Abstract
The effects of FLT3/FLK-2 ligand (FL) and KIT ligand (KL) on in vitro expansion of hematopoietic stem cells were studied using lineage-negative (Lin−)Sca-1–positive (Sca-1+) c-kit–positive (c-kit+) marrow cells from 5-fluorouracil (5-FU)–treated mice. As single agents, neither FL nor KL could effectively support the proliferation of enriched cells in suspension culture. However, in combination with interleukin-11 (IL-11), both FL and KL enhanced the production of nucleated cells and progenitors. The kinetics of stimulation by FL was different from that by KL in that the maximal expansion by FL of the nucleated cell and progenitor pools required a longer incubation than with KL. We then tested the reconstituting abilities of cells cultured for 1, 2, and 3 weeks by transplanting the expanded Ly5.1 cells together with “compromised” marrow cells into lethally irradiated Ly5.2 mice. Cells that had been expanded with either cytokine combination were able to maintain the reconstituting ability of the original cells. Only cells that had been incubated with KL and IL-11 for 21 days had less reconstituting ability than fresh marrow cells. These results indicate that there can be significant expansion of progenitors in vitro without compromising the reconstituting ability of stem cells. Addition of IL-3 to permissive cytokine combinations significantly reduced the ability of cultured cells to reconstitute the hematopoiesis of irradiated hosts. These observations should provide a basis for a rational approach to designing cytokine combinations for in vitro expansion of hematopoietic stem cells.
BOTH FLT3/FLK-2 and KIT receptor tyrosine kinases belong to the type III receptor tyrosine kinase family that includes FMS and platelet-derived growth factor receptors.1-9 The ligands for several of these receptors stimulate the proliferation of hematopoietic cells.7,8,10-12 FLT3/FLK-2 transcripts have been detected in murine and human cell populations enriched for hematopoietic stem cells and progenitors and are absent in more mature cells.1-4 In addition, targeted disruption of the flt3/flk-2 gene led to deficiencies in primitive hematopoietic progenitors.13 The FLT3/FLK-2 ligand (FL) is similar to KIT ligand (KL) in that both proteins stimulate the proliferation of primitive hematopoietic progenitors.14-24 Neither factor has much stimulatory activity on its own, but each factor synergizes with other early-acting cytokines such as interleukin-6 (IL-6), IL-11, IL-12, and granulocyte colony-stimulating factor (G-CSF ).17,18,25 26
Currently, there is significant interest in hematology in the in vitro (ex vivo) expansion of hematopoietic stem cells and progenitors.27-40 A number of investigators have already shown that it is possible to increase the number of hematopoietic progenitors in culture by using combinations of early-acting cytokines.41 In studies of murine lymphohematopoietic progenitors in culture, we observed that a combination of FL and IL-11 stimulates production of cells with a blast-like appearance in suspension culture for a longer time than KL-containing cytokine combinations.17 We report here the results of our studies of the effects of FL or KL on the long-term engrafting capability of stem cells. Although kinetic differences exist, both cytokines are capable of yielding committed and uncommitted progenitors without compromising stem cell reconstituting capability in lethally irradiated hosts.
MATERIALS AND METHODS
Growth factors.Recombinant soluble human FL was produced in yeast and purified as previously described.14 Purified recombinant murine KL was provided by Kirin Brewery Co (Tokyo, Japan). Medium conditioned by Chinese hamster ovary cells that had been genetically engineered to produce murine IL-3 at a high titer (70,000 U/mL) was a gift from T. Sudo of the Biomaterial Institute (Yokohama, Japan). Purified recombinant human IL-6 was a gift from M. Naruto of Toray Industries (Kamakura, Japan). Purified recombinant human IL-11 was a gift from P. Schendel of the Genetics Institute (Cambridge, MA). Purified recombinant erythropoietin (Ep) was a gift from F.-K. Lin of Amgen (Thousand Oaks, CA). Unless otherwise specified, concentrations of the cytokines used were as follows: FL 100 ng/mL, KL 100 ng/mL, IL-3 200 U/mL, IL-6 100 ng/mL, IL-11 20 ng/mL, and Ep 2 U/mL.
Cell preparations.Cells from 2- to 5-month-old BDF1 and C57B1/6 mice (Charles River Laboratories, Raleigh, NC) were used in suspension culture, and cells from 2- to 3-month-old C57B1/6 mice (Jackson Laboratories, Bar Harbor, ME) that are congenic for Ly5 allotypes were used in transplantation experiments. 5-Fluorouracil (5-FU; Adria Laboratories, Columbus, OH) was administered intravenously through the tail vein at 150 mg/kg body weight, and bone marrow cells were harvested 2 days later. Single cell suspensions were prepared from pooled femurs and tibiae, and the cells with densities between 1.063 and 1.077 g/mL were collected with gradients of Metrizamide (Accurate Chemical & Scientific Corp, Westburg, NY). The cells were further enriched for progenitors by negative immunomagnetic selection with a mixture of lineage-specific antibodies.42 Lineage-negative (Lin−) cells were incubated with fluorescein isothiocyanate–conjugated monoclonal antibody D7 (anti-Sca-1)43 and biotin-conjugated monoclonal ACK4 (anti-c-kit)44 for 15 minutes on ice. Isotype controls were fluorescein isothiocyanate–conjugated rat IgG2a and biotin-conjugated rat IgG2a. The cells were washed twice with Ca2+, Mg2+-free phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA; Fraction V; Sigma Chemical Co, St Louis, MO) and incubated with R-phycoerythrin–conjugated Streptavidin (Jackson ImmunoResearch Laboratories, West Grove, PA) for 15 minutes on ice. The cells were washed twice, resuspended in PBS/BSA, and kept on ice until cell sorting. Flow cytometric analysis and cell sorting were performed on a FACStarPlus (Becton Dickinson, San Jose, CA).
Suspension and clonal cell cultures.One thousand Lin− Sca-1–positive (Sca-1+) c-kit–positive (c-kit+) cells were incubated in each well of a 24-well plate (Falcon, Lincoln Park, NJ) in suspension culture. The culture medium contained α-medium, 20% (vol/vol) fetal calf serum (Intergen, Purchase, NY), 1% deionized Fraction V BSA, 1 × 10−4 mol/L 2-mercaptoethanol (Sigma), and combinations of cytokines. On day 7 of incubation, the cultured cells were diluted, replated in freshly prepared media in 24-well plates at 2,000 or 5,000 cells/mL, and incubated for 7 more days. On day 14, the cultured cells were diluted, replated in freshly prepared media in 24-well plates at 5 or 10 × 104 cells/mL, and incubated for 7 more days. At each time of replating, aliquots were analyzed for colony formation in 35-mm suspension culture dishes (Falcon) containing α-medium, 1.2% 1,500-cp methylcellulose (Shinetsu Chemical, Tokyo, Japan), 30% fetal calf serum, 1% BSA, 1 × 10−4 mol/L 2-mercaptoethanol, KL, IL-3, IL-11, and Ep. Total colony-forming units in culture (CFU-C) and the progenitors for granulocyte/erythrocyte/macrophage/megakaryocyte (CFU-GEMM) were determined on day 8 of incubation by in situ observation of the plates on an inverted microscope.45 46
Expansion of Cell and Progenitor Populations in Suspension Culture
| Cytokines . | Day 7 . | Day 14 . | ||||
|---|---|---|---|---|---|---|
| . | TNC . | CFU-C . | CFU-GEMM . | TNC . | CFU-C . | CFU-GEMM . |
| FL | <1,000 | ND | ND | <1,000 | ND | ND |
| KL | 3.4 × 103 | 400 | 50 | 3.2 × 103 | 110 | 10 |
| IL-11 | 0 | 0 | 0 | 0 | 0 | 0 |
| FL,KL | 5.6 × 103 | 580 | 30 | 1.3 × 104 | 110 | 10 |
| FL, IL-11 | 5.6 × 104 | 7,800 | 180 | 2.2 × 107 | 228,000 | 2,500 |
| KL, IL-11 | 2.2 × 105 | 33,700 | 1,050 | 1.1 × 108 | 706,000 | 12,000 |
| Cytokines . | Day 7 . | Day 14 . | ||||
|---|---|---|---|---|---|---|
| . | TNC . | CFU-C . | CFU-GEMM . | TNC . | CFU-C . | CFU-GEMM . |
| FL | <1,000 | ND | ND | <1,000 | ND | ND |
| KL | 3.4 × 103 | 400 | 50 | 3.2 × 103 | 110 | 10 |
| IL-11 | 0 | 0 | 0 | 0 | 0 | 0 |
| FL,KL | 5.6 × 103 | 580 | 30 | 1.3 × 104 | 110 | 10 |
| FL, IL-11 | 5.6 × 104 | 7,800 | 180 | 2.2 × 107 | 228,000 | 2,500 |
| KL, IL-11 | 2.2 × 105 | 33,700 | 1,050 | 1.1 × 108 | 706,000 | 12,000 |
One thousand Lin−Sca-1+c-kit+ cells were cultured in the presence of the designated cytokines. On day 7, the cells were washed and then diluted in freshly prepared media, and the incubation continued for 7 more days. On both day 7 and day 14 of culture, cells were analyzed for CFU-C and CFU-GEMM in methylcellulose culture.
Abbreviation: ND, not determined.
Time Course Analysis of Expansion of TNC and CFU-C in Suspension Culture
| Cytokines . | Day 7 . | Day 14 . | Day 21 . | |||
|---|---|---|---|---|---|---|
| . | TNC . | CFU-C . | TNC . | CFU-C . | TNC . | CFU-C . |
| FL, KL | 3.8 × 103 | 970 | 1.6 × 104 | 180 | 1.5 × 104 | 30 |
| FL, IL-11 | 5.6 × 104 | 8,600 | 4.5 × 106 | 53,000 | 4.0 × 107 | 232,200 |
| KL, IL-11 | 8.6 × 105 | 105,300 | 2.2 × 108 | 453,800 | 5.2 × 108 | 36,300 |
| Cytokines . | Day 7 . | Day 14 . | Day 21 . | |||
|---|---|---|---|---|---|---|
| . | TNC . | CFU-C . | TNC . | CFU-C . | TNC . | CFU-C . |
| FL, KL | 3.8 × 103 | 970 | 1.6 × 104 | 180 | 1.5 × 104 | 30 |
| FL, IL-11 | 5.6 × 104 | 8,600 | 4.5 × 106 | 53,000 | 4.0 × 107 | 232,200 |
| KL, IL-11 | 8.6 × 105 | 105,300 | 2.2 × 108 | 453,800 | 5.2 × 108 | 36,300 |
One thousand Lin−Sca-1+c-kit+ cells were cultured in the presence of the designated cytokines. On day 7 and day 14 of culture, the cells were washed and then diluted in freshly prepared media, and the incubation continued for 7 more days. At each time of replating, aliquots were analyzed for colony formation.
In vivo reconstitution experiments.Female C57B1/6-Ly5.2 mice were administered single 850-cGy total-body irradiation via a 4 × 106 V linear accelerator. After total-body irradiation of the recipient mice, sorted fresh marrow cells of male C57B1/6-Ly5.1 mice were injected into the tail vein of the recipients together with 4 × 105 “compromised” marrow cells of female C57B1/6-Ly5.2 mice in a final volume of 0.2 mL PBS containing 0.1% BSA. “Compromised” cells had been subjected to two previous rounds of transplantation and regeneration in female mice.47 After 1 to 3 weeks' incubation, fractions of cultured cells were injected into female C57B1/6-Ly5.2 mice together with “compromised” cells. Peripheral blood was obtained from the retro-orbital venous plexus using heparin-coated micropipettes (Drummond Scientific Co, Broomall, PA) 2, 4, and/or 6 months after transplantation. Red blood cells were lysed by 0.15 mol/L NH4Cl. The samples were then used for flow cytometric analysis of donor-derived cells by staining with anti-Ly5.1 (A20-1.7). In all experiments, cultures were initiated with the same number of cells as the “fresh sample.” In designated experiments, duplicate cultures were performed so that the entire sample and fractions equivalent to 1/10 and 1/100 of the sample were injected.
RESULTS
Effects of FL on the expansion of progenitor cells.First, we determined the optimal concentrations of FL and KL for stimulation of growth of Lin−Sca-1+c-kit+ marrow cells of 5-FU–treated mice. Varying concentrations of FL and KL were added to suspension cultures containing 20 ng/mL IL-11. The optimal doses of both FL and KL were determined to be 100 ng/mL.
Next, we studied the effects of FL as a single factor and in combination with KL and/or IL-11 on the proliferation of Lin−Sca-1+ c-kit+ cells in suspension culture. The results presented in Table 1 are representative of two experiments. As single factors, FL, KL, and IL-11 had little or no effect on the production of total nucleated cells (TNC). When FL or KL were combined with IL-11, significant expansion of both cells and progenitors was observed. However, direct comparison of FL and KL is not meaningful, since the initial cells that were expanded were c-kit+ cells and thus may not represent the physiologic composition of primitive progenitors. KL did not synergize with FL.
Later effects of FL on the expansion of progenitor cells.We have recently observed that many blast cells persist on day 13 of methylcellulose culture containing FL and IL-11.17 Therefore, in the next set of experiments, we extended suspension culture to 3 weeks and analyzed the effects of two-factor combinations (FL and KL, FL and IL-11, and KL and IL-11) on the expansion of cells and progenitors. The results of one of two similar experiments are presented in Table 2. Again, both the combination of FL and IL-11 and the combination of KL and IL-11 significantly increased the number of cells and CFU-C, whereas the combination of FL and KL showed little synergy. Proliferation of cells and progenitors appeared slower in culture with FL and IL-11 than with KL and IL-11. However, by day 21 of incubation, the number of CFU-C in FL-containing cultures was significantly higher than in KL-containing cultures, even though there were fewer TNC in FL-containing cultures than in KL-containing cultures.
Reconstituting Ability of Fresh Bone Marrow Cells and Day-14 Cultured Cells
| A. Quantitation of Donor Cells . | ||||
|---|---|---|---|---|
| . | . | . | . | . |
| Cytokines in Culture . | No. of Cells Injected . | No. of CFU-C Injected . | % Ly5.1 (donor) PB Cells3-150 . | |
| . | . | . | 2 Months . | 6 Months . |
| Summary Data | ||||
| Fresh marrow | 100 | 41 | 45.1 ± 7.0 | 41.1 ± 9.8 |
| 10 | 11.0 ± 13.6 | 7.1 ± 8.9 | ||
| 1 | 2.5 ± 3.2 | 2.7 ± 4.1 | ||
| FL, KL | 660 | ND | 0.5 ± 0.1 | 0.6 ± 0.1 |
| FL, IL-11 | 5.0 × 105 | 4,630 | 59.0 ± 15.8 | 72.3 ± 23.9 |
| 5.0 × 104 | 11.8 ± 15.7 | 18.5 ± 26.9 | ||
| 5.0 × 103 | 1.1 ± 0.9 | 1.1 ± 0.6 | ||
| KL, IL-11 | 2.0 × 106 | 15,300 | 57.1 ± 19.6 | 50.0 ± 27.2 |
| 2.0 × 105 | 4.6 ± 2.4 | 1.9 ± 0.7 | ||
| 2.0 × 104 | 0.3 ± 0.1 | 0.6 ± 0.1 | ||
| Individual Recipient Data | ||||
| Fresh marrow | 100 | 35.6 | 27.9 | |
| 55.2 | 57.2 | |||
| 41.2 | 40.3 | |||
| 50.7 | 35.1 | |||
| 42.8 | 44.8 | |||
| FL, IL-11 | 5.0 × 105 | 79.3 | 92.8 | |
| 69.3 | 84.0 | |||
| 32.8 | 28.8 | |||
| 60.7 | 90.8 | |||
| 53.0 | 65.0 | |||
| KL, IL-11 | 2.0 × 106 | 82.1 | 80.2 | |
| 42.2 | 15.4 | |||
| 78.1 | 80.1 | |||
| 32.8 | 23.6 | |||
| 50.2 | 50.5 | |||
| B. Surface Phenotype of Donor Cells | ||||
| Cytokines in Culture | % Mac-1+Gr-1+ Cells | % B 220+ Cells | % Thy-1+ Cells | |
| Fresh marrow | 3.1 | 30.5 | 20.8 | |
| 2.5 | 22.3 | 13.2 | ||
| FL, IL-11 | 8.5 | 42.0 | 28.5 | |
| 6.0 | 48.0 | 25.2 | ||
| KL, IL-11 | 2.1 | 32.5 | 26.5 | |
| 4.0 | 25.3 | 21.5 | ||
| A. Quantitation of Donor Cells . | ||||
|---|---|---|---|---|
| . | . | . | . | . |
| Cytokines in Culture . | No. of Cells Injected . | No. of CFU-C Injected . | % Ly5.1 (donor) PB Cells3-150 . | |
| . | . | . | 2 Months . | 6 Months . |
| Summary Data | ||||
| Fresh marrow | 100 | 41 | 45.1 ± 7.0 | 41.1 ± 9.8 |
| 10 | 11.0 ± 13.6 | 7.1 ± 8.9 | ||
| 1 | 2.5 ± 3.2 | 2.7 ± 4.1 | ||
| FL, KL | 660 | ND | 0.5 ± 0.1 | 0.6 ± 0.1 |
| FL, IL-11 | 5.0 × 105 | 4,630 | 59.0 ± 15.8 | 72.3 ± 23.9 |
| 5.0 × 104 | 11.8 ± 15.7 | 18.5 ± 26.9 | ||
| 5.0 × 103 | 1.1 ± 0.9 | 1.1 ± 0.6 | ||
| KL, IL-11 | 2.0 × 106 | 15,300 | 57.1 ± 19.6 | 50.0 ± 27.2 |
| 2.0 × 105 | 4.6 ± 2.4 | 1.9 ± 0.7 | ||
| 2.0 × 104 | 0.3 ± 0.1 | 0.6 ± 0.1 | ||
| Individual Recipient Data | ||||
| Fresh marrow | 100 | 35.6 | 27.9 | |
| 55.2 | 57.2 | |||
| 41.2 | 40.3 | |||
| 50.7 | 35.1 | |||
| 42.8 | 44.8 | |||
| FL, IL-11 | 5.0 × 105 | 79.3 | 92.8 | |
| 69.3 | 84.0 | |||
| 32.8 | 28.8 | |||
| 60.7 | 90.8 | |||
| 53.0 | 65.0 | |||
| KL, IL-11 | 2.0 × 106 | 82.1 | 80.2 | |
| 42.2 | 15.4 | |||
| 78.1 | 80.1 | |||
| 32.8 | 23.6 | |||
| 50.2 | 50.5 | |||
| B. Surface Phenotype of Donor Cells | ||||
| Cytokines in Culture | % Mac-1+Gr-1+ Cells | % B 220+ Cells | % Thy-1+ Cells | |
| Fresh marrow | 3.1 | 30.5 | 20.8 | |
| 2.5 | 22.3 | 13.2 | ||
| FL, IL-11 | 8.5 | 42.0 | 28.5 | |
| 6.0 | 48.0 | 25.2 | ||
| KL, IL-11 | 2.1 | 32.5 | 26.5 | |
| 4.0 | 25.3 | 21.5 | ||
Irradiated recipients (5 mice per group) were transplanted with 100, 10, or one cell equivalents of fresh Lin−Sca-1+c-kit+ cells or with whole or fractions of expanded cultures initiated with 100 Lin−Sca-1+c-kit+. Levels of engraftment at 2 and 6 months are shown both as a summary of groups and as individual data. Lineage phenotypes of the donor cells were analyzed at 6 months in 2 mice per group.
Mean ± SD % donor-derived nucleated peripheral blood cells identified by anti-Ly5.1. Background (peripheral blood cells of nontransplanted female mice) staining was <1.0%.
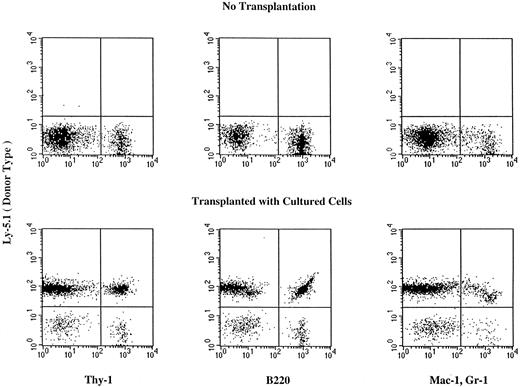
Reconstituting ability of expanded cells.We then tested the in vivo reconstituting ability of cells expanded with combinations of cytokines. The suspension cultures were initiated with Lin−Sca-1+c-kit+ C57B1/6-Ly5.1 cells. The cultures were serially transferred to new flasks containing fresh media every 7 days to keep cell concentrations at less than 1 × 106/mL as described in the methods. We then transplanted the whole sample or fractions of the sample of cells expanded from 100 enriched cells into lethally irradiated C57B1/6-Ly5.2 mice. As controls, we also transplanted 100, 10, and one enriched marrow cells. The results of analyses of day 14 expanded cells are presented in Table 3. Incubation with the combination of FL and KL barely supported cell proliferation and failed to maintain the population of stem cells. Both the combination of FL and IL-11 and the combination of KL and IL-11 maintained the reconstituting ability of the expanded cells. Monocytes, granulocytes, and T and B lymphocytes of donor cell type were detected in the peripheral blood of mice injected with the expanded cells (Fig 1 and Table 3). Because of the observation (Table 2) that the combination of FL and IL-11 supports maintenance of progenitor cells longer than the combination of KL and IL-11, we next compared the reconstitution ability of cells that were expanded for 7 or 21 days under two different cytokine conditions (Table 4). The cells expanded with FL or KL and IL-11 for 7 days had almost the same reconstituting ability as fresh cells. However, on day 21 of culture, the combination of FL and IL-11 maintained the reconstituting ability of cultured cells much better than the combination of KL and IL-11.
Hematopoietic reconstitution by cells cultured for 14 days in the presence of FL and IL-11. Nucleated cells of the peripheral blood were analyzed using flow cytometry 6 months after transplantation. Thy-1+ cells, B220+ cells, and Mac-1+, Gr-1+ cells of donor (Ly5.1) origin are seen in the peripheral blood of the recipient. Analyses of additional samples are presented in Table 3.
Hematopoietic reconstitution by cells cultured for 14 days in the presence of FL and IL-11. Nucleated cells of the peripheral blood were analyzed using flow cytometry 6 months after transplantation. Thy-1+ cells, B220+ cells, and Mac-1+, Gr-1+ cells of donor (Ly5.1) origin are seen in the peripheral blood of the recipient. Analyses of additional samples are presented in Table 3.
Reconstituting Ability of Fresh Bone Marrow and Day 7 or Day 21 Cultured Cells
| Cytokines . | Days of Incubation . | No. of Cells Injected . | No. of CFU-C Injected . | % Ly5.1 (donor) PB Cells4-150 . | |
|---|---|---|---|---|---|
| . | . | . | . | 2 mo . | 4 mo . |
| Fresh marrow | 100 | 42 | 51.3 ± 14.1 | 52.3 ± 20.8 | |
| FL, IL-11 | 7 | 1.6 × 104 | ND | 50.5 ± 8.3 | 40.1 ± 8.60 |
| KL, IL-11 | 7 | 2.0 × 105 | ND | 63.4 ± 12.2 | 44.7 ± 16.9 |
| FL, IL-11 | 21 | 3.4 × 107 | 34,100 | 47.3 ± 8.5 | 36.2 ± 16.8 |
| KL, IL-11 | 21 | 3.2 × 107 | 9,200 | 16.3 ± 2.5 | 11.7 ± 2.2 |
| Cytokines . | Days of Incubation . | No. of Cells Injected . | No. of CFU-C Injected . | % Ly5.1 (donor) PB Cells4-150 . | |
|---|---|---|---|---|---|
| . | . | . | . | 2 mo . | 4 mo . |
| Fresh marrow | 100 | 42 | 51.3 ± 14.1 | 52.3 ± 20.8 | |
| FL, IL-11 | 7 | 1.6 × 104 | ND | 50.5 ± 8.3 | 40.1 ± 8.60 |
| KL, IL-11 | 7 | 2.0 × 105 | ND | 63.4 ± 12.2 | 44.7 ± 16.9 |
| FL, IL-11 | 21 | 3.4 × 107 | 34,100 | 47.3 ± 8.5 | 36.2 ± 16.8 |
| KL, IL-11 | 21 | 3.2 × 107 | 9,200 | 16.3 ± 2.5 | 11.7 ± 2.2 |
Irradiated recipients (5 mice per group) were transplanted with 100 fresh Lin−Sca-1+c-kit+ cells or with the whole fractions of day 7 or day 21 cultured cells that had been initiated with 100 Lin−Sca-1+c-kit+ cells.
Mean ± SD % donor-derived nucleated peripheral blood cells identified by anti-Ly5.1. Background (peripheral blood cells of nontransplanted female mice) staining was <1.0%.
Finally, we examined the in vivo reconstituting ability of cells expanded in the presence of multiple cytokines. The four cytokine combinations with FL or KL did not enhance the in vivo reconstituting ability of cultured cells versus fresh cells (Table 5, experiment 1). We previously reported that IL-3 and IL-1 possess negative regulatory effects on early B lymphopoiesis,48 T lymphopoiesis,49 and in vitro expansion of stem cells with long-term reconstitution capabilities.50 Therefore, in one experiment, we examined the addition of IL-3 to multiple cytokines. When added to four-cytokine combinations consisting of KL, IL-6, IL-11, and Ep, IL-3 significantly reduced the in vivo reconstituting ability of cultured cells, whereas FL did not (Table 5, experiment 2).
Effects of Multiple Cytokines on the Reconstituting Ability of Cultured Cells
| Cytokines in Culture . | No. of Cells Injected . | No. of CFU-C Injected . | % Ly5.1 (donor) PB Cells5-150 . | |
|---|---|---|---|---|
| . | . | . | 2 mo . | 6 mo . |
| Experiment 1 | ||||
| Fresh marrow | 100 | 48 | 47.1 ± 22.8 | 54.6 ± 29.8 |
| FL,IL-6,IL-11,Ep | 2.7 × 104 | 4,500 | 58.7 ± 19.1 | 42.0 ± 18.7 |
| KL,IL-6,IL-11,Ep | 3.3 × 105 | 30,000 | 72.9 ± 11.2 | 48.3 ± 26.2 |
| Experiment 2 | ||||
| Fresh marrow | 500 | 240 | 71.3 ± 17.8 | 65.1 ± 28.3 |
| KL,IL-6,IL-11,Ep | 8.0 × 105 | 182,000 | 69.5 ± 17.5 | 63.5 ± 30.6 |
| FL,KL,IL-6,IL-11,Ep | 1.5 × 106 | 281,000 | 80.5 ± 16.2 | 73.8 ± 22.6 |
| IL-3,KL,IL-6,IL-11,Ep | 3.3 × 106 | 35,000 | 19.3 ± 11.1 | 14.5 ± 11.5 |
| Cytokines in Culture . | No. of Cells Injected . | No. of CFU-C Injected . | % Ly5.1 (donor) PB Cells5-150 . | |
|---|---|---|---|---|
| . | . | . | 2 mo . | 6 mo . |
| Experiment 1 | ||||
| Fresh marrow | 100 | 48 | 47.1 ± 22.8 | 54.6 ± 29.8 |
| FL,IL-6,IL-11,Ep | 2.7 × 104 | 4,500 | 58.7 ± 19.1 | 42.0 ± 18.7 |
| KL,IL-6,IL-11,Ep | 3.3 × 105 | 30,000 | 72.9 ± 11.2 | 48.3 ± 26.2 |
| Experiment 2 | ||||
| Fresh marrow | 500 | 240 | 71.3 ± 17.8 | 65.1 ± 28.3 |
| KL,IL-6,IL-11,Ep | 8.0 × 105 | 182,000 | 69.5 ± 17.5 | 63.5 ± 30.6 |
| FL,KL,IL-6,IL-11,Ep | 1.5 × 106 | 281,000 | 80.5 ± 16.2 | 73.8 ± 22.6 |
| IL-3,KL,IL-6,IL-11,Ep | 3.3 × 106 | 35,000 | 19.3 ± 11.1 | 14.5 ± 11.5 |
Irradiated recipients (5 mice per group) were transplanted with 100 (experiment 1) or 500 (experiment 2) freshly prepared Lin−Sca-1+c-kit+ cells or with the whole fractions of day 7 cultured cells that had been initiated with 100 or 500 Lin−Sca-1+c-kit+ cells.
Mean ± SD % donor-derived nucleated peripheral blood cells identified by anti-Ly5.1. Background (peripheral blood cells of nontransplanted female mice) staining was <1.0%.
DISCUSSION
Previously, we reported that combinations of FL and either IL-6, IL-11, or G-CSF support proliferation of primitive hematopoietic progenitors including lymphohematopoietic progenitors.17 In this regard, FL and KL are similar in hematopoietic function, although the number and size of the colonies supported by FL-containing cytokine combinations were smaller than those supported by KL-containing cytokine combinations.17 During the same study, we noted that blast-like cells persist longer in incubation with FL than with KL. FL and KL signal through different but related tyrosine kinase receptors.1,2 14 Because of their similarities and dissimilarities, we compared their effects on the in vitro expansion of progenitors and hematopoietic stem cells with reconstituting capability. Proliferation of the progenitors appeared slower but persisted longer in suspension culture with FL and IL-11 than in culture with KL and IL-11.
We then compared the ability of FL and KL to support in suspension culture the in vivo long-term reconstituting cells. In the presence of IL-11, both factors maintained the reconstituting cells in culture for 14 days. Although there was no apparent expansion of the stem cells, the standard deviations of the means of the results were large. Neither were there apparent differences between the combination of FL and IL-11 and the combination of KL and IL-11. However, there were kinetic differences in the peak production of CFU-C in that for KL it occurred at day 14 and for FL at day 21 or longer. This time course of the reconstituting cells appears to be consistent with that of the progenitors. In our previous studies, we noted a gradual decline of stem cell functions in 2-week suspension cultures with KL and IL-11.50 However, in that series of experiments, we sorted the cultured cells on day 7 for cells with stem cell phenotypes and recultured the enriched cells for 7 more days. The serial dilution technique used in the current studies allowed maintenance of stem cell functions and appeared to be better than the resorting strategies we used previously. It is possible that stem cells change phenotypes during suspension culture. Alternatively, the resorting of cultured cells may have traumatized the stem cells.
As stated in the introduction, many investigators have already shown that it is possible to expand the population of cells and colony-forming cells in suspension culture in the presence of combinations of early-acting cytokines. However, attempts to expand the population of cells that are capable of long-term reconstitution have met with variable success.28,31-33 Recently, Peters et al51 reported that a 48-hour suspension culture of murine marrow cells in the presence of IL-3, IL-6, IL-11, and KL results in impairment of the engrafting capability of the cultured cells. They proposed that expansion of the progenitors may produce a defective long-term repopulating capability of the stem cells. We believe that the reason for their observation is that IL-3 abrogates the long-term reconstitution capability of cultured cells. Results presented herein and in a previous publication50 indicate that cells expanded in the absence of IL-3 or IL-1 maintain reconstituting ability.
In vitro expansion of hematopoietic stem cells promises to be important in clinical stem cell transplantation. We have shown that both FL and KL are capable of expanding the progenitor cell pool without compromising engraftment. Significant kinetic differences appear to exist between the two cytokines, but we have not determined which factor combination is superior. Again, we noted negative effects of IL-3 on the ability of cultured cells to engraft the marrow of recipient mice. Although it is not wise to translate findings in murine systems directly to human cells, cytokine combinations containing IL-3 need to be evaluated carefully for in vitro expansion of human stem cells.
ACKNOWLEDGMENT
We thank P.N. Pharr, A.G. Leary, and N.D. Grant for assistance in preparing the manuscript and H.Q. Zeng in FACS cell sorting.
Supported by National Institutes of Health grants no. DK/HL48714 and DK32294, the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, and a grant from Amgen Inc.
Address reprint requests to Makio Ogawa, MD, PhD, VA Medical Center, 109 Bee St, Charleston, SC 29401-5799.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal