of defined markers of monocytic differentiation can be uncoupled from suppression of proliferation.
PARVOVIRIDAE IS a large family of small animal viruses that have been isolated from many species, from insects to humans.1-3 The H-1 virus used in the present study belongs to the subgroup of autonomously replicating parvoviruses of vertebrates and contains a single-stranded, linear DNA genome of about 5 kb.4-8 The parvovirus life cycle depends on cellular factors that are expressed as a function of cell proliferation and differentiation.6 In contrast to DNA tumor viruses, parvoviruses are unable to force quiescent infected cells into the S phase of the cell cycle. Cancer cells can be assumed to provide these viruses with an environment beneficial to their multiplication. Indeed, several parvoviruses appear to be oncotropic.9,10 In agreement with this, our previous studies have shown that in vitro transformation of various cell lines correlated with their sensitization to parvovirus-induced killing and with an increased capacity to sustain certain steps of the viral life-cycle as compared to their untransformed counterparts.11 In particular, the production and the toxic activity of the nonstructural protein NS1, a key effector of parvovirus replication and cytopathogenicity, is stimulated in oncogene-transformed cells.12-14 This modulation may account, at least in part, for the fact that parvoviruses are not only devoid of oncogenic activity, but can even exert an oncosuppressive effect in vivo.9 The mechanism of this anticancer surveillance is poorly understood at present. To unravel the pathways of tumor suppression at the molecular level, H-1 virus was used for isolating from the human K562 erythroleukemic cell line rare variants (designated KS) that resisted virus infection.15 These variants were distinguishable from the parental cells not only by their insensitivity to the cytopathic effect of H-1 virus, but also by a much reduced malignant phenotype correlating with the reactivation of wild-type (wt) p53 tumor suppressor gene expression. Furthermore, transformed rat embryo fibroblasts producing high levels of a p53 mutant protein, able to oligomerize and inactivate endogenous wt p53, were shown to be sensitized to H-1 virus infection.15 Therefore, a close interconnection appears to exist between the commitment of cells to neoplastic transformation and their susceptibility to parvovirus infection.
The striking host cell specificity of parvoviruses prompted us to investigate the interaction of H-1 virus with the myeloid leukemia cell line U937 derived from a human histiocytic lymphoma.16 Like K562 and the human promyelocytic HL-60 cell line, U937 cells do not express detectable levels of p53.17,18 Myc, known to be involved in the regulation of cell proliferation, is overexpressed in these cells.19 Monocytic differentiation of U937 cells can be induced in vitro by treating cultures with several agents and in particular with the tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA).20,21 This differentiation is accompanied with a rapid decrease of c-myc expression and the arrest of cell proliferation.19,22,23 Recent observations point to the fact that vitamin D3 may induce U937 cells to differentiate through the transcriptional activation of the cyclin-dependent kinase inhibitor protein p21 (WAF1/CIP1) production in the absence of p53.24 In addition, another key player in the regulation of the cell cycle is the retinoblastoma tumor suppressor gene product RB, which is present in U937 cells and may block proliferation by inhibiting the activity of RNA polymerase I transcription factor UBF.25 Moreover, recent evidence indicates that RB regulates the activity of the proto-oncogene c-myc.19,22,23 26 It is worth noting that, though normally associated with proliferation arrest, cell commitment to differentiation can be uncoupled from this process.23,27 Indeed, a variant of the chronic myelogenous leukemia cell line RWLeu-4 has recently been reported to be resistant to the antiproliferative effects of 1-α-25(OH)2-vitamin D3, while exhibiting a pattern of differentiation-specific antigens similar to that of the parental line after commitment to maturation in the presence of this agent.27
As stated above, parvoviruses have specific requirements for proliferating and usually poorly differentiated host cells. The human promonocytic leukemia cell line U937 fulfills these conditions and is very sensitive to H-1 virus infection. It was further reasoned that the uncoupling of cell growth and differentiation could be investigated by determining whether proliferating but H-1 virus-resistant U937 variant subclones could be isolated and, should that be the case, by characterizing their phenotypic traits. A marker of particular interest consisted of the p53 protein, given the key role of this protein in cell proliferation and differentiation28,29 and its reactivation in H-1 virus-resistant KS variants of human erythroleukemic cells.15 Our results show that growing U937 subclones that resist H-1 virus infection (designated RU) can indeed be found. The RU subclones analyzed differ from KS cells by the lack of a detectable change in p53 status compared with the respective H-1 virus-sensitive parental lines, pointing to the fact that multiple pathways may lead to parvovirus resistance. RU cells exhibit a unique activated phenotype including the constitutive production of active oxygen species. Altogether, these data uncover a novel mechanism by which cells may become resistant to parvovirus infection, and identify specific monocytic differentiation traits that can be uncoupled from cell proliferation arrest.
MATERIALS AND METHODS
Cells and reagents.The human promonocytic cell line U93716 and its various subclones were cultured in RPMI 1640 medium (Life Technologies, Eggenstein, Germany) supplemented with 10% heat-inactivated fetal calf serum (FCS) in a 5% CO2 atmosphere at 37°C. The simian virus 40–transformed human kidney cell line NB-E30 was cultured in minimal essential medium supplemented with 5% FCS. Exponentially growing cultures were used for all experiments. Treatment of cells with TPA and 4-hydroxypyrazolo(3,4-d)pyrimidine (Allopurinol; Sigma, Deisenhofen, Germany) was performed at a final concentration of 50 ng/mL and 3 mmol/L, respectively. Human superoxide dismutase (SOD; Sigma) was added to a final concentration of 500 IU/mL. NG-monomethyl-L-arginine (L-NMA; Calbiochem-Navabiochem GmbH, Badsoden, Germany) was used at a concentration of 0.5 mmol/L. Enhanced chemiluminescence (ECL) and α-naphthyl acetate esterase (NAE) kits were purchased from Amersham Life Science (Buckinghamshire, UK) and Sigma Diagnostics (Sigma), respectively. The protein assay kit was obtained from Bio-Rad Laboratories GmbH (Munich, Germany).
Virus production and cell infection.H-1 parvovirus was propagated in NB-E cells and purified as described by Chen et al.31 H-1 virus inoculation and titration by plaque-assay were performed according to published methods.31 The multiplicity of infection (MOI) is given by the number of plaque-forming units (pfu) inoculated per cell. Radiolabeled virus was obtained by incubating NB-E cells for 24 hours with either [35S]methionine (1,025 Ci/mmol; 50 μCi/mL; Amersham) or [3H]thymidine (5.0 Ci/mmol; 5 mCi/mL; Amersham) 15 hours after H-1 virus infection (1 pfu/cell).
Selection of U937 cell variants resisting H-1 parvovirus infection.Selection of H-1 virus-resistant U937 variants was performed essentially as described for K562 cells.15 Briefly, eight different U937 cultures, each derived from a single clone obtained by limiting dilution of the U937 parental line, were infected with H-1 virus at an MOI of 100 pfu/cell (Fig 1) and further incubated in culture medium without removing the inoculum. The cytopathic effect of the virus led to a massive cell death, with a reduction of the number of living cells to less than 0.1% of mock-treated cells after 2 days. Resistant subclones were isolated by dilution of infected cultures in flat-bottom 96-well plates (Nunc, Roskilde, Denmark) and further incubation for another 3 weeks. This infection and selection procedure was repeated twice under the same conditions, leading to the isolation of U937 cell variants that were stably resistant to parvovirus H-1. Four such variants (designated RU1, RU2, RU3, and RU4, and each derived from a different subclone of the original U937 cell line) were chosen for further analysis.
Selection of RU clones from the U937 cell line. Starting with 106 U937 cells, three successive rounds of infection with parvovirus H-1 were performed at a MOI of 100 pfu/cell. Cell viability was determined microscopically 4 days after infection, using the Trypan blue dye (0.2%) exclusion procedure. Only subclones displaying a survival to H-1 virus infection higher than 50% were considered. Four clones (RU1-4) whose resistance to H-1 infection was above 90% were selected.
Selection of RU clones from the U937 cell line. Starting with 106 U937 cells, three successive rounds of infection with parvovirus H-1 were performed at a MOI of 100 pfu/cell. Cell viability was determined microscopically 4 days after infection, using the Trypan blue dye (0.2%) exclusion procedure. Only subclones displaying a survival to H-1 virus infection higher than 50% were considered. Four clones (RU1-4) whose resistance to H-1 infection was above 90% were selected.
Viral DNA and RNA extraction.Low-molecular-weight DNA was selectively extracted from cells 40 hours after infection, using a modified Hirt procedure.32 Briefly, recovered cells were washed with phosphate-buffered saline (PBS), resuspended in lysis buffer (0.6% sodium dodecyl sulfate [SDS], 10 mmol/L Tris-HCl [pH 7.5], 0.1 mmol/L EDTA), and incubated for 3 hours at 37°C with proteinase K at 100 μg/mL. NaCl was then added to a final 1 mol/L concentration and the extract was incubated on ice overnight. After pelleting the precipitated high-molecular-weight DNA by centrifugation, the supernatant was recovered, treated first with RNAse A at 100 μg/mL, and then with proteinase K at 100 μg/mL (37°C, 30 minutes), extracted twice with phenol/chloroform and chloroform, ethanol-precipitated, and redissolved in water.
Total cellular RNA was isolated 48 hours postinfection using the modified guanidium isothiocyanate method described by Chomczynski and Sacchi.33 Briefly, 2 × 106 cells were collected and resuspended in 0.5 mL of 4 mol/L guanidium thiocyanate, 25 mmol/L sodium citrate (pH 7), 0.5% sarcosyl, and 0.1 mol/L 2-mercaptoethanol. After adding 0.5 mL water-saturated phenol and 0.1 mL chloroform, the sample was mixed and further incubated on ice for 20 minutes. The aqueous phase containing RNA was separated by centrifugation, supplemented with an equal volume of isopropanol, and precipitated at −20°C. After centrifugation, the RNA pellet was redissolved in formamide at 50°C for 15 minutes and kept at −70°C.
Southern and Northern blotting.Purified DNA and RNA were fractionated by electrophoresis on 0.8% agarose and 1% agarose/formaldehyde gels, respectively. After transfer under high salt conditions to a Hybond-N+ nylon filter (Amersham), samples were hybridized overnight at 42°C with a specific randomly primed 32P-labeled H-1 virus DNA probe in the presence of 50% formamide and 5% dextran sulfate. Membranes were then washed under highly stringent conditions and autoradiographed.
Immunoblot analysis.Cultures (5 × 106 cells) were grown in the absence or presence of TPA (50 ng/mL) for 36 hours, washed twice in ice-cold PBS, and incubated for 30 minutes on ice with RIPA buffer (10 mmol/L Tris-HCl [pH 7.5], 150 mmol/L NaCl, 1 mmol/L EDTA, 1% Nonidet P-40 [Boehringer Mannheim, Mannheim, Germany], 0.5% sodium deoxycholate, and 0.5% SDS) containing 10 μg/mL aprotinin and 10 μg/mL phenylmethylsulfonyl fluoride. Further cell disruption and homogenization was accomplished by forcing the extract through a 21-gauge needle and sonication on ice. After determining the protein concentration (Bio-Rad assay), the sample was diluted with an equal volume of 100 mmol/L Tris-HCl (pH 6.8), 5% SDS, 10% 2-mercaptoethanol, 20% glycerol, and 0.1% bromophenol blue. Approximately 50 μg of protein were subjected to 8% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and then electrotransferred onto an Immobilon-P membrane (Millipore Corp, Bedford, MA), using standard protocols. Nonspecific binding sites were blocked by incubating blots for 2 hours in PBS containing 10% Blotto powdered milk and 0.05% Tween-20 (Sigma). Blots were further incubated with indicated antibodies and revealed using the ECL kit and horseradish peroxidase–conjugated second antibody from Amersham. Antibodies specific for c-Myc (clone 9E10), RB (Ab-3), and p53 (Ab-3) were purchased from Dianova (Oncogene Science, Hamburg, Germany). Antibodies directed against c-Jun (D), c-Fos (2G9C3), and p21 (L-17) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Immunoprecipitation.Exponentially growing cultures, infected or not with H-1 virus (5 pfu/cell), were incubated for 2 hours at 37°C in methionine-free medium supplemented with [35S]L-methionine (1,055 Ci/mmol, 0.2 mCi/mL; Amersham). Cells were lysed in RIPA buffer and the suspension was sonicated. Samples of total proteins (107 cpm) were immunoprecipitated with rabbit antiserums directed against parvoviral nonstructural (αNS1) or capsid (αVP) proteins, using Protein G-plus/Protein A-agarose as recommended by the manufacturer (Oncogene Science). Immunoprecipitated proteins were analyzed by 8% SDS-PAGE and revealed by fluorography after soaking the gels for 30 minutes in Amplify (Amersham).
Proliferation assay.The proliferation capacity of parental U937 cells and RU clones was assayed by incorporation of labeled thymidine. Exponentially growing cultures in flat-bottom 96-well plates (104 cells per well in 100 μL of RPMI containing 10% FCS with or without 50 ng/mL of TPA) were supplemented with 1 μCi of [methyl-3H]thymidine (79 Ci/mmol; Amersham) for 4 hours. Cells from triplicate samples were collected by means of a Cell Harvester (Inotech, Dottikon, Switzerland), and incorporated radioactivity was measured in a liquid scintillation counter.
Flow cytometric analysis.Surface markers of U937 and RU clones were analyzed by indirect immunofluorescence as described by López-Guerrero et al.34 Briefly, cells were incubated for 30 minutes at 4°C with appropriate monoclonal antibodies (MoAbs) in PBS containing 0.1% bovine serum albumin (BSA) and 0.04% sodium azide (PBS-BSA). MoAbs directed to the cell-surface antigens CD71 (T1/6), CD11b (Bear 1), CD11c (HC1/1), CD14 (Bear 2), CD4 (HP2/6), and CD54 (RR1/1) have been described elsewhere.35 36 MoAbs directed to CD11a (TS1/11), CD18 (TS1/18), and CD29 (TS2/16) were a generous gift of Dr F. Sánchez-Madrid (Hospital de la Princesa, Madrid, Spain). MoAb directed to CD31 (HC1/6) was kindly provided by Dr C. Bernabeu (Centro de Investigaciones Biológicas, Madrid, Spain). After two washing steps with PBS-BSA, cells were incubated with fluorescein isothiocyanate-labeled F(ab′)2 rabbit anti-mouse IgG (Dianova GmbH, Hamburg, Germany) for 30 minutes in the dark at 4°C in PBS-BSA. Cells were finally washed three times and analyzed by means of a FACSort cytofluorometer (Becton Dickinson, Erembodegem-Aalst, Belgium). P3x63 myeloma culture supernatant was used as a negative control whose background fluorescence was substracted from that given by the above-mentioned antibodies.
Nonspecific esterase assay.Cultures (106 cells) were incubated for 48 hours with or without 50 ng/mL of TPA, collected, and rinsed twice with PBS. Nonspecific esterase (NAE) production was determined according to the protocols supplied with the reagent kits (Sigma Diagnostics).
Superoxide anion production.The ability of U937 and RU cells to generate O−2⋅ was quantitated as described by Rook et al.37 Briefly, triplicate cultures were seeded in 96-well plates (2 × 106 viable cells in 0.2 mL RPMI/well) and supplemented with an additional 0.2 mL of RPMI containing 2 mg/mL of nitroblue tetrazolium (NBT; Sigma), with or without TPA at a final concentration of 10 μg/mL. After incubation at 37°C in a 5% CO2 atmosphere for 30 minutes, the medium was removed after centrifugation and the cells were washed thoroughly with methanol and allowed to air dry. The total blue formazan precipitated was solubilised by successively adding 0.45 mL of 2 mol/L KOH and 0.55 mL of dimethyl sulfoxide (DMSO; Sigma) to each well. The OD620-450 of 0.2 mL from this solution was determined with an MKII titertek Multiskan PLUS microplate spectrophotometer (Labsystems, Helsinki, Finland), using as a blank a well without cells but incubated with NBT and subjected to the same steps. A standard curve was obtained by dissolving known quantities of NBT in KOH and DMSO, allowing the OD620-450 to be expressed in nanomoles of reduced NBT/well.
NO production.The formation of NO was measured as the sum of nitrite (NO−2 ) and nitrate (NO−3 ) as described by Green et al.38 Cells were washed twice with minimal essential medium and resuspended at 106 cells/mL in the same medium. Aliquots (0.2 mL) of cell suspension were incubated in flat-bottom 96-well culture plates, at 37°C in a 5% CO2 atmosphere for 48 hours. After centrifugation at 400g for 5 minutes, the supernatants were collected. For nitrite determination, 0.1 mL of supernatant was mixed with the same volume of Greiss reagent (1:1 mixture 0.1% naphthyl-ethylenediamine dihydrochloride in distilled water and 1% sulfanilamide in 5% phosphoric acid), incubated in a 96-well microplate for 10 minutes at room temperature and optically read (OD690-550 ) with a spectrophotometer (Labsystems). Nitrate was measured after reduction to nitrite with nitrate reductase (Boehringer Mannheim) in the presence of NADPH.
In vivo tumorigenicity.To measure the in vivo tumorigenicity of U937 and RU cells, scid/scid mice (6 to 7 weeks old) were inoculated subcutaneously in the flank fat pads with cell suspensions. The tumorigenicity was expressed as the number of mice developing tumors within 2 months.
RESULTS
Selection of H-1 virus-resistant U937 variants (RU).The promonocytic cell line U937 is highly sensitive to the cytopathic effect of parvovirus H-1. The fraction of living cells was reduced to less than 0.1% at 4 days after infection with 100 pfu/cell (Fig 2A). To isolate rare virus-resistant cell variants, U937 cultures derived from single clones by limiting dilution were inoculated with 100 H-1 pfu/cell. After infection, a progressive degeneration of the cultures was observed, but maintenance of these cultures for 3 weeks led to the growth of some resistant subclones. These rare resistant variants were expanded and subjected to two more rounds of infection, as described in Materials and Methods. The descendants of subclones that were at least 50% resistant to H-1 virus during the second and third cycles of infection were expanded. Among these, we selected for further analysis four lines that were more than 90% resistant to H-1 virus, each derived from a different original U937 subclone, and designated as RU1, RU2, RU3, and RU4 (Figs 1 and 2A). RU cells were found to be stably resistant to H-1 virus infection over a period of 36 months. The U937 origin of RU clones was ascertained by DNA fingerprinting upon enzymatic digestion with the restriction enzyme Hinf I and subsequent hybridization with a multilocus C probe (data not shown). Limiting dilution assays showed a very low level of persistent infection in RU1, RU3, and RU4 clones shortly after the selection procedure. Yet, this carrier state was transient and infectious virus could not longer be detected by plaque assay in RU culture supernatants after a few months. The RU cells involved in the present study and analyzed more than 8 months after selection were free of any detectable viral imprint, as determined by polymerase chain reaction (PCR) using primers specific for NS1 and VP DNA sequences. No viral DNA species could be amplified from 1 μg of total DNA (1.5 × 105 cells) under conditions allowing the detection of 1 pg of viral DNA, indicating that RU cultures reproducibly contained less than 1 viral genome per cell.
Analysis of viral parameters in parental U937 and resistant RU cells after H-1 virus infection. (A) The survival of U937 and RU cells was determined by counting viable cells 4 days after infection with H-1 virus (MOI = 100 pfu/cell), using the Trypan blue exclusion method, and expressed as a percentage of the value measured in mock-infected cultures. Results from a typical experiment are shown. (B) Infectious particles recovered 48 hours after inoculation of 106 cells with H-1 virus (5 pfu/cell) were measured by plaque-assay. Data shown are from a typical experiment. Similar relative values were obtained in three independent experiments (SD < 22%). (C) The virus uptake was assayed after infection with [3H]thymidine-labeled H-1 virus (2 × 103 pfu [105 cpm]/106 cells), followed by a washing step with 1 mmol/L EDTA to remove noninternalized particles (upper part). Internalized [35S]methionine-labeled capsids were revealed by autoradiography after immunoprecipitation and SDS-PAGE analysis of VP1 and VP2 proteins extracted 2 hours after infection with H-1 virus (4 × 106 pfu [106 cpm]/106 cell) and washing with EDTA (lower part). (D) Amplification of viral DNA was determined by Southern blotting. After virus (10 pfu/cell) or mock infection, cultures were incubated for 40 hours in complete medium. DNA samples extracted by the Hirt procedure were analyzed by agarose gel electrophoresis, blotted, and hybridized with a virus-specific 32P-labeled DNA probe. RFI and RFII refer to viral DNA replicative forms of monomer and dimer length, respectively. Viral DNA species were positioned relative to HindIII-digested phage λ DNA used as size markers and revealed by ethidium bromide staining. (E) The viral transcripts were detected by Northern blotting. At 48 hours postinfection (10 pfu/cell), total RNA was isolated by the guanidinium thiocyanate method, fractionated by agarose/formaldehyde gel electrophoresis, blotted, and hybridized with a virus-specific 32P-labeled DNA probe. (F ) The accumulation of viral proteins was determined by immunoprecipitation. At 36 hours postinfection (5 pfu/cell) cultures were incubated for 2 hours in the presence of [35S]methionine and processed for immunoprecipitation with antiserums directed against the viral products NS-1 or VP-1 and VP-2, SDS-PAGE, and autoradiography.
Analysis of viral parameters in parental U937 and resistant RU cells after H-1 virus infection. (A) The survival of U937 and RU cells was determined by counting viable cells 4 days after infection with H-1 virus (MOI = 100 pfu/cell), using the Trypan blue exclusion method, and expressed as a percentage of the value measured in mock-infected cultures. Results from a typical experiment are shown. (B) Infectious particles recovered 48 hours after inoculation of 106 cells with H-1 virus (5 pfu/cell) were measured by plaque-assay. Data shown are from a typical experiment. Similar relative values were obtained in three independent experiments (SD < 22%). (C) The virus uptake was assayed after infection with [3H]thymidine-labeled H-1 virus (2 × 103 pfu [105 cpm]/106 cells), followed by a washing step with 1 mmol/L EDTA to remove noninternalized particles (upper part). Internalized [35S]methionine-labeled capsids were revealed by autoradiography after immunoprecipitation and SDS-PAGE analysis of VP1 and VP2 proteins extracted 2 hours after infection with H-1 virus (4 × 106 pfu [106 cpm]/106 cell) and washing with EDTA (lower part). (D) Amplification of viral DNA was determined by Southern blotting. After virus (10 pfu/cell) or mock infection, cultures were incubated for 40 hours in complete medium. DNA samples extracted by the Hirt procedure were analyzed by agarose gel electrophoresis, blotted, and hybridized with a virus-specific 32P-labeled DNA probe. RFI and RFII refer to viral DNA replicative forms of monomer and dimer length, respectively. Viral DNA species were positioned relative to HindIII-digested phage λ DNA used as size markers and revealed by ethidium bromide staining. (E) The viral transcripts were detected by Northern blotting. At 48 hours postinfection (10 pfu/cell), total RNA was isolated by the guanidinium thiocyanate method, fractionated by agarose/formaldehyde gel electrophoresis, blotted, and hybridized with a virus-specific 32P-labeled DNA probe. (F ) The accumulation of viral proteins was determined by immunoprecipitation. At 36 hours postinfection (5 pfu/cell) cultures were incubated for 2 hours in the presence of [35S]methionine and processed for immunoprecipitation with antiserums directed against the viral products NS-1 or VP-1 and VP-2, SDS-PAGE, and autoradiography.
Life-cycle of H-1 virus in RU cells.Consistent with their selection procedure, RU clones were highly resistant to H-1 virus, with 90% to 98% of the cells surviving high-multiplicity infection compared with less than 0.1% for the parental U937 line (Fig 2A). Infection of U937 cells was productive, leading to the release of a burst of progeny particles, the size of which was close to 100 pfu/cell (Fig 2B). In contrast, little infectious virus could be recovered from similarly infected RU cultures (<1 pfu/cell). This residual infectivity was in line with the decay of input virions (data not shown), arguing for the failure of RU clones to sustain a productive H-1 virus infection. To define the restriction to H-1 virus replication in this system, RU and parental U937 cells were compared for their ability to support various steps of the viral life cycle.
Virus uptake was determined after cell incubation with [3H]thymidine-labeled particles (MOI: 2 × 103 pfu [105 cpm]/106 cells) for 1 hour. Infected cultures were washed with the chelating agent EDTA (1 mmol/L) to release noninternalized virus before the measurement of cell-associated radioactivity. As shown in Fig 2B (upper part), labeled virus was taken up by U937 cells and all RU clones. Yet, the cell lines differed in the yields of internalized particles, which were significantly reduced in RU3 and RU4 clones compared with parental U937, RU1, and RU2 cells. This difference was confirmed by infecting cells with [35S] methionine-labeled particles (MOI: 4 × 106 pfu [106 cpm]/106 cells) and visualizing input viral capsid proteins by autoradiography after immunoprecipitation with a specific antiserum and SDS-PAGE (Fig 2B, lower part). RU3 and RU4 clones could again be distinguished by their lower virus content, which may be due to the reduced uptake or the increased intracellular degradation of incoming virions. Altogether, these results indicate that the insensitivity of RU cells to H-1 virus cannot be ascribed to the lack of functional receptors for virion adsorption, although a block at the entry level may still take some part in the resistance of RU3 and RU4 clones to parvovirus infection.
Subsequent events of the parvoviral life-cycle were then analyzed. Formation and amplification of viral DNA replicative forms of monomer (RFI) and dimer (RFII) length could be detected by Southern blotting in infected RU clones at a level that was similar (RU2, RU4), slightly lower (RU3), or significantly reduced (RU1) compared with U937 cells (Fig 2D). Therefore, the above-mentioned restriction to virus entry in RU3 and RU4 cells did not appear to constitute a major limiting factor for the accumulation of viral DNA replicative intermediates. It is noteworthy that viral SS DNA was hardly detectable in Southern blots, even for the permissive U937 cells. This may be due to the poor efficiency of the Hirt extraction procedure in denaturing progeny viral particles and to the imbalance between RF and SS DNA synthesis as previously reported for other transformed cell lines.11 Northern blotting experiments showed that RU cells were also able to produce the viral transcripts R1-3 in amounts parallel to the respective viral DNA contents of the clones (Fig 2F ). It should be stated that at equivalent viral DNA levels, the four RU clones had a lower capacity for viral mRNA accumulation than parental U937 cells.
Interestingly, an even more pronounced impairment of RU versus U937 cells was also observed with regard to the production of viral nonstructural (NS) and capsid (VP) proteins as measured by immunoprecipitation (Fig 2F ). Altogether, these results argue for at least two (post)transcriptional barriers limiting parvovirus gene expression in infected RU cells. This limitation was especially striking for the NS1 polypeptide that was hardly detectable in RU clones, except for RU2. The residual NS1 and VP contents of RU cultures may be ascribed to the fact that all cells from a given clone produced the viral proteins in small amounts, or that a minority of cells accumulated a normal level of these proteins while the majority was deficient in this respect. To discriminate between these possibilities, U937 and RU cultures were infected with H-1 virus (MOI = 10 pfu/cell), incubated for 24 hours, and examined after immunofluorescence staining with antibodies directed against NS1 or VP proteins. RU cultures comprised 5% (RU1, RU3, and RU4) to 15% (RU2) of cells that gave a positive signal (data not shown). The intensity of this signal was comparable to the fluorescence exhibited by most infected U937 cells, arguing for an all-or-nothing type of inhibition of parvovirus gene expression in RU cells. The reduction of the fraction of RU cells able to accumulate NS1 most likely contributed to the above-mentioned resistance of RU clones to H-1 virus-induced killing, knowing that a cytotoxic function was assigned to this viral product.39
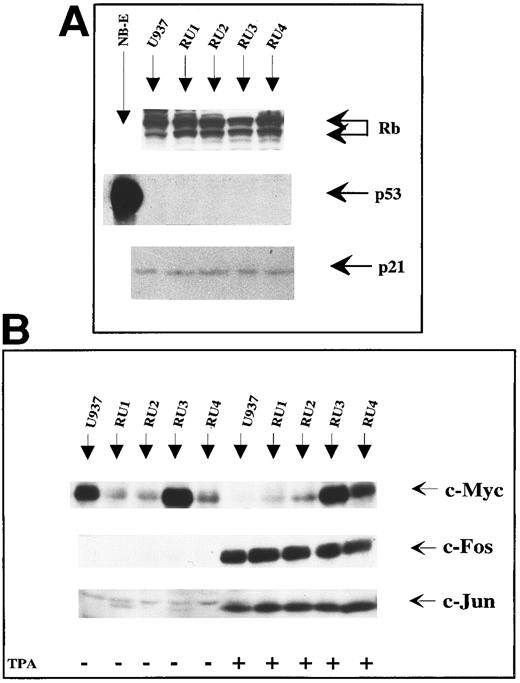
Proliferation and differentiation states of RU cells.A previous study concerning the human erythroleukemia cell line K562 showed that derivatives selected for their resistance to parvovirus H-1, named KS, exhibited reactivation of wt p53 expression.15 This may contribute to the survival of H-1 virus-infected KS cells. Indeed, the reverse change, ie, the functional inactivation of the p53 tumor suppressor gene product, was found to correlate with the sensitization of rat fibroblasts to the parvovirus.15 Like K562 cells, the U937 line analyzed in the present work failed to produce p53 at a detectable level18 (Fig 3A). This prompted us to determine by Western blotting whether the RU clones were also characterized by changes in the expression of p53, its downstream effector p21, or the other tumor suppressor gene product RB. In contrast with the above-mentioned KS cells, RU clones behaved like parental U937 cells in that they gave no detectable p53 signal in Western blots (Fig 3A). Furthermore, a search for p53 transcripts by reverse transcriptase-PCR using specific primers failed to reveal any reactivation of p53 gene expression in RU cells (data not shown). No significant difference between RU and U937 cells was detected either with regard to p21 and RB steady-state levels. Therefore, the accumulation of these products does not appear to be responsible for the modulation of the sensitivity of U937 and variant cells to H-1 virus. Our results suggest, in particular, that at least some cells may become resistant to the parvovirus independently of p53 expression.
Immunoblot analysis of tumor suppressor gene and oncogene products. (A) Comparison of U937 and RU cells for steady-state levels of the tumor suppressor gene products RB, p53, and p21. After cell lysis in RIPA buffer, 50 μg of total proteins were separated by SDS-PAGE and electrotransferred onto polyvinylidene difluoride membranes. Indicated proteins were detected by means of specific antibodies (1 μg/mL), and revealed by ECL after treatment with horseradish peroxidase-conjugated second antibody. The NB-E cell line was included as control for endogenous p53 protein production. (B) Effect of TPA on the production of c-Myc, c-Fos, and c-Jun oncoproteins in U937 and RU cells. Cultures (107 cells) were grown in the presence (+) or absence (−) of TPA (50 ng/mL) for 36 hours and processed for immunobloting as described in (A), using antibodies specific for the indicated proteins.
Immunoblot analysis of tumor suppressor gene and oncogene products. (A) Comparison of U937 and RU cells for steady-state levels of the tumor suppressor gene products RB, p53, and p21. After cell lysis in RIPA buffer, 50 μg of total proteins were separated by SDS-PAGE and electrotransferred onto polyvinylidene difluoride membranes. Indicated proteins were detected by means of specific antibodies (1 μg/mL), and revealed by ECL after treatment with horseradish peroxidase-conjugated second antibody. The NB-E cell line was included as control for endogenous p53 protein production. (B) Effect of TPA on the production of c-Myc, c-Fos, and c-Jun oncoproteins in U937 and RU cells. Cultures (107 cells) were grown in the presence (+) or absence (−) of TPA (50 ng/mL) for 36 hours and processed for immunobloting as described in (A), using antibodies specific for the indicated proteins.
It has previously been reported that sensitivity to parvovirus infection depends on the proliferation and differentiation states of host cells.6 This prompted us to compare U937 and RU cells with regard to these parameters. It is noteworthy that the maturation of U937 cells can be triggered in vitro by various inducers, such as the phorbol ester TPA,40 and is normally accompanied by the arrest of proliferation.19 The colony-formation assay used in the present work to isolate surviving RU clones imposed maintenance of the cell proliferation capacity. Therefore, it was of particular interest to determine whether the concomitant selection for resistance to H-1 virus uncoupled the expression of distinct differentiation traits from cell-cycle arrest. This possibility was tested by comparing U937 and RU cells in the presence and absence of TPA.
The downmodulation of c-myc and upregulation of c-fos and c-jun gene expression are among the earliest events induced by the exposure of U937 cells to TPA41,42 (Fig 3B). In the absence of TPA, c-Myc could be detected by immunoblot analysis in the RU clones, at a similar (RU3) or much reduced (RU1, RU2, and RU4) level compared with parental U937 cells (Fig 3B left-hand part). Knowing the involvement of c-Myc in neoplastic transformation,43 the RU clones were compared between themselves and with the U937 cell line for their tumor-forming ability after implantation into immunodeficient mice. As shown in Table 1, the clones expressing reduced c-Myc levels (RU1, RU2, and RU4) were less tumorigenic than U937 cells, ie, they gave rise to fewer (or even no) tumors that grew at a much slower rate. In contrast, the RU3 clone proved to be proficient in both c-Myc accumulation (Fig 3B, left-hand part) and neoplastic growth (Table 1), arguing for a possible correlation between these features in the U937 cell system.
Tumorigenic Properties of U937 and RU Cell Clones in scid/scid Mice
| Clone* . | Fraction of Animals With Tumors† . | Tumorigenic Capacity (%)‡ . | Delay (d)ρ . | Tumor Section (mm2 )1-155 . |
|---|---|---|---|---|
| U937 | 20/25 | 80 ± 20 | 14 | 68 |
| RU1 | 8/29 | 28 ± 17 | 18 | 14 |
| RU2 | 11/29 | 38 ± 17 | 15 | 6 |
| RU3 | 21/25 | 84 ± 21 | 13 | 107 |
| RU4 | 0/15 | 0 | NR | NR |
| Clone* . | Fraction of Animals With Tumors† . | Tumorigenic Capacity (%)‡ . | Delay (d)ρ . | Tumor Section (mm2 )1-155 . |
|---|---|---|---|---|
| U937 | 20/25 | 80 ± 20 | 14 | 68 |
| RU1 | 8/29 | 28 ± 17 | 18 | 14 |
| RU2 | 11/29 | 38 ± 17 | 15 | 6 |
| RU3 | 21/25 | 84 ± 21 | 13 | 107 |
| RU4 | 0/15 | 0 | NR | NR |
Abbreviation: NR, not relevant.
Ten million cells injected subcutaneously in the flank fat pads.
Number of animals developing a palpable tumor within 2 months over total number of treated animals (pooled data from three experiments).
Percentage of tumor-bearing animals (average value and SD from three experiments).
ρ Average time after which at least half of the tumor appeared in a series.
Product of main diameters.
Interestingly, treatment with TPA for up to 36 hours had little effect on the accumulation of c-Myc in all four RU clones, while this product became undetectable in U937 cells (Fig 3B, right-hand part). On the other hand, TPA induced accumulation of the c-Fos and c-Jun oncoproteins to a similar extent in the U937 and RU cell lines. Given the role of c-Myc in the regulation of cell proliferation,43 44 these results raised the possibility that RU cells may resist the antiproliferative effect of maturation-inducing agents.
This was tested by measuring the incorporation of [3H]thymidine by U937 and RU cells pretreated, or not, with TPA for various times. As shown in Fig 4, similar levels of incorporation were achieved by all cell lines in the absence of phorbol ester. In contrast, the RU clones could be distinguished from U937 cells by their greater resistance to the cytostatic effect of TPA. Although the incorporation of DNA precursors was virtually abolished in U937 cells after 3 days of treatment with TPA, it remained detectable in all RU clones for at least 5 days, confirming the ability of these variants to proliferate under differentiation-prone conditions.
Effect of TPA on the proliferation capacity of U937 and RU cells. U937 or RU cultures (104 cells) were grown in 0.1 mL of complete medium with or without TPA (50 ng/mL). At indicated times, cultures were pulsed for 4 hours with 1 μCi of [3H]thymidine, and incorporated radioactivity was subsequently measured in a liquid scintillation counter (average values and SD bars from three independent experiments, each done in triplicate).
Effect of TPA on the proliferation capacity of U937 and RU cells. U937 or RU cultures (104 cells) were grown in 0.1 mL of complete medium with or without TPA (50 ng/mL). At indicated times, cultures were pulsed for 4 hours with 1 μCi of [3H]thymidine, and incorporated radioactivity was subsequently measured in a liquid scintillation counter (average values and SD bars from three independent experiments, each done in triplicate).
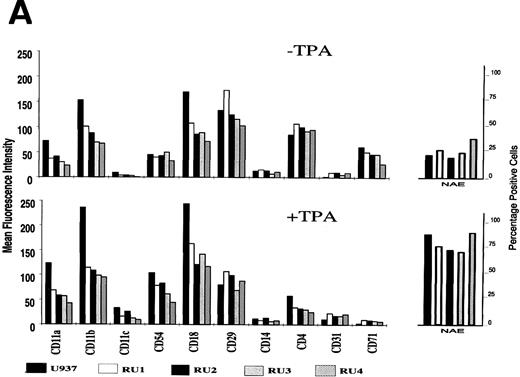
Previous reports have shown that U937 cells committed to differentiate become adherent and exibit various phenotypic changes.35,45,46 This prompted us to investigate whether TPA also induced RU clones to express distinct differentiation markers despite maintenance of concomitant cell proliferation. To this end, U937 and RU cells were compared for the expression of various surface antigens and nonspecific esterases, as measured by flow-cytometric and enzymatic (NAE) analysis, respectively. In the absence of TPA, U937 and RU cells exhibited a similar pattern of protein markers corresponding to the monocytic lineage of the parental line (Fig 5A). Only minor quantitative differences were detected with regard to integrins CD11a, CD11b, and CD18, which were displayed in higher amounts by U937 compared with RU cells. Treatment with TPA for 48 hours led to similar modifications of the expression of most of these markers in U937 and RU cells, arguing for the competence of the latter clones in progressing along the monocyte differentiation pathway (Fig 5A). Some of the markers were upregulated (nonspecific esterases, CD11c, CD54, CD31), whereas others were downmodulated (CD29, CD4, CD71) in the presence of TPA. CD14 expression failed to be affected by TPA to a significant extent in any of the cells tested. Interestingly, display of the above-mentioned integrins CD11a, CD11b, and CD18 was also stimulated by TPA in both U937 and RU cells. Yet, the level of these integrins in TPA-treated RU cells remained lower than in untreated parental U937 cells. Given the role of integrins in cell-adhesion processes,45 this result may account for the failure of TPA to trigger homotypic aggregation of RU cells under conditions inducing this phenomenon in the U937 line (Fig 5B). It should also be stated that the striking inhibition of CD71 expression by TPA in RU clones came as a surprise. Indeed, this antigen is considered as a marker of a TPA-resistant trait of RU cells, namely proliferation.45 Altogether, these features of RU clones point to the fact that monocytic differentiation can take place, at least to some extent, independently of the arrest of proliferation that normally accompanies maturation.
Effect of TPA on the maturation of U937 and RU cells. (A) Cultures (5 × 105 cells) were grown in 5 mL of complete medium for 48 hours, in the absence (upper part) or presence (lower part) of 50 ng/mL of TPA, and harvested. Cell-surface antigen display and NAE activity were determined as described in Materials and Methods. Results shown are average values from three independent experiments (SD < 20%). (B) Homotypic aggregation of cells was visualized by phase contrast microscopy after incubation for 48 hours with TPA. U937 and RU cultures were indistinguishable from each other in the absence of TPA.
Effect of TPA on the maturation of U937 and RU cells. (A) Cultures (5 × 105 cells) were grown in 5 mL of complete medium for 48 hours, in the absence (upper part) or presence (lower part) of 50 ng/mL of TPA, and harvested. Cell-surface antigen display and NAE activity were determined as described in Materials and Methods. Results shown are average values from three independent experiments (SD < 20%). (B) Homotypic aggregation of cells was visualized by phase contrast microscopy after incubation for 48 hours with TPA. U937 and RU cultures were indistinguishable from each other in the absence of TPA.
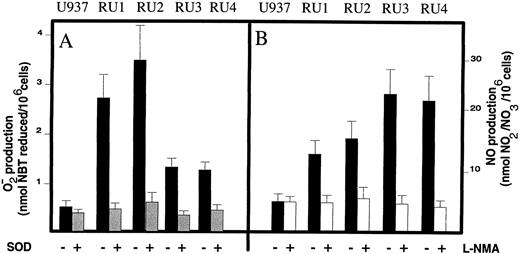
Activation steady-state of U937 and RU cells.A characteristic sign of monocytic maturation consists in the production of oxygen metabolites.47-50 The apparent perturbation of the proliferation and differentiation programs in the RU clones prompted us to investigate whether these cells could also be distinguished from the U937 line with regard to the generation of NO and O−2⋅ species. Parental U937 cells produced little amounts of these metabolites in the absence of monocyte activators (Fig 6A and B). In contrast, all four RU clones had a constitutive capacity for releasing significant amounts of active oxygen species in the absence of any pretreatment (Fig 6A and B). The levels of spontaneous NO and O−2⋅ production in RU cells were comparable to (and even higher than) the one induced in parental U937 cells by a standard activation procedure (ie, a 3-day treatment with TPA [50 ng/mL] or interferon-γ [IFN-γ] [100 IU/mL]) (data not shown). The specificity of this constitutive activation was confirmed by showing its suppression by the O−2⋅ detoxifier superoxide dismutase and the NO production inhibitor L-NMA. Therefore, the growing RU clones appear to be in a constitutive activation steady-state, which is normally a hallmark of monocyte maturation and proliferation arrest, exemplifying again the uncoupling of these processes in the variant cells. Active oxygen species have been reported to be involved in cellular defence mechanisms51 and, in particular, to participate in antiviral responses.52-55 This raised the possibility that selection for H-1 virus resistance led to the isolation of activated RU variants at least in part because of a direct protection given by oxygen metabolites against parvovirus infection. This hypothesis was tested by determining whether RU cells could be sensitized to H-1 virus by incubating them, from 24 hours preinfection onward, with the inhibitor of NO production L-NMA56 and/or the xanthine oxidase inhibitor allopurinol that suppresses O−2⋅ production.57 As indicated in Table 2, the survival of H-1 virus-infected (versus mock-infected) RU cells was indeed decreased by the active oxygen antagonists. The various RU clones differed in the extent of their sensitization to parvovirus infection, with RU4 being most susceptible (ninefold enhancement of H-1 virus-induced killing in the presence of both L-NMA and allopurinol). In contrast, the survival of infected parental U937 cells was in the 0.1% to 0.2% range, irrespective of whether O−2⋅ and NO inhibitors were added.
Analysis of the activation steady-state of U937 and RU clones. (A) O−2⋅ production was quantitated by measuring the reduction of NBT, after incubation of the cultures (5 × 105 cells/mL) for 5 hours in RPMI supplemented with 10% FCS in the presence () or absence (▪) of 500 IU/mL of human SOD. (B) NO production was determined by quantitating NO−2/NO−3 in supernatants of cultures (2 × 105 cells/mL) incubated for 36 hours in complete medium in the presence (□) or absence (▪) of 0.5 mmol/L L-NMA. Average values from triplicates cultures are shown with standard derivatives bars.
Analysis of the activation steady-state of U937 and RU clones. (A) O−2⋅ production was quantitated by measuring the reduction of NBT, after incubation of the cultures (5 × 105 cells/mL) for 5 hours in RPMI supplemented with 10% FCS in the presence () or absence (▪) of 500 IU/mL of human SOD. (B) NO production was determined by quantitating NO−2/NO−3 in supernatants of cultures (2 × 105 cells/mL) incubated for 36 hours in complete medium in the presence (□) or absence (▪) of 0.5 mmol/L L-NMA. Average values from triplicates cultures are shown with standard derivatives bars.
Effect of O−2⋅ and NO Inhibitors on the Susceptibility of U937 and RU Clones to H-1 Virus Infection
| Pretreatment . | Cell Survival to H-1 Virus Infection (%) . | Recovered Virus (pfu/cell) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | U937 . | RU1 . | RU2 . | RU3 . | RU4 . | U937 . | RU1 . | RU2 . | RU3 . | RU4 . |
| None | 0.14 | 93 | 90 | 98 | 91 | 90 | 0.20 | 0.72 | 0.23 | 0.11 |
| Allopurinol (3 mmol/L) | 0.16 | 62 | 57 | 49 | 31 | 77 | 3.1 | 5.4 | 2.4 | 2.8 |
| L-NMA (0.5 mmol/L) | 0.21 | 77 | 64 | 62 | 57 | 95 | 2.3 | 4.7 | 3.2 | 2.1 |
| Allopurinol + L-NMA | 0.13 | 53 | 45 | 44 | 22 | 79 | 3.9 | 6.0 | 3.3 | 3.2 |
| Pretreatment . | Cell Survival to H-1 Virus Infection (%) . | Recovered Virus (pfu/cell) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | U937 . | RU1 . | RU2 . | RU3 . | RU4 . | U937 . | RU1 . | RU2 . | RU3 . | RU4 . |
| None | 0.14 | 93 | 90 | 98 | 91 | 90 | 0.20 | 0.72 | 0.23 | 0.11 |
| Allopurinol (3 mmol/L) | 0.16 | 62 | 57 | 49 | 31 | 77 | 3.1 | 5.4 | 2.4 | 2.8 |
| L-NMA (0.5 mmol/L) | 0.21 | 77 | 64 | 62 | 57 | 95 | 2.3 | 4.7 | 3.2 | 2.1 |
| Allopurinol + L-NMA | 0.13 | 53 | 45 | 44 | 22 | 79 | 3.9 | 6.0 | 3.3 | 3.2 |
Cultures (2 × 105 cells) pretreated for 24 hours with indicated agents were infected with H-1 virus at an MOI of 5 pfu/cell and further grown in the presence of the respective drug. Cultures were processed for the measurement of cell survival and virus production at 2 days postinfection. Survival of H-1 virus-infected cells is expressed relative to similarly pretreated but mock-infected cultures. Average values from 3 experiments are given (SD < 25%).
Parallel to the determination of the toxic effect of H-1 virus, the amount of infectious units that could be collected from the various cultures was quantitated by plaque assays. As shown in Table 2, L-NMA and allopurinol not only potentiated RU cells killing by the parvovirus, but also enhanced the capacity of RU cultures for releasing infectious particles. Indeed, treatment with NO/O−2⋅ inhibitors led to the recovery of 8 (RU2) to over 30 (RU4) times more virus from infected RU cultures, while having no significant effect on H-1 virus production in parental U937 cells. However, it should be stated that even in the presence of these inhibitors, the virus capacity of RU clones was much lower than that of U937 cells.
Altogether, these results indicate that in the monocytic lineage, H-1 virus can be used to select for a constitutively activated cell phenotype that is compatible with continuing proliferation and is likely to place some direct restriction(s) on parvovirus replication and cytopathic effect.
DISCUSSION
The human promonocytic cell line U937 can be induced to exhibit some characteristics of functionally mature monocytes under in vitro culture conditions,58 and serves in this respect as a useful model to study the mechanisms of cell proliferation and differentiation.22 46 Using the H-1 autonomous parvovirus as a selective agent, we have isolated four H-1 virus-resistant U937 variants that were designated RU and are characterized by three main properties: (1) uncoupling of the commitment into monocyte/macrophage differentiation from the arrest of proliferation; (2) alteration of the control of c-Myc oncoprotein accumulation; and (3) correlation of H-1 virus resistance with the constitutive production of oxygen metabolites (activation steady-state).
(1) Continuing proliferation of differentiating RU cells.The commitment of U937 cells into monocytic differentiation is typically accompanied by the arrest of proliferation.19 Similarly to U937 cells, the RU clones were induced by the maturating agent TPA to exhibit molecular signs of differentiation, including activation of the early responding genes c-fos and c-jun, accumulation of nonspecific esterases and upmodulation or downmodulation of a number of cells surface antigens. However, RU cells could be distinguished from the parental U937 line by the fact that these phenotypic changes were not associated with a cessation of proliferation, although the growth rate of RU cells was reduced in the presence of TPA. Therefore, the usual coupling of monocytic differentiation and proliferation arrest appears to be disrupted in the RU clones, indicating that the latter is not necessary for at least some steps of the former. Though normally not seen in the absence of selection, this uncoupling is not without precedents. Indeed, Lasky et al27 have recently isolated a human chronic myelogenous leukemia cell strain, JMRD3, which exhibited characteristics of differentiated cells, but failed to become quiescent and adherent in the presence of 1,25-vitamin D3. Furthermore, Hass et al59 60 have characterized a U937 variant resistant to TPA. Treatment of these cells with the phorbol ester did not induce growth arrest, but markedly reduced CD71 expression, like in the RU clones. The present work indicates that parvovirus H-1 constitutes an interesting tool to select for such rare variants, in which monocytic differentiation can go on with continuing cell proliferation. Our data broaden the list of differentiation markers whose expression is compatible with cell proliferation, and show for the first time that it includes a hallmark of monocytic maturation, namely the activation state (see below).
(2) Disregulation of c-Myc oncoprotein accumulation in RU cells.U937 cells are characterized by a high constitutive production of the c-Myc oncoprotein19 (Fig 3B). This may contribute to the sustained proliferation of the U937 line, given the known involvement of c-Myc in cell-cycle progression.61 It is worth noting in this respect that one of the variants analyzed in this study (RU3) retained the high constitutive c-Myc expression characteristic of the parental U937 cells, while the other clones (RU1, RU2, and RU4) accumulated c-Myc to a significantly lower extent. These differences in constitutive c-Myc expression do not seem to be essential to the resistance of host cells to H-1 virus because all four RU clones survived parvovirus infection. On the other hand, the reduced capacity of RU1, RU2, and RU4 clones for c-Myc accumulation was accompanied with a striking suppression of their ability to form tumors in recipient animals, compared with RU3 and U937 cells. Although no definite conclusion can be drawn from the limited number of RU clones studied, this observation agrees with other evidence43 to support a role of c-Myc in neoplastic growth. The role of c-Myc in the control of U937 cell growth is further supported by the fact that TPA inhibits both the proliferation of these cultures and their capacity for accumulating c-Myc19 (Fig 3B). This correlation is substantiated by the present work, which shows that the RU subclones are resistant to both effects of TPA, ie, they continue to proliferate in the presence of the phorbol ester while their c-Myc content does not decrease significantly. Interestingly, the U937 variant reported by Hass et al60 to resist the antiproliferative action of TPA also keeps accumulating c-Myc under these conditions. Given the other evidence of c-Myc participation in cell exit from the quiescent state,61 these data support the idea that c-Myc is a TPA target whose downmodulation contributes to prevent responsive cells from proliferating in the presence of the phorbol ester. Therefore, the disregulation making c-Myc production insensitive to TPA in RU clones is likely to mediate, at least in part, the resistance of these cells to the cytostatic effect of the drug.
(3) Resistance to H-1 virus and constitutive activation of RU cells.In other systems, presence of the functional tumor suppressor gene product p53 was found to correlate with enhanced cell resistance to parvovirus infection.15 Endogenous p53 is not expressed at a detectable level in the H-1 virus-sensitive U937 line. This prompted us to investigate whether selection for H-1 virus resistance in the U937 system led to the isolation of variants that recovered the capacity for p53 production, as recently reported for the K562 erythroleukemic cell line.15 This is not the case of the RU subclones that remain p53-negative and are indistinguishable from the parental U937 line with respect to two other tumor suppressor gene products, p21 (a downstream effector of p53, which can also act independently of p5317,62) and RB. Therefore, no simple relationship appears to exist between parvovirus resistance and tumor suppressor gene expression.
On the other hand, RU clones display lower levels of some integrins involved in cell-cell interactions (eg, CD11a, CD11b, and CD18), compared with the U937 line. To test whether these quantitative changes in surface antigens may modulate the sensitivity of target cells to H-1 virus, U937 cultures were treated with various antibodies directed against integrins before parvovirus infection. Although most of these antibodies were able to abolish homotypic aggregation upon addition of TPA, no effect on the outcome of H-1 virus infection was detected (data not shown). Therefore, these surface alterations are unlikely to be responsible for the differential sensitivity of RU versus U937 cells to the parvovirus.
Another distinct feature of RU clones, compared with the parental U937 line, consists of their activation steady-state, ie, their constitutive capacity for producing oxygen metabolites, in particular O−2⋅ and NO. This raised the question of the possible relevance of activation to the resistance of RU clones to H-1 virus. Indeed, oxidative responses were reported to play a protective role against some viral infections.52-55,63,64 NO is a free radical gaseous molecule that can mediate vital physiologic functions, including host defense mechanisms.47 Its interaction with superoxide anion yields the highly toxic peroxynitrite ONOO−. Implications of NO in antiviral activities were recently discovered.52-55 Inhibition of NO synthesis in mice leads ectromelia virus to cause fulminant mousepox.53 In addition, epithelial cells that fail to sustain a detectable NO synthesis become resistant to this virus when treated with organic compounds that generate NO. On the other hand, the replication of herpes simplex type 1 in murine macrophage-like RAW264.7 cells is drastically reduced after NO induction by IFN-γ.54 To test whether the resistance of RU clones to H-1 virus may be related to the constitutive production of O−2⋅ and/or NO, infected cells were incubated with compounds that scavenge these metabolites or inhibit their formation. Antioxidative treatments were found to sensitize RU cells to parvovirus toxicity and to increase the yields of infectious virus that can be procured from infected cultures. Because these yields are still very low compared with the H-1 virus burst released by permissive U937 cells, it is presently unclear whether antioxidants allow either a genuine virus production in RU cultures, or the recovery of a greater fraction of the inoculum due to the stabilization of incoming particles. Irrespective of whether parvovirus infection becomes productive or not, the cytopathic effect of H-1 virus on RU cells appears to be enhanced under antioxidative conditions. Although side effects of the O−2⋅/NO inhibitors used cannot be ruled out, these results raise the possibility that the activation steady-state may confer on RU cells some protection against parvovirus infection. A major restriction to the life cycle of H-1 virus in RU cells takes place at the level of viral gene expression. Further studies are required to determine how oxygen metabolites may interfere with this step of parvovirus replication.
Chemical NO donors and NO gas were shown to inhibit the proliferation and to induce some differentiation events in HL-60 cells.51 In contrast, the proliferation of RU cells was not decreased in comparison with U937 cells. Furthermore, after induction of differentiation with TPA, RU cells showed some maturation features while keeping the capacity for proliferation. Taking into account the fact that all four RU strains were isolated independently, their active proliferation and constitutive production of NO and O−2⋅ may be traced back to the procedure followed to select these clones. On the one hand, active cell growth was required by the colony-formation assay used. On the other hand, constitutive NO and O−2⋅ production may mediate at least part of the resistance of RU cells to H-1 virus. It would be interesting to assess the role of these metabolites in the defense of cells belonging to other lineages to parvovirus infection.
ACKNOWLEDGMENT
We are indebted to Drs V. Umansky and M. Rocha for NO assays; Drs C. Bernabeu and F. Sánchez-Madrid for the generous gift of monoclonal antibodies directed against surface antigens; Drs B. Alarcón and P. Lastres for flow cytometry measurements; Dr C. Streydio for fingerprint analyses; Dr T. Dupressoir for his kind help with animal studies; Dr J.J. Cornelis for stimulating discussions; G. Balboni for her excellent technical assistance; and Dr E. Casademunt for reading the manuscript.
Supported in part by a grant from the Commission of the European Communities (Biomedicine and Health Research program). J.A.L.-G. was supported by a fellowship from the Commission of the European Communities (Human Capital and Mobility) and by the German Cancer Research Center. B.R. is the holder of a fellowship from the Belgian Fonds National de la Recherche Scientifique.
Address reprint requests to Jean Rommelaere, PhD, Deutsches Krebsforschungszentrum, Abt. 0610 and INSERM U375, Im Neuenheimer Feld 242, D-69120 Heidelberg, Germany.

![Fig. 2. Analysis of viral parameters in parental U937 and resistant RU cells after H-1 virus infection. (A) The survival of U937 and RU cells was determined by counting viable cells 4 days after infection with H-1 virus (MOI = 100 pfu/cell), using the Trypan blue exclusion method, and expressed as a percentage of the value measured in mock-infected cultures. Results from a typical experiment are shown. (B) Infectious particles recovered 48 hours after inoculation of 106 cells with H-1 virus (5 pfu/cell) were measured by plaque-assay. Data shown are from a typical experiment. Similar relative values were obtained in three independent experiments (SD < 22%). (C) The virus uptake was assayed after infection with [3H]thymidine-labeled H-1 virus (2 × 103 pfu [105 cpm]/106 cells), followed by a washing step with 1 mmol/L EDTA to remove noninternalized particles (upper part). Internalized [35S]methionine-labeled capsids were revealed by autoradiography after immunoprecipitation and SDS-PAGE analysis of VP1 and VP2 proteins extracted 2 hours after infection with H-1 virus (4 × 106 pfu [106 cpm]/106 cell) and washing with EDTA (lower part). (D) Amplification of viral DNA was determined by Southern blotting. After virus (10 pfu/cell) or mock infection, cultures were incubated for 40 hours in complete medium. DNA samples extracted by the Hirt procedure were analyzed by agarose gel electrophoresis, blotted, and hybridized with a virus-specific 32P-labeled DNA probe. RFI and RFII refer to viral DNA replicative forms of monomer and dimer length, respectively. Viral DNA species were positioned relative to HindIII-digested phage λ DNA used as size markers and revealed by ethidium bromide staining. (E) The viral transcripts were detected by Northern blotting. At 48 hours postinfection (10 pfu/cell), total RNA was isolated by the guanidinium thiocyanate method, fractionated by agarose/formaldehyde gel electrophoresis, blotted, and hybridized with a virus-specific 32P-labeled DNA probe. (F ) The accumulation of viral proteins was determined by immunoprecipitation. At 36 hours postinfection (5 pfu/cell) cultures were incubated for 2 hours in the presence of [35S]methionine and processed for immunoprecipitation with antiserums directed against the viral products NS-1 or VP-1 and VP-2, SDS-PAGE, and autoradiography.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/5/10.1182_blood.v89.5.1642/3/m_bl_0021f2a.jpeg?Expires=1767741946&Signature=bhG5OKyl~lTb1k0p4VWxb04NBRznYmlWihzzdmRLeXHwoRe5NHnls13ik4NB4g0ZTSaYudHlnJ2S4pFvx5NM6yWXVcGvLMoJWRgk7-2tdzMP8jfBEBOJLxXtm3Hl~FXPemYpkvjcMzLkkcGac6iVKF2MecOUNFJ9175-3eKEXod9mfEUEYPwqF~10X-DDjM71oWxuiPuRBsfLGWdWLwM2LZKAuaVIJEUqOfRQt42CqYQj4-5GY4fyZ3svR73VHB5YLpnlHy1HX~aFg4JHJFgweRmzlByZkzoiuL5Co1wY07hOgbOS9XAZCTOuPgd7zdYygcT3b1cPY-setmigHL5Lg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Analysis of viral parameters in parental U937 and resistant RU cells after H-1 virus infection. (A) The survival of U937 and RU cells was determined by counting viable cells 4 days after infection with H-1 virus (MOI = 100 pfu/cell), using the Trypan blue exclusion method, and expressed as a percentage of the value measured in mock-infected cultures. Results from a typical experiment are shown. (B) Infectious particles recovered 48 hours after inoculation of 106 cells with H-1 virus (5 pfu/cell) were measured by plaque-assay. Data shown are from a typical experiment. Similar relative values were obtained in three independent experiments (SD < 22%). (C) The virus uptake was assayed after infection with [3H]thymidine-labeled H-1 virus (2 × 103 pfu [105 cpm]/106 cells), followed by a washing step with 1 mmol/L EDTA to remove noninternalized particles (upper part). Internalized [35S]methionine-labeled capsids were revealed by autoradiography after immunoprecipitation and SDS-PAGE analysis of VP1 and VP2 proteins extracted 2 hours after infection with H-1 virus (4 × 106 pfu [106 cpm]/106 cell) and washing with EDTA (lower part). (D) Amplification of viral DNA was determined by Southern blotting. After virus (10 pfu/cell) or mock infection, cultures were incubated for 40 hours in complete medium. DNA samples extracted by the Hirt procedure were analyzed by agarose gel electrophoresis, blotted, and hybridized with a virus-specific 32P-labeled DNA probe. RFI and RFII refer to viral DNA replicative forms of monomer and dimer length, respectively. Viral DNA species were positioned relative to HindIII-digested phage λ DNA used as size markers and revealed by ethidium bromide staining. (E) The viral transcripts were detected by Northern blotting. At 48 hours postinfection (10 pfu/cell), total RNA was isolated by the guanidinium thiocyanate method, fractionated by agarose/formaldehyde gel electrophoresis, blotted, and hybridized with a virus-specific 32P-labeled DNA probe. (F ) The accumulation of viral proteins was determined by immunoprecipitation. At 36 hours postinfection (5 pfu/cell) cultures were incubated for 2 hours in the presence of [35S]methionine and processed for immunoprecipitation with antiserums directed against the viral products NS-1 or VP-1 and VP-2, SDS-PAGE, and autoradiography.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/5/10.1182_blood.v89.5.1642/3/m_bl_0021f2d.jpeg?Expires=1767741946&Signature=nX30y-3o5tdKsRBFGDN4IZjcor5aZ9R0nmiPk~NqCr5V2UGg2-1hfNjP30YWKXSaz6uyDtL71WtVE9cctEK714W1f~UMl4~KFklAbC0GaH2QuLjyBEi5AjIcJbH6C7mhrr4L4cP1tY~z0OvtMn51IJsVEPonIoSOR-DfsKS5DbiS9KTonlefNU88FWXzXNp6-cNFKtn5VOmm7V2oZJCnm8LUi89c8yea8yCgLzY63oPZPXZSfm2oFMHtfByI1h7hpMsA-f4XrcsZ4xH-mKvxz4~b6pd3oUtcsAqFdvSLhO1i8V6o-lSKTE2q5AYK3yRrNFSh9MzbFm2cQ6ZViAykTA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of TPA on the proliferation capacity of U937 and RU cells. U937 or RU cultures (104 cells) were grown in 0.1 mL of complete medium with or without TPA (50 ng/mL). At indicated times, cultures were pulsed for 4 hours with 1 μCi of [3H]thymidine, and incorporated radioactivity was subsequently measured in a liquid scintillation counter (average values and SD bars from three independent experiments, each done in triplicate).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/5/10.1182_blood.v89.5.1642/3/m_bl_0021f4.jpeg?Expires=1767741946&Signature=DIAcjGiDaKjbponjKyXfIHGf5qEbkZ8fBNUpf680fTXfy8JeCKNEv5hQAKKYxFCEpF8ZluuP8GlxqzGQdjOB5vKlhaN6jhRNfGSFjKRdIdCRI9ZDO38lcMPaXqAB3eoSbl4PJBqKxrz3-v460e8sa1W~75CIwtUE~eUwbZerOKmdy5t-8wnOTfEEjRP5ODRHX-I8SGH8pU~OzQIwCxkw2KgmnD6krF2giQR4H6cqI~wFy4eYUaahYUAbVXjW9p9ejMZpR5j8P-32cUbd5fetfAuH0bj~GjHoUsg1Wu83oNf8~jQ-gy4tla6vp5mxeinI7F9Dtgs2FZIAozSL6H968A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal