Abstract
Familial and congenital polycythemia, not due to high oxygen affinity hemoglobin or reduced 2,3-diphosphoglycerate in erythrocytes, is common in the Chuvash population of the Russian Federation. Hundreds of individuals appear to be affected in an autosomal recessive pattern. We studied six polycythemic Chuvash patients <20 years of age from unrelated families and 12 first-degree family members. Hemoglobins were markedly elevated in the index subjects (mean ± standard deviation [SD] of 22.6 ± 1.4 g/dL), while platelet and white blood cell counts were normal. Although performed in only three of the index subjects, serum erythropoietin concentrations determined by both radioimmune and functional assays were significantly higher in polycythemic patients compared with first-degree family members with normal hemoglobin concentrations. Southern blot analysis of the Bgl 2 erythropoietin gene polymorphism showed that one polycythemic subject was a heterozygote, suggesting the absence of linkage of polycythemia with the erythropoietin gene, assuming autosomal recessive inheritance. Polymerase chain reaction (PCR) amplification of the GGAA and GA minisatellite polymorphic regions of the erythropoietin receptor gene showed no evidence of linkage of phenotype with this gene. We conclude that Chuvash polycythemia may represent a secondary form of familial and congenital polycythemia of as yet unknown etiology. This condition is the only endemic form of familial and congenital polycythemia described.
FAMILIAL AND CONGENITAL polycythemias refer to an uncommon group of inherited disorders that are characterized by an increase in the red blood cell mass. The term erythrocytosis is synonymous and also frequently used. These conditions may either be primary or secondary to elevated erythropoietin concentrations. Most cases of congenital polycythemia are secondary disorders caused by hemoglobin mutations that result in increased affinity for oxygen and consequently tissue hypoxia and elevated erythropoietin levels.1,2 More rarely, decreased production of 2,3-diphosphoglycerate, a molecule that facilitates the release of oxygen from hemoglobin to tissues,3,4 or inappropriate erythropoietin expression5-7 may lead to increased erythropoietin concentrations. In primary familial and congenital polycythemias, erythropoietin levels are not elevated, but erythroid progenitors have heightened sensitivity to erythropoietin.8-10 Polycythemia seems to result from mutations causing the excessive growth of erythroid progenitor cells.9 For example, mutations involving the erythropoietin receptor gene or its regulation may lead to autosomal dominant polycythemia in the presence of normal or low erythropoietin concentrations by an enhanced signal transduction, ie, gain-of-function mutation.8,11,12 Autosomal dominant mutations of genes other than the erythropoietin receptor gene affecting postreceptor responses may also lead to primary familial and congenital polycythemia.13,14 The primary and secondary congenital polycythemias described to date in the western medical literature occur sporadically, without a seeming predilection for a particular population. Not widely reported in the west, over the past 25 years Russian and Chuvash scientists have studied a form of congenital polycythemia that is endemic in the Chuvash population of the Russian Federation.15-17
The Chuvash Autonomous Republic is located on the west bank of the Volga River in the central part of European Russia. It is 18,300 square kilometers in area and has an altitude of less than 500 meters. The population was about 1,300,000 in 1979 with 70% of Chuvash ethnicity. The first reports of congenital polycythemia in Chuvashia appeared in the 1970s.15 16 One hundred and three cases of polycythemia that seemed to be familial in nature and that did not fit the classification of polycythemia vera were collected over a period of 10 years. All of these patients were Chuvash by nationality and inhabitants of the northeastern region of Chuvashia. More than two thirds of the patients were less than 20 years of age at the time of diagnosis (Table 1) and slightly more than one half were females. As shown in Table 2, most of the 103 Chuvash patients with polycythemia gave a history of fatigue and headaches and all demonstrated plethora on physical examination. Basic hematological laboratory findings are summarized in Table 3. In addition, the bone marrow, examined histologically in 62 patients, showed hypercellularity in 48 patients, normal cellularity in 13, and hypoplasia in one patient who had been treated with 32P. In all cases, there was absolute or relative erythroid hyperplasia, while megakaryocytes were not increased in number.
Age at Diagnosis of 103 Chuvash Patients With Polycythemia
| Age at Diagnosis (yr) . | No. . | % . |
|---|---|---|
| 2 to 4 | 6 | 5.8 |
| 5 to 9 | 17 | 16.5 |
| 10 to 14 | 18 | 17.5 |
| 15 to 19 | 33 | 32.0 |
| 20 to 24 | 8 | 7.8 |
| 25 to 29 | 6 | 5.8 |
| 30 to 34 | 5 | 4.9 |
| 35 to 39 | 6 | 5.8 |
| 40 and older | 4 | 3.9 |
| Age at Diagnosis (yr) . | No. . | % . |
|---|---|---|
| 2 to 4 | 6 | 5.8 |
| 5 to 9 | 17 | 16.5 |
| 10 to 14 | 18 | 17.5 |
| 15 to 19 | 33 | 32.0 |
| 20 to 24 | 8 | 7.8 |
| 25 to 29 | 6 | 5.8 |
| 30 to 34 | 5 | 4.9 |
| 35 to 39 | 6 | 5.8 |
| 40 and older | 4 | 3.9 |
Symptoms and Signs in 103 Polycythemic Chuvash Patients
| Finding . | No. . | % . |
|---|---|---|
| Symptoms | ||
| Headache | 68 | 66.0 |
| Fatigue | 53 | 51.5 |
| Abdominal pain | 21 | 20.4 |
| Pain in the lower extremities | 20 | 19.4 |
| Chest pain | 18 | 17.5 |
| No complaints | 26 | 25.2 |
| Signs | ||
| Plethora | 103 | 100 |
| Mild hepatomegaly | 34 | 33.0 |
| Clubbing | 31 | 30.1 |
| Bleeding tendency or hemorrhage | 25 | 24.3 |
| Delayed physical development | 23 | 22.3 |
| Varicose veins | 19 | 18.4 |
| Slight splenomegaly | 13 | 12.6 |
| Thrombosis | 10 | 9.7 |
| Peptic ulcer | 7 | 6.8 |
| Finding . | No. . | % . |
|---|---|---|
| Symptoms | ||
| Headache | 68 | 66.0 |
| Fatigue | 53 | 51.5 |
| Abdominal pain | 21 | 20.4 |
| Pain in the lower extremities | 20 | 19.4 |
| Chest pain | 18 | 17.5 |
| No complaints | 26 | 25.2 |
| Signs | ||
| Plethora | 103 | 100 |
| Mild hepatomegaly | 34 | 33.0 |
| Clubbing | 31 | 30.1 |
| Bleeding tendency or hemorrhage | 25 | 24.3 |
| Delayed physical development | 23 | 22.3 |
| Varicose veins | 19 | 18.4 |
| Slight splenomegaly | 13 | 12.6 |
| Thrombosis | 10 | 9.7 |
| Peptic ulcer | 7 | 6.8 |
Laboratory Values in 103 Chuvash Patients With Polycythemia
| . | Mean ± SD . | Normal Range . |
|---|---|---|
| Hemoglobin (g/dL)3-150 | 21.8 ± 2.5 | 12-16, F; 14-18, M |
| Hematocrit3-151 (%; range) | 76.5 ± 7.6 | 37-47, F; 40-53, M |
| Red blood cells (×10−6/μL) | 7.3 ± 0.8 | 3.8-5.2, F; 4.4-5.9, M |
| Leukocytes (×10−3/μL) | 6.1 ± 1.9 | 4.0-10.0 |
| Neutrophils (%) | 63 ± 14 | 46-74 |
| Blood viscosity | 15 ± 4.5 | 3.9-5.3 |
| Leukocyte alkaline phosphatase | 11.2 ± 6.7 | 25-139 |
| Bilirubin (mg/dL) | 1.2 ± 0.8 | 0.1-1.0 |
| . | Mean ± SD . | Normal Range . |
|---|---|---|
| Hemoglobin (g/dL)3-150 | 21.8 ± 2.5 | 12-16, F; 14-18, M |
| Hematocrit3-151 (%; range) | 76.5 ± 7.6 | 37-47, F; 40-53, M |
| Red blood cells (×10−6/μL) | 7.3 ± 0.8 | 3.8-5.2, F; 4.4-5.9, M |
| Leukocytes (×10−3/μL) | 6.1 ± 1.9 | 4.0-10.0 |
| Neutrophils (%) | 63 ± 14 | 46-74 |
| Blood viscosity | 15 ± 4.5 | 3.9-5.3 |
| Leukocyte alkaline phosphatase | 11.2 ± 6.7 | 25-139 |
| Bilirubin (mg/dL) | 1.2 ± 0.8 | 0.1-1.0 |
Hemoglobin and bilirubin were determined spectrophotometrically. Hematocrit was measured by centrifugation. Red blood cells, leukocytes, and percentage of neutrophils were determined microscopically. Blood viscosity was measured with a viscometer VK-4. Leukocyte alkaline phosphatase was determined by scoring the degree of staining of precipitated alkaline phosphatase in white cells in a peripheral blood smear.
N = 95.
Over 10 years of observation, 11 of 103 Chuvash patients with polycythemia died. The causes of death in the first eight patients to die are shown in Table 4. Their ages ranged from 16 years to 58 years, and thrombosis was the leading cause of death. Genealogical studies were performed in 79 families with a total of 339 siblings. All of them were from phenotypically normal parents, and the children of the patients were also phenotypically normal. The hypothesis of autosomal recessive inheritance was tested using the “a priori” method of Hogben.18 As shown in Table 5, polycythemia appeared to follow an autosomal recessive pattern of inheritance. More recently, we have observed two pedigrees in which affected subjects are present in two generations, ie, a maternal grandmother and a grandchild in each of these families. Consanguinity is not common among the Chuvash people.
Causes of Death in 8 Chuvash Patients With Polycythemia
| . | No. . | % . |
|---|---|---|
| Cerebral vein thrombosis | 3 | 37.5 |
| Cerebral hemorrhage | 2 | 25.0 |
| Lower extremity venous thrombosis | 1 | 12.5 |
| Cardiovascular decompensation | 1 | 12.5 |
| Carcinoma of the stomach | 1 | 12.5 |
| . | No. . | % . |
|---|---|---|
| Cerebral vein thrombosis | 3 | 37.5 |
| Cerebral hemorrhage | 2 | 25.0 |
| Lower extremity venous thrombosis | 1 | 12.5 |
| Cardiovascular decompensation | 1 | 12.5 |
| Carcinoma of the stomach | 1 | 12.5 |
Segregation Analysis of 79 Chuvash Families With Polycythemia Using the “A Priori” Method of Hogben18
| Size of Sibship . | No. of Sibships . | Total No. of Individuals . | No. of Affected Individuals . | Variance . | |
|---|---|---|---|---|---|
| . | . | . | Observed . | Expected . | . |
| 2 | 15 | 30 | 16 | 17.142 | 1.837 |
| 3 | 11 | 33 | 14 | 14.270 | 2.893 |
| 4 | 18 | 72 | 22 | 26.330 | 7.561 |
| 5 | 16 | 80 | 24 | 26.222 | 9.468 |
| 6 | 11 | 66 | 20 | 20.073 | 8.535 |
| 7 | 6 | 42 | 9 | 12.118 | 5.821 |
| 8 | 2 | 16 | 6 | 4.445 | 2.345 |
| Totals | 79 | 339 | 111 | 120.600 | 38.460 |
| SE = 6.202 | |||||
| Size of Sibship . | No. of Sibships . | Total No. of Individuals . | No. of Affected Individuals . | Variance . | |
|---|---|---|---|---|---|
| . | . | . | Observed . | Expected . | . |
| 2 | 15 | 30 | 16 | 17.142 | 1.837 |
| 3 | 11 | 33 | 14 | 14.270 | 2.893 |
| 4 | 18 | 72 | 22 | 26.330 | 7.561 |
| 5 | 16 | 80 | 24 | 26.222 | 9.468 |
| 6 | 11 | 66 | 20 | 20.073 | 8.535 |
| 7 | 6 | 42 | 9 | 12.118 | 5.821 |
| 8 | 2 | 16 | 6 | 4.445 | 2.345 |
| Totals | 79 | 339 | 111 | 120.600 | 38.460 |
| SE = 6.202 | |||||
The patients were members of 81 families, but in two families, the patient with polycythemia was the only child. The expected number of siblings with polycythemia was 120.6 compared with the observed number of 111. The difference between the expected and observed numbers of affected siblings of 9.6 was less than two times the standard error of the variance (<2 × 6.202), and the hypothesis of autosomal recessive inheritance was not rejected (χ2 = 2.33, df = 6, P > .8).
Some pathogenic features of Chuvash polycythemia have also been characterized.16,17 The plasma volume was determined in 19 patients (11 men and 8 women) by injecting albumin labeled with 131I and determining its dilution. The volume of circulating whole blood and the red blood cell mass were then estimated according to the hematocrit. The volume of plasma was normal or slightly decreased at 42.6 ± 3.2 mL/kg, while the estimated blood volume was elevated (103.3 ± 6.5 mL/kg) as was the estimated red blood cell mass (60.7 ± 5.4 mL/kg).17 Polyacrylamide gel hemoglobin electrophoresis was performed in 19 Chuvash patients with polycythemia, and an abnormal hemoglobin fraction was not identified.16 The methemoglobin concentration was measured spectrophotometrically in 10 patients and was <1% in all.16 The concentration of 2,3-diphosphoglycerate in erythrocytes was analyzed enzymatically in 11 patients and 10 healthy controls. The results for patients with Chuvash polycythemia ranged from 4.3 to 6.1 μmol 2,3-diphosphoglycerate/L erythrocytes, and the mean ± standard error (SE) of 5.2 ± 0.2 μmol/L erythrocytes did not differ significantly from healthy controls (4.7 ± 0.09).17 Analyses of arterial blood gases and O2 dissociation curves in 22 patients with polycythemia and 10 healthy controls showed that pCO2 , pO2 , and O2 saturation were similar between the patients and the controls, while the pH was lower in the patients and the O2 dissociation curve was shifted slightly to the right (Table 6).17 Erythropoietin levels were estimated in 20 Chuvash subjects with polycythemia before and after phlebotomy therapy and in 10 healthy controls. The method used was an in vivo bioassay based on the reticulocyte response in polycythemic Swiss mice.19 Erythropoietin levels tended to be in the normal to elevated range in patients with polycythemia before phlebotomy therapy, and they increased significantly with phlebotomy (Table 7).17
Arterial Blood Gases and P50 in Chuvash Patients With Polycythemia and in Controls (Mean ± SD)
| Group . | pH . | pCO2 (mmHg) . | pO2 (mmHg) . | O2 Saturation (%) . | p50 (mmHg) . |
|---|---|---|---|---|---|
| Polycythemia (n = 22) | 7.32 ± .03 | 56 ± 6 | 81 ± 7 | 96 ± 1 | 30.8 ± .4 |
| Healthy (n = 10) | 7.35 ± .03 | 51 ± 4 | 85 ± 10 | 95 ± 2 | 28.6 ± .9 |
| Group . | pH . | pCO2 (mmHg) . | pO2 (mmHg) . | O2 Saturation (%) . | p50 (mmHg) . |
|---|---|---|---|---|---|
| Polycythemia (n = 22) | 7.32 ± .03 | 56 ± 6 | 81 ± 7 | 96 ± 1 | 30.8 ± .4 |
| Healthy (n = 10) | 7.35 ± .03 | 51 ± 4 | 85 ± 10 | 95 ± 2 | 28.6 ± .9 |
Analyzed with an OsM-I analyzer (Radiometer, Denmark).
Erythropoietin Levels Before and After Phlebotomy Therapy (N = 20)
| . | Before Phlebotomies . | After Phlebotomies . | P . |
|---|---|---|---|
| . | Median (range) . | Median (range) . | . |
| Hematocrit (%) | 66 (55-77) | 48 (37-60) | <.001 |
| Erythropoietin (U/mL)7-150 | 51 (23-649) | 121 (29-649) | .007 |
| . | Before Phlebotomies . | After Phlebotomies . | P . |
|---|---|---|---|
| . | Median (range) . | Median (range) . | . |
| Hematocrit (%) | 66 (55-77) | 48 (37-60) | <.001 |
| Erythropoietin (U/mL)7-150 | 51 (23-649) | 121 (29-649) | .007 |
Mean ± SD of 51 ± 23 U/mL in 10 healthy controls (range, 20-85).
To summarize, the studies from Russia and Chuvashia indicate that Chuvash polycythemia is a true absolute polycythemia not due to an abnormal hemoglobin, methemoglobinemia, pulmonary disease, or reduced activity of 2,3-diphosphoglycerate. The age, sex distribution, symptoms, physical findings, certain laboratory values, and familial pattern differ from those of polycythemia vera.20 21 The serum erythropoietin levels performed with a semiquantitative biological assay are inconclusive, but may be consistent with a secondary form of familial and congenital polycythemia. The mortality of more than 10% over 10 years of observation in a very young cohort of patients indicates that this form of polycythemia is not benign.
In the present report, we provide results based on studies of several polycythemic subjects and nonpolycythemic family members from Chuvashia that bear on the pathophysiology of Chuvash polycythemia. Additionally we suggest the appropriate classification of this polycythemic disorder.
MATERIALS AND METHODS
Clinical Studies of Chuvash Patients With Polycythemia and Their Family Members
Six patients with polycythemia and 12 first-degree family members from six unrelated families were studied in Cheboksary, Chuvashia, or Moscow, Russia after giving informed consent. The first-degree family members included eight parents and four siblings. Each subject was examined clinically and had peripheral blood samples drawn by venipuncture. Four of the index subjects underwent echocardiography and had ultrasound examination of the abdomen. Complete blood counts were determined on EDTA anticoagulated blood using automated cell counters. The percentage of reticulocytes was determined by microscopic examination of peripheral blood smears stained with brilliant cresyl blue. The absolute reticulocyte counts were estimated by multiplying the percent of reticulocytes and the red blood cell count determined by the automated cell counter. The percentage of white blood cell subtypes was determined by automated cell sorting or by differential counting of a peripheral blood smear microscopically, and the absolute counts were determined by multiplying these percentages by the white blood cell counts.
Assessment of Hemoglobin and Hemoglobin Association
Standard procedures were used for the preparation of erythrocyte hemolysates, the electrophoresis of hemoglobins on cellulose acetate and in citrate agar, and the electrophoresis of globins at alkaline and acid pH.22 Two affected subjects had the hemoglobin dissociation curve and dissociation constant measured by cooximeter.8 Measurement of red blood cell 2,3-diphosphoglycerate was performed in one polycythemic subject as described by Beutler.23
Determination of Serum Erythropoietin Concentrations
Erythropoietin concentrations were determined in the sera of three patients with polycythemia by a radioimmunoassay performed by Dr J. Goldwasser's laboratory (University of Chicago, Chicago, IL)24 and by a functional assay involving antierythropoietin antibodies bound to magnetic beads (Dr Gerald Krystal, The Terry Fox Laboratory, Vancouver, British Columbia).25 26 Erythropoietin concentrations were measured in the sera from six first-degree family members by the radioimmunoassay and from eight first-degree family members by the functional assay.
DNA Studies
Genomic DNA was isolated from peripheral blood leukocytes by a standard procedure.27
Erythropoietin gene.Southern blot analyses were performed in six unrelated Chuvash patients with polycythemia and 11 first-degree family members according to standard procedures.27 Genomic DNA samples were examined with the enzymes Hinf I28 and Bgl II (originally described as a polymorphism of a tightly linked type I collagen gene29 30 ) to assess two defined restriction fragment polymorphisms of the erythropoietin gene. Both probes were a gift of Dr Fu-Kuen Lin (Amgen, Thousand Oaks, CA).
Erythropoietin receptor gene.Polymorphisms of the erythropoietin receptor gene were studied in three unrelated Chuvash patients with polycythemia and eight first-degree family members. Two microsatellite markers consisting of GGAA and GA repeats were amplified by polymerase chain reaction (PCR) as described31 in two cycles using identical PCR conditions. Briefly, the primer sequences for the detection of (GGAA)n were 5′-GCCAGGTGCAGTGGGAAGATTGCT-3′ (forward primer) and 5′-TCTCTCTCTCCTTTCCTTCCTTCC-3′ (reverse primer). The primer sequences for the detection of (GA)n were 5′-GAAGGAAGGAAGGAAGGAAGGAAAGG-3′ (forward primer) and 5′-GCCCTGGACAGGTGACTTACCTTA-3′ (reverse primer). The PCR reaction was performed in a 10-μL reaction mixture (50 mmol/L KCl, 10 mmol/L Tris-HCl [pH 9.0], 1.5 mmol/L MgCl2 , 200 μmol/L each deoxynucleotide triphosphate (dNTP), 0.01% Triton X-100, 3 pmol/L each primer, 20 ng genomic DNA, 0.5 U Taq polymerase [Promega Corp, Madison, WI], 1.5 pmol/L end-labeled primers with 32P[dATP]). Genomic DNA was amplified for 30 cycles using a Perkin Elmer-Cetus DNA Thermal Cycler 480 (Perkin Elmer-Cetus Corp, Norwalk, CT), denaturation 45 seconds at 94°C, annealing 30 seconds at 60°C, and elongation 30 seconds at 72°C. The initial denaturation step was performed at 94°C for 5 minutes. The PCR products were analyzed on a 6% denaturing polyacrylamide gel. The number of repeats was verified by sequencing of subcloned PCR fragments into the pGEM-3Zf+ plasmid vector (Promega Corp). Several independent clones from each sequenced allele were analyzed.
Statistical Analyses
The Student's t-test was used to compare continuous variables between index subjects and first-degree family members. Fisher's exact test was used to compare proportions. Serum erythropoietin concentrations were compared after log transformation.
RESULTS
Clinical Studies
The six index subjects who form the basis of the present study reported intermittent symptoms of headache and malaise and one man gave a history of seizures. In all of these subjects, there was historical evidence of plethora and/or elevated hemoglobin concentrations within the first year of life. In two individuals, elevated hemoglobin concentrations had been noted in the neonatal period before discharge from the hospital. On physical examination, all of the patients had plethora, while none of them had hepatomegaly or splenomegaly. There were no signs of cardiac or pulmonary disease. Among the four index subjects who underwent echocardiography and abdominal ultrasonography, no evidence of valvular or septal heart defects was found, and there were no hepatic or renal masses. The demographic and laboratory features of the six Chuvash patients with polycythemia and the 12 first-degree family members we studied are shown in Table 8.
Demographic and Clinical Features of Chuvash Patients With Polycythemia and Their First-Degree Relatives
| . | Index Subjects . | First-Degree Family Members (N = 12) . | P . |
|---|---|---|---|
| . | (N = 6) . | . | . |
| Age (years) | 11 ± 3 | 33 ± 13 | <.0005 |
| Females (no. and %) | 4 (67) | 6 (50) | .6 |
| Hemoglobin (g/dL) | 22.6 ± 1.4 | 14.5 ± 1.1 | <.0005 |
| Hematocrit (%) | 66.6 ± 3.9 | 42.7 ± 3.4 | <.0005 |
| Red blood cells (×10−6/μL) | 7.5 ± 0.5 | 4.7 ± 0.5 | <.0005 |
| Mean corpuscular volume (fL) | 89 ± 4 | 90 ± 5 | .6 |
| Mean corpuscular hemoglobin (pg) | 30.1 ± 1.3 | 30.6 ± 1 | .5 |
| Red blood cell distribution width (%) | 14.0 ± 0.88-150 | 13.0 ± 0.68-151 | .012 |
| Reticulocytes (×10−3/μL) | 66.3 ± 39.28-152 | 41.7 ± 15.6ρ | .3 |
| White blood cells (×10−3/μL) | 7.2 ± 1.6 | 7.3 ± 1.5 | .8 |
| Neutrophils (×10−3/μL) | 4.1 ± 1.4 | 3.8 ± 1.2 | .7 |
| Lymphocytes (×10−3/μL) | 2.2 ± 1.0 | 2.4 ± 0.9 | .7 |
| Monocytes (×10−3/μL) | 0.6 ± 0.6 | 0.9 ± 0.6 | .4 |
| Eosinophils (×10−3/μL) | 0.2 ± 0.2 | 0.2 ± 0.1 | .6 |
| Platelets (×10−3/μL) | 213 ± 38 | 255 ± 53 | .1 |
| . | Index Subjects . | First-Degree Family Members (N = 12) . | P . |
|---|---|---|---|
| . | (N = 6) . | . | . |
| Age (years) | 11 ± 3 | 33 ± 13 | <.0005 |
| Females (no. and %) | 4 (67) | 6 (50) | .6 |
| Hemoglobin (g/dL) | 22.6 ± 1.4 | 14.5 ± 1.1 | <.0005 |
| Hematocrit (%) | 66.6 ± 3.9 | 42.7 ± 3.4 | <.0005 |
| Red blood cells (×10−6/μL) | 7.5 ± 0.5 | 4.7 ± 0.5 | <.0005 |
| Mean corpuscular volume (fL) | 89 ± 4 | 90 ± 5 | .6 |
| Mean corpuscular hemoglobin (pg) | 30.1 ± 1.3 | 30.6 ± 1 | .5 |
| Red blood cell distribution width (%) | 14.0 ± 0.88-150 | 13.0 ± 0.68-151 | .012 |
| Reticulocytes (×10−3/μL) | 66.3 ± 39.28-152 | 41.7 ± 15.6ρ | .3 |
| White blood cells (×10−3/μL) | 7.2 ± 1.6 | 7.3 ± 1.5 | .8 |
| Neutrophils (×10−3/μL) | 4.1 ± 1.4 | 3.8 ± 1.2 | .7 |
| Lymphocytes (×10−3/μL) | 2.2 ± 1.0 | 2.4 ± 0.9 | .7 |
| Monocytes (×10−3/μL) | 0.6 ± 0.6 | 0.9 ± 0.6 | .4 |
| Eosinophils (×10−3/μL) | 0.2 ± 0.2 | 0.2 ± 0.1 | .6 |
| Platelets (×10−3/μL) | 213 ± 38 | 255 ± 53 | .1 |
Values are mean ± SD unless otherwise indicated.
N = 5.
N = 10.
N = 4.
ρ N = 6.
Laboratory Studies
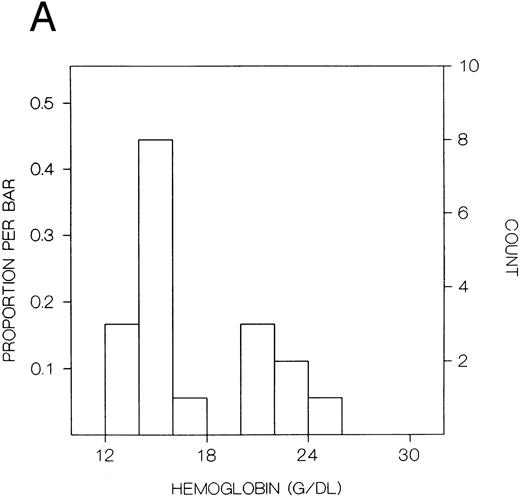
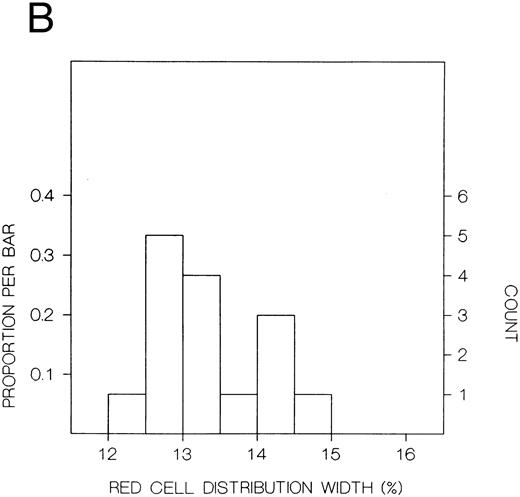
The values for hemoglobin, hematocrit, red blood cells, and red blood cell distribution width were significantly higher in the index subjects than the first-degree relatives, but there were no significant differences in white blood cells or platelets (Table 8). The frequency distributions of hemoglobin concentration and red blood cell distribution width for the 18 subjects included in this study are shown in Fig 1. There were distinct bimodal distributions to these measures with the index subjects all falling in the upper modes and the first-degree relatives in the lower modes.
Frequency distributions of (A) hemoglobin in six Chuvash index subjects with polycythemia and 12 first-degree family members and (B) red blood cell distribution width in five index subjects and 10 first-degree relatives. For each measure, there is a distinct bimodal distribution; all of the index subjects are in the higher distribution and all of the first-degree relatives are in the lower distribution.
Frequency distributions of (A) hemoglobin in six Chuvash index subjects with polycythemia and 12 first-degree family members and (B) red blood cell distribution width in five index subjects and 10 first-degree relatives. For each measure, there is a distinct bimodal distribution; all of the index subjects are in the higher distribution and all of the first-degree relatives are in the lower distribution.
Assessment of Hemoglobin and Hemoglobin Association
No electrophoretically distinguishable hemoglobin variant was detected. In two index subjects, the p50s were 26.8 and 27.4 mm Hg (normal, 24 to 30) and the Hill dissociation constant was 2.81 in both cases (normal, 2.72 to 2.91). The erythrocyte 2,3-diphosphoglycerate concentration was 11.9 μmol/g hemoglobin in one index subject (normal, 12.3 ± 1.9).
Erythropoietin Levels
Geometric mean values for radioimmunoassay and functional measurements of erythropoietin are shown in Table 9. Erythropoietin levels were significantly higher in the index subjects than in the first-degree family members, even though the index subjects had much higher hematocrits.
Serum Erythropoietin and Hemoglobin Levels in Chuvash Patients With Polycythemia and in First-Degree Relatives
| . | Index Subjects . | First-Degree Relatives (N = 8) . | P . |
|---|---|---|---|
| . | (N = 3) . | . | . |
| Hemoglobin (g/dL; mean ± SD) | 22.4 ± 0.7 | 14.2 ± 0.9 | <.0005 |
| Erythropoietin by radioimmunoassay (mU/mL)9-150 | 17 (8-37) | 7 (4-11)9-151 | .045 |
| Erythropoietin by functional assay (mU/mL)9-152 | 85 (58-124) | 39 (24-64) | .036 |
| . | Index Subjects . | First-Degree Relatives (N = 8) . | P . |
|---|---|---|---|
| . | (N = 3) . | . | . |
| Hemoglobin (g/dL; mean ± SD) | 22.4 ± 0.7 | 14.2 ± 0.9 | <.0005 |
| Erythropoietin by radioimmunoassay (mU/mL)9-150 | 17 (8-37) | 7 (4-11)9-151 | .045 |
| Erythropoietin by functional assay (mU/mL)9-152 | 85 (58-124) | 39 (24-64) | .036 |
Results are geometric mean and SD range.
Normal 21 ± 6 mU/mL.
N = 6.
Normal < 50 mU/mL.
DNA Studies
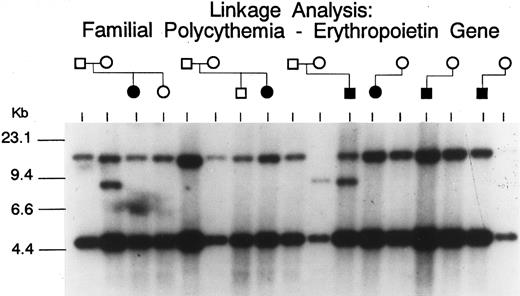
Erythropoietin gene.The Hinf I and Bgl II polymorphisms of the erythropoietin gene were examined in six Chuvash pedigrees comprising six patients with polycythemia and 11 first-degree family members. The Bgl II polymorphism was found to be informative in these studies. Five patients were homozygotes for this polymorphism, but one was a heterozygote. The heterozygous polycythemic patient's mother, an obligate carrier assuming autosomal recessive inheritance, was a homozygote for a different allele than the patient's father and the five other unrelated polycythemic patients (Fig 2).
Southern blot analysis of the erythropoietin gene (digested with Bgl II) in six polycythemic Chuvash patients and some of their first-degree relatives. The higher molecular weight Bgl II fragment is polymorphic, with the ∼15 kb wild allele having an 8 kb partner. A mother of the polycythemic patient in the third pedigree (an obligate carrier assuming autosomal recessive inheritance) is homozygous for the poymorphic allele, while her affected son is heterozygous. These data suggest that neither allele is linked to polycythemia.
Southern blot analysis of the erythropoietin gene (digested with Bgl II) in six polycythemic Chuvash patients and some of their first-degree relatives. The higher molecular weight Bgl II fragment is polymorphic, with the ∼15 kb wild allele having an 8 kb partner. A mother of the polycythemic patient in the third pedigree (an obligate carrier assuming autosomal recessive inheritance) is homozygous for the poymorphic allele, while her affected son is heterozygous. These data suggest that neither allele is linked to polycythemia.
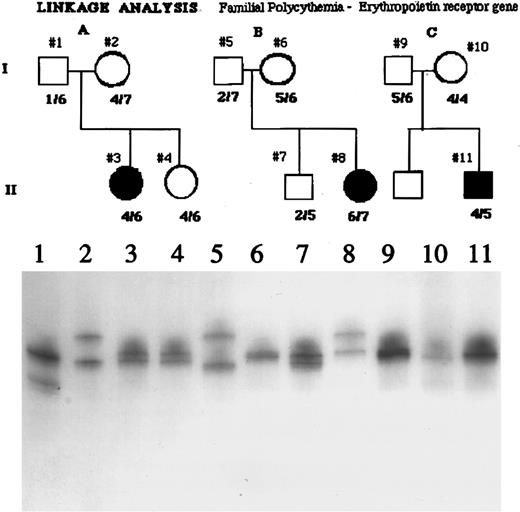
Erythropoietin receptor gene.Minisatellite polymorphisms of the erythropoietin receptor were examined in three families comprising three patients and eight first-degree family members. Because of higher polymorphic allelic frequency GGAA minisatellite polymorphisms, not all of the families depicted in Fig 2 were examined for erythropoietin receptor GA minisatellite polymorphisms. As shown in Fig 3, several different erythropoietin receptor alleles were present in the affected subjects, and these subjects were not homozygotes for one or a restricted number of alleles.
Analysis of the GGAA minisatellite repeat of the erythropoietin receptor gene in three polycythemic Chuvash patients and some of their first-degree relatives. As shown in the autoradiogram of the PCR analysis of this locus, several different alleles are present in the affected subjects, in their parents (obligate carriers assuming autosomal recessive inheritance), and in siblings. Because the subjects with polycythemia are not homozygous for one or a restricted number of alleles, these data do not suggest linkage of polycythemia to the erythropoietin receptor gene.
Analysis of the GGAA minisatellite repeat of the erythropoietin receptor gene in three polycythemic Chuvash patients and some of their first-degree relatives. As shown in the autoradiogram of the PCR analysis of this locus, several different alleles are present in the affected subjects, in their parents (obligate carriers assuming autosomal recessive inheritance), and in siblings. Because the subjects with polycythemia are not homozygous for one or a restricted number of alleles, these data do not suggest linkage of polycythemia to the erythropoietin receptor gene.
DISCUSSION
Polycythemia was detected in the Chuvash population of the mid-Volga River region of Russia in the 1960s15 and by 1977, 103 cases from 81 families had been described.16 Since then, more cases have come to light, and we estimate that hundreds of children may suffer from this condition. Virtually all of the individuals with this form of polycythemia live in the Chuvash Autonomous Region and other nearby geographical areas in the mid-Volga River region of the Russian Federation. The condition causes thrombotic and hemorrhagic vascular complications, which lead to early mortality, and survival beyond the age of 40 years is uncommon.16 Thus, this disease has substantial morbidity that may be even higher than that observed in the primary familial and congenital polycythemias that were initially considered to be benign conditions.32 As reviewed above, affected patients do not fit the picture of polycythemia vera and they tend to have normal blood gases. Children of both sexes are born with the disease to normal parents, and the occurrence of the condition within sibships is consistent with autosomal recessive inheritance. Thus, Chuvash polycythemia appears to fall in the category of a familial and congenital polycythemia, and it is probably the most common congenital polycythemia in the world.
In our present clinical studies of six Chuvash families (Table 8), we confirm that children with polycythemia have extreme elevations of hemoglobin (mean, 23 g/dL) and hematocrit (mean, 67%) in the absence of splenomegaly, cardiac or pulmonary disease, or any other systemic process than polycythemia. Mean values for hemoglobin and hematocrit are normal in first-degree family members. When the frequency distribution of hemoglobin or hematocrit is examined in these pedigrees, a clear-cut bimodal pattern is observed (Fig 1) with all patients falling in the upper mode and all first-degree family members falling in the lower mode. This pattern is more suggestive of a genetic influence on blood counts than some environmental influence, which would be expected to affect the results in other household members as well.
Our finding of elevated erythropoietin levels in the index subjects compared with first-degree family members (Table 9) would suggest that Chuvash polycythemia may represent a secondary form of familial and congenital polycythemia. Both the previous investigations performed in Chuvashia and our present findings indicate that this condition is not due to an abnormal hemoglobin with heightened affinity to oxygen or to decreased erythrocyte levels of 2,3-diphosphoglycerate. Our present investigation seems to rule out structural abnormalities of the heart, lungs, liver, or kidneys as being responsible.
Because one could hypothesize abnormal regulation of the erythropoietin gene to be implicated in the pathogenesis of Chuvash polycythemia, we analyzed the Bgl II polymorphism of the erythropoietin gene in the present study (Fig 2). The lack of consistent homozygosity for this restriction fragment length polymorphism (RFLP) in the patients with polycythemia is evidence against linkage of this phenotype with the erythropoietin gene. Due to the small size of the Chuvash population and its ethnic homogeneity, it is likely that Chuvash polycythemia represents a founder effect and that all affected individuals within this population would carry the same disease allele. While less likely as an explanation for the pathogenesis of Chuvash polycythemia (in view of the elevated levels of erythropoietin), we also examined the possibility that a mutation of the erythropoietin receptor gene could be associated with increased responsiveness of erythroid progenitors to erythropoietin in this condition. We have recently identified frequent polymorphisms of the erythropoietin receptor gene involving two closely linked regions consisting of GGAA and GA repeats in the 5′ untranslated region.31 We studied these erythropoietin receptor polymorphisms, detectable by PCR amplification, in three unrelated Chuvash families with congenital polycythemia, and found no evidence of linkage with these polymorphisms (Fig 3). These results suggest that mutations of genes other than the genes for erythropoietin and the erythropoietin receptor may be responsible for the disease phenotype.
In summary, Chuvash polycythemia appears to be the only endemic form of familial and congenital polycythemia yet described. The finding of elevated erythropoietin concentrations suggests that this is a secondary rather than primary form of familial and congenital polycythemia. Because Chuvash polycythemia does not appear to be related to structural organ defects, an abnormal hemoglobin, low erythrocyte concentrations of 2,3-diphosphoglycerate or alterations in the erythropoietin and erythropoietin receptor genes, the possibility that this condition is caused by an abnormal oxygen sensing mechanism6 7 will need to be investigated in future studies. The condition is not benign, and efforts to understand the pathophysiology of this disorder and to develop effective therapeutic measures will benefit a substantial proportion of the Chuvash population. Furthermore, the identification of the molecular basis of this congenital disease should enhance our understanding of the physiology of normal hematopoiesis and may also lead to a better understanding of pathological hemopoietic states.
ACKNOWLEDGMENT
We are grateful to Drs Goldwasser and Krystal for performing the erythropoietin determinations and to Dr Fu-Kuen Lin for providing the probes for erthropoietin gene polymorphisms.
Supported in part by a grant from the Office of Minority Health to the Cell Biology and Metabolism Branch, National Institute of Child Health and Human Development (V.R.G.), by a Veterans Administration Hospital Merit grant (J.T.P.) and by United States Public Health Service Grants No. HL51650 and HL50077 (J.T.P.).
Address reprint requests to Victor R. Gordeuk, MD, Department of Medicine, The George Washington University Medical Center, Suite 3-428, 2150 Pennsylvania Ave, NW, Washington DC 20037.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal