Abstract
Administration of kit-ligand (KL) before and after doses of 5-fluorouracil (5-FU) results in marrow failure in mice, presumably because of enhanced KL-induced cycling of stem cells, which makes them more susceptible to the effects of 5-FU. In attempt to capitalize on this effect on stem cells, we studied the ability of KL and 5-FU to allow stable donor engraftment of congenically marked marrow in a C57BL/6 (B6) mouse model. KL was administered subcutaneously at 50 μg/kg, 21 hours and 9 hours before and 3 hours after each of two doses of 5-FU (125 mg/kg) given 7 days apart to B6-recipients. Animals then received three injections of 107 congenic B6-Gpi-1a-donor bone marrow cells at 24, 48, and 72 hours after the second 5-FU dose. A separate group of animals received a single dose of either 1 × 107 or 3 × 107 donor marrow cells 24 hours after the last 5-FU dose. The level of engraftment was measured from Gpi-phenotyping at 1, 3, 6, and 8 months in red blood cells (RBCs) and at 8 months by phenotyping cells from the thymus, spleen, and marrow. Percent donor engraftment in RBCs appeared stable after 6 months. The percent donor engraftment in RBCs at 8 months was significantly higher in KL + 5-FU prepared recipients (33.0 ± 2.7), compared with 5-FU alone (18.5 ± 2.6, P < .0005), or saline controls (17.8 ± 1.7, P < .0001). In an additional experiment, granulocyte colony-stimulating factor (100 μg/dose) was added to a reduced dose of KL (12.5 μg/dose); engraftment was similar to KL alone. At 8 months after transplantation the levels of engraftment in other tissues such as bone marrow, spleen, and thymus correlated well with erythroid engraftment to suggest that multipotent long-term repopulating stem cells had engrafted in these animals. There are concerns for the toxicity of total body irradiation (TBI)- or busulfan-based regimens in young recipients of syngeneic or transduced autologous marrow who are transplanted for correction of genetic disease. In these recipients complete donor engraftment may not be needed. The results with KL and 5-FU are encouraging for the further refinement of non-TBI, nonbusulfan techniques to achieve stable mixed chimerism.
THE PREPARATION of patients that undergo bone marrow transplantation (BMT) often involves total body irradiation (TBI) or busulfan-based regimens to provide for stem cell ablation, immune suppression, and, in case of leukemia, eradication of malignant cells. In addition to the deleterious effects of TBI and busulfan on hematopoietic stem cells, they can also be hazardous to nonhematologic tissues leading to unacceptable toxicities especially in young children. In experimental murine BMT, no drugs other than busulfan and derivatives have been found to allow long-term engraftment without unacceptable normal tissue side effects.1-3 However, correction of genetic hematologic disorders with syngeneic or transduced autologous cells may require only partial stem cell ablation and milder conditioning may thus suffice. In addition, preferential engraftment along the defected lineage as reported for genetically anemic W/Wv mice,4-6 thalassemic mice,7-9 and also in patients with genetic hematologic disorders10-13 have indicated a selective growth advantage of healthy stem cells into the defective lineage so that successful reconstitution may be achieved after partial stem cell ablation. To explore alternative conditioning protocols not including TBI or busulfan, we prepared recipients with a combination of 5-fluorouracil (5-FU) and kit-ligand (KL). KL exacerbates marrow failure if administered before 5-FU,14 suggesting that KL stimulates the cycling of hematopoietic cells making them more vulnerable to the effects of 5-FU. A second 5-FU dose given within 4 to 7 days after the first dose also appears to sensitize primitive stem cells as shown in a competitive repopulation assay.15 Therefore, a 2-week schedule where KL was administered in combination with 5-FU was evaluated as a preparative regimen for BMT.
The ligand for the c-kit receptor (KL) synergizes with other hematopoietic growth factors to induce proliferation of BM progenitors in vitro.16,17 Furthermore, KL has the ability to mobilize hematopoietic stem cells from the BM into the peripheral blood (PB), especially when given in combination with granulocyte colony-stimulating factor (G-CSF ).18-21 It also protects animals from lethal irradiation when given before irradiation,22-24 and KL enhances recovery from radiation-induced aplasia.25 Because KL has the ability to protect against lethal irradiation, it was expected that KL would also protect from 5-FU, a drug that preferentially kills cycling cells. However, recent reports show that the opposite is the case; KL added to 5-FU appears to increase damage to the hematopoietic progenitor pool.14 Similar results with the radioprotector interleukin-1 (IL-1) have also been reported.26 These reports only described effects on committed progenitors in short-term assays, such as 30-day survival and colony-forming unit-spleen (CFU-S) frequencies. Therefore, we measured the effects on the more primitive long-term repopulating ability (LTRA)-subset with the cobblestone area forming cell (CAFC)-assay and in a competitive repopulation assay. Finally, we compared LTRA-survival after several variations on the KL/5-FU combination protocol with the ability of these protocols to allow for permanent long-term engraftment.
MATERIALS AND METHODS
Mice and treatment.C57BL/6J (B6) mice and C57BL/6J-CAST-Gpi-1a/Gpi-1a (B6-Gpi-1a) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Male C57BL/6J (B6) mice were used as recipients and B6-Gpi-1a mice as donors. Recipient mice were prepared by subcutaneous administration of KL (50 μg/kg/injection at −21, −9, and +3 hours relative to 5-FU [125 mg/kg] given intravenously) in 2 consecutive weeks according to the design shown in Fig 1. In some experiments G-CSF (100 μg/kg/injection) was combined with KL given at a lower dosage (12.5 μg/kg/injection). KL and G-CSF were generously provided by Amgen Inc (Thousand Oaks, CA).
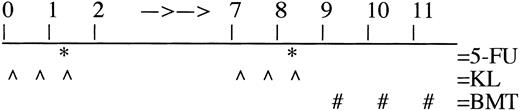
Design of conditioning with 5-FU and KL. Recipient mice were prepared by administration of KL (50 μg/kg/injection at −21, −9, and +3 hours relative to 5-FU (125 mg/kg)) in 2 consecutive weeks. Ten million donor BM cells were injected into the tail vein at 24, 48, and 72 hours after the last 5-FU injection. See Materials and Methods for more detailed explanation.
Design of conditioning with 5-FU and KL. Recipient mice were prepared by administration of KL (50 μg/kg/injection at −21, −9, and +3 hours relative to 5-FU (125 mg/kg)) in 2 consecutive weeks. Ten million donor BM cells were injected into the tail vein at 24, 48, and 72 hours after the last 5-FU injection. See Materials and Methods for more detailed explanation.
BM from B6-Gpi-1a donors was collected by flushing the hind limbs with sterile phosphate-buffered saline (PBS). Single-cell suspensions were obtained and nucleated cells were counted in Trypan Blue on a hemocytometer. Ten million viable cells were injected into the tail vein at 24, 48, and 72 hours after the last 5-FU injection. This procedure was used since it was shown that large daily doses of BM cells could optimize engraftment in unconditioned animals.27 Separate groups of animals received only one injection of either 1 × 107 or 3 × 107 donor marrow cells at 24 hours after the second dose of 5-FU.
Recipients were bled 1, 3, and 6 months after BMT and ratios of donor (Gpi-1a) to recipient type (Gpi-1b) red blood cells were determined by Gpi-electrophoresis as described.28 At 8 months after BMT mice were killed and the level of engraftment in other hematopoietic tissues such as BM, spleen, and thymus was compared with engraftment in erythrocytes.
Competitive repopulation of treated BM.In the competitive repopulation assay the long-term repopulating ability of a stem cell population relative to normal BM cells is measured. Briefly, varying numbers of BM cells from 5-FU– and KL-treated B6 (Gpi-1b) mice were mixed with a constant number (5 × 105) of normal control (B6-Gpi-1a) marrow cells. The mixtures were then injected into lethally irradiated (1,250 cGy split-dose TBI; 750 and 500 cGy 3 hours apart) B6 recipients. Endogenous marrow repopulation was determined by injecting a group of mice with control Gpi-1a cells only and was found to be less than 5% (4.1 ± 2.4). Recipients were killed at 6 months after BMT and ratios of test (Gpi-1b) to control (Gpi-1a) cells determined by electrophoresis of BM cell extracts. A measure of long-term repopulating cells, the repopulating unit, was calculated according to the formula:
where C is the number of RU in control (Gpi-1a) marrow and 1 RU is defined as the repopulating ability of 105 normal BM cells.29
CAFC assay of treated BM.BM cells treated according to the schedule were collected at 24 hours after the second 5-FU injection and assayed in a CAFC-assay.30-32 Each hind limb was carefully dissected, crushed, and filtered through a metal sieve to obtain a single BM cell suspension in PBS. The cell yield was determined by counting cells in a hematocytometer and then the cells were diluted in Iscove's medium (GIBCO-BRL, Gaithersburg, MD) supplemented with 20% horse serum, 100 U penicillin/mL, 0.1 mg streptomycin/mL, 10−5 mol/L hydrocortisone, and 10−5 mol/L 2-mercaptoethanol before overlaying the cells on a preestablished stromal layer of FBMD-1 stromal cells (generously provided by Dr Steve Neben, Genetics Institute, Cambridge, MA). In each treatment group 8 dilutions with 15 wells per dilution were used. The cells were maintained at 33°C and 5% CO2 . Half of the medium (100 μL) was replaced weekly. Wells were evaluated for cobblestone areas at day 7, day 14, day 21, day 28, and day 35. CAFC frequencies were calculated as described.32 33
RESULTS
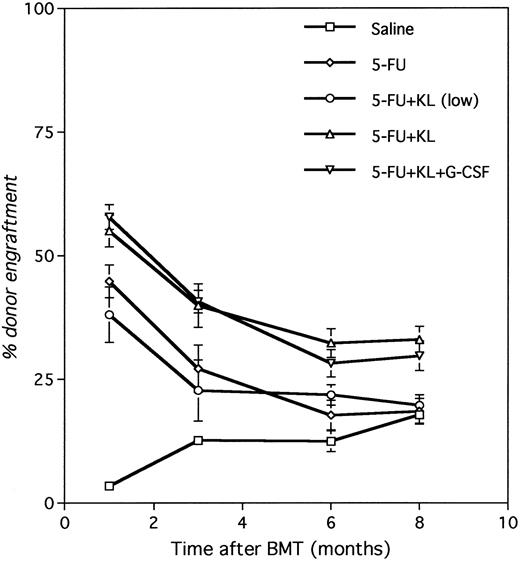
Engraftment after conditioning with 5-FU and KL.Pretreatment of mice with KL and 5-FU allowed for engraftment of syngeneic (congenically marked) BM. Figure 2 shows that the level of engraftment was more than 50% at 1 month after BMT and stabilized at 30% to 40% between 6 and 8 months in the groups where KL was included in the 2-week conditioning protocol. Engraftment with 5-FU and KL was significantly higher than saline (P < .0001) and 5-FU alone (P < .002). When only 5-FU was administered the percentage chimerism dropped from about 40% at 1 month to 19% at 8 months post-BMT, similar to the saline control group (18% at 8 months). Table 1 shows the level of engraftment evaluating several alternative conditioning regimens including 5-FU with or without either the normal or a reduced dose of KL, and 1-week cycles versus 2-week cycles. Among animals receiving 3 × 107 cells and only a single dose of 5-FU with KL, engraftment at 6 to 8 months was not different from saline or 5-FU alone (P = .11 and P = .79, respectively), supporting the efficacy of two sequential (weekly) doses of 5-FU with KL. With a fourfold reduction in KL dose combined with two doses of 5-FU, the level of engraftment at 8 months was not different from 5-FU alone or saline, demonstrating a dose-response effect for KL. However, G-CSF added to this reduced dose had the effect of increasing engraftment to about 60% at 1 month after BMT and about 30% at 8 months post-BMT (P = .004 v saline; P = .01 v 5-FU; P = .01 v 5-FU/KL [low dose] and P = .43 v 5-FU/KL [normal dose]), suggesting that the addition of G-CSF to lower doses of KL could provide the same level of engraftment as the normal dose of KL.
Development of chimerism after conditioning with 5-FU and KL. Recipients prepared with two weekly doses of saline, 5-FU, 5-FU + KL, 5-FU + KL (low dose), or 5-FU + KL (low dose) + G-CSF were transplanted with 3 × 107 BM cells divided over 3 consecutive days (24, 48, and 72 hours after the last 5-FU injection). The level of chimerism was determined at various times after BMT by Gpi-phenotyping of PB erythrocytes.
Development of chimerism after conditioning with 5-FU and KL. Recipients prepared with two weekly doses of saline, 5-FU, 5-FU + KL, 5-FU + KL (low dose), or 5-FU + KL (low dose) + G-CSF were transplanted with 3 × 107 BM cells divided over 3 consecutive days (24, 48, and 72 hours after the last 5-FU injection). The level of chimerism was determined at various times after BMT by Gpi-phenotyping of PB erythrocytes.
Engraftment After 5-FU/KL Ablation
| Treatment . | No. of Weekly Cycles . | % Gpi-1a Engraftment (PB) . | |||
|---|---|---|---|---|---|
| . | . | 1 mo . | 3 mo . | 6 mo . | 8 mo . |
| Three injections of 107 BM cells: | |||||
| Saline | 2 | 3.4 ± 0.9 (15)‡ | 12.6 ± 0.9 (14) | 12.4 ± 2.1 (15) | 17.8 ± 1.7 (15) |
| 5-FU | 1 | 26.7 ± 2.5 (5) | 10.9 ± 2.1 (5) | 14.4 ± 1.7 (5) | 15.0 ± 1.5 (5) |
| 5-FU | 2 | 44.8 ± 3.3 (13) | 27.1 ± 4.8 (12) | 17.7 ± 3.0 (15) | 18.5 ± 2.6 (15) |
| 5-FU + KL* | 1 | 39.0 ± 3.4 (10) | 20.2 ± 2.1 (10) | 16.8 ± 1.6 (10) | 13.5 ± 1.0 (10) |
| 5-FU + KL (12.5 μg/kg) | 2 | 38.1 ± 5.6 (10) | 22.7 ± 6.2 (10) | 21.8 ± 2.1 (10) | 19.7 ± 2.1 (10) |
| 5-FU + KL* | 2 | 55.0 ± 3.2 (18) | 39.9 ± 4.4 (18) | 32.3 ± 2.9 (18) | 33.0 ± 2.7 (18) |
| 5-FU + KL + G-CSF† | 2 | 57.8 ± 2.5 (9) | 40.7 ± 2.3 (9) | 28.2 ± 2.8 (9) | 29.7 ± 3.0 (9) |
| One injection of 107 BM cells: | |||||
| 5-FU | 1 | 17.7 ± 1.9 (5) | 5.4 ± 1.0 (5) | 12.2 ± 0.6 (5) | 6.5 ± 0.6 (5) |
| 5-FU | 2 | 21.6 ± 2.4 (5) | 2.2 ± 1.7 (5) | 9.6 ± 1.4 (5) | 9.8 ± 1.2 (5) |
| 5-FU + KL* | 2 | 46.2 ± 3.9 (10) | 22.7 ± 3.9 (10) | 21.6 ± 2.9 (10) | 15.0 ± 2.5 (10) |
| One injection of 3 × 107 BM cells: | |||||
| 5-FU + KL* | 2 | 60.2 ± 1.3 (5) | 51.2 ± 3.0 (5) | 28.6 ± 3.9 (5) | 30.5 ± 1.5 (5) |
| Treatment . | No. of Weekly Cycles . | % Gpi-1a Engraftment (PB) . | |||
|---|---|---|---|---|---|
| . | . | 1 mo . | 3 mo . | 6 mo . | 8 mo . |
| Three injections of 107 BM cells: | |||||
| Saline | 2 | 3.4 ± 0.9 (15)‡ | 12.6 ± 0.9 (14) | 12.4 ± 2.1 (15) | 17.8 ± 1.7 (15) |
| 5-FU | 1 | 26.7 ± 2.5 (5) | 10.9 ± 2.1 (5) | 14.4 ± 1.7 (5) | 15.0 ± 1.5 (5) |
| 5-FU | 2 | 44.8 ± 3.3 (13) | 27.1 ± 4.8 (12) | 17.7 ± 3.0 (15) | 18.5 ± 2.6 (15) |
| 5-FU + KL* | 1 | 39.0 ± 3.4 (10) | 20.2 ± 2.1 (10) | 16.8 ± 1.6 (10) | 13.5 ± 1.0 (10) |
| 5-FU + KL (12.5 μg/kg) | 2 | 38.1 ± 5.6 (10) | 22.7 ± 6.2 (10) | 21.8 ± 2.1 (10) | 19.7 ± 2.1 (10) |
| 5-FU + KL* | 2 | 55.0 ± 3.2 (18) | 39.9 ± 4.4 (18) | 32.3 ± 2.9 (18) | 33.0 ± 2.7 (18) |
| 5-FU + KL + G-CSF† | 2 | 57.8 ± 2.5 (9) | 40.7 ± 2.3 (9) | 28.2 ± 2.8 (9) | 29.7 ± 3.0 (9) |
| One injection of 107 BM cells: | |||||
| 5-FU | 1 | 17.7 ± 1.9 (5) | 5.4 ± 1.0 (5) | 12.2 ± 0.6 (5) | 6.5 ± 0.6 (5) |
| 5-FU | 2 | 21.6 ± 2.4 (5) | 2.2 ± 1.7 (5) | 9.6 ± 1.4 (5) | 9.8 ± 1.2 (5) |
| 5-FU + KL* | 2 | 46.2 ± 3.9 (10) | 22.7 ± 3.9 (10) | 21.6 ± 2.9 (10) | 15.0 ± 2.5 (10) |
| One injection of 3 × 107 BM cells: | |||||
| 5-FU + KL* | 2 | 60.2 ± 1.3 (5) | 51.2 ± 3.0 (5) | 28.6 ± 3.9 (5) | 30.5 ± 1.5 (5) |
KL = 50 μg/kg/dose.
KL = 12.5 μg/kg/dose; G-CSF = 100 μg/kg/dose.
Mean ± SEM (n).
A single dose of 1 × 107 BM cells at 24 hours after 5-FU + KL (2 cycles) resulted in about 15% engraftment at 8 months (P < .0001 v three injections). An increase in the number of BM cells to 3 × 107 given in one single injection at 24 hours after 5-FU + KL (2 cycles) led to a level of engraftment comparable to three separate injections of 1 × 107 BM cells on three consecutive days after 5-FU + KL (P = .46), suggesting that the number of transplanted cells and not the number of injections influenced engraftment. Thus, the factors most important for the percentage of chimerism are the use of 2 cycles of 5-FU + KL, the dose of KL, and the number of cells transplanted.
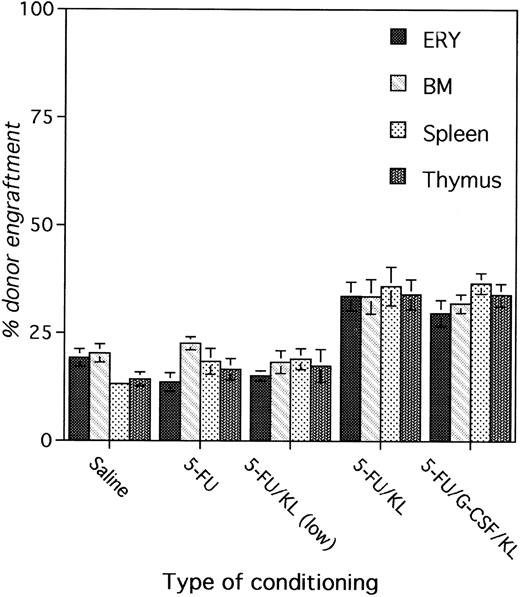
Figure 3 shows the levels of engraftment in erythrocytes, BM cells, spleen cells, and thymocytes at 8 months after BMT in various treatment groups. The level of chimerism in the different hematopoietic tissues is comparable within each group, suggesting stable and permanent engraftment of long-term repopulating cells with multilineage differentiation potential. Other groups also had comparable levels of engraftment in all hematopoietic tissues at 8 months after BMT (data not shown).
Correlation between erythroid chimerism and chimerism in BM, spleen, and thymus. Recipients prepared with two weekly doses of saline, 5-FU, 5-FU + KL, 5-FU + KL (low dose), or 5-FU + KL (low dose) + G-CSF were transplanted with 3 × 107 BM cells divided over 3 consecutive days (24, 48, and 72 hours after the last 5-FU injection). The level of chimerism was determined at 8 months after BMT by Gpi-phenotyping of erythrocytes, BM cells, splenocytes, and thymocytes.
Correlation between erythroid chimerism and chimerism in BM, spleen, and thymus. Recipients prepared with two weekly doses of saline, 5-FU, 5-FU + KL, 5-FU + KL (low dose), or 5-FU + KL (low dose) + G-CSF were transplanted with 3 × 107 BM cells divided over 3 consecutive days (24, 48, and 72 hours after the last 5-FU injection). The level of chimerism was determined at 8 months after BMT by Gpi-phenotyping of erythrocytes, BM cells, splenocytes, and thymocytes.
Competitive repopulation of treated BM.BM cells treated according to the schedule mentioned in Materials and Methods were collected at 24 hours after the second 5-FU injection and assayed in a competitive repopulation assay. Because the cell loss after treatment is considerable, it is preferable to express competitive repopulation as repopulating units (RU) per hind limb. Table 2 shows that 5-FU alone depleted the RU to 84% of normal, but the combination of KL and 5-FU depleted the RU to 16% of normal. Combining G-CSF with a reduced dose of KL had a comparable effect on the number of surviving RU.
Survival of LTRA 24 Hours After 5-FU and KL as Measured in the Competitive Repopulation Assay
| Treatment . | No. of Weekly Cycles . | RU/Hind Limb . | % of Control . |
|---|---|---|---|
| Saline | 2 | 127 | 100 |
| 5-FU alone | 2 | 106 | 84 |
| 5-FU + KL | 2 | 20 | 16 |
| 5-FU + KL + G-CSF | 2 | 17 | 14 |
| Treatment . | No. of Weekly Cycles . | RU/Hind Limb . | % of Control . |
|---|---|---|---|
| Saline | 2 | 127 | 100 |
| 5-FU alone | 2 | 106 | 84 |
| 5-FU + KL | 2 | 20 | 16 |
| 5-FU + KL + G-CSF | 2 | 17 | 14 |
Repopulation units were calculated from chimerism in lethally irradiated recipients at 8 months after transplantation of BM cells from treated BM mixed with a constant number of untreated BM. The repopulation units (RU) were calculated according to the formula: RU = (% of Gpi-1b * C)/(100-% of Gpi-1b), where C is the number of RU in control (Gpi-1a) marrow and 1 RU is defined as the repopulating ability of 105 normal BM cells.29
CAFC assay of treated BM.In addition to the competitive repopulation in vivo, treated BM cells were tested in vitro in the CAFC assay. Figure 4 shows survival of CAFC after 5-FU or 5-FU combined with KL. The data are presented as the number of CAFC per hind limb (Fig 4A) and as a percentage of control, saline-treated marrow (Fig 4B). The data are presented in more detail in Table 3. It can be seen that protocols with two cycles of 5-FU + KL (high dose) or 5-FU + KL (low dose) + G-CSF have a dramatic effect on early CAFC (CAFC-7), the subset that correlates with progenitors such as CFU-S day 8,31 leading to a level of survival of only 0.03% to 0.06%. Survival of late CAFC (CAFC-28/CAFC-35; similar to long-term repopulating stem cells) was 10% to 16%, comparable to the level of survival of primitive stem cells measured in the competitive repopulation assay. In contrast, 2 cycles of 5-FU without KL resulted in 4% and 49% survival of CAFC-7 and CAFC-28, respectively. Table 3 shows the results of other treatments with 5-FU with or without KL on CAFC-survival. One dose of 5-FU did not significantly reduce CAFC-28 numbers where two doses of 5-FU 7 days apart reduced CAFC-28 numbers to about 50% of control (P < .05 v saline). There was no significant additional CAFC-28 depletion when one dose of 5-FU was combined with KL.
CAFC-survival after conditioning with 5-FU and KL. Mice (2 to 3 per group) were treated with two weekly doses of saline, 5-FU, or 5-FU + KL, and killed 24 hours after the last 5-FU dose to test CAFC-survival. (A) Residual CAFC per hind limb; (B) the percentage of surviving CAFC relative to control (saline treated) both as a function of CAFC-day type. Early CAFC (day 7) correlate with committed progenitors like CFU-S day 8 and late CAFC (day 28 and later correlate with long-term repopulating stem cells (LTRA).31 (▪), control marrow; (♦), 5-FU marrow; (•), 5-FU + KL.
CAFC-survival after conditioning with 5-FU and KL. Mice (2 to 3 per group) were treated with two weekly doses of saline, 5-FU, or 5-FU + KL, and killed 24 hours after the last 5-FU dose to test CAFC-survival. (A) Residual CAFC per hind limb; (B) the percentage of surviving CAFC relative to control (saline treated) both as a function of CAFC-day type. Early CAFC (day 7) correlate with committed progenitors like CFU-S day 8 and late CAFC (day 28 and later correlate with long-term repopulating stem cells (LTRA).31 (▪), control marrow; (♦), 5-FU marrow; (•), 5-FU + KL.
CAFC Survival 24 Hours After 5-FU/KL Ablation
| Treatment . | No. of Weekly Cycles . | CAFC Survival . | |||
|---|---|---|---|---|---|
| . | . | CAFC-7/HL . | (%) . | CAFC-28/HL . | (%) . |
| Saline | 2 | 81,592 (61,131-108,901)ρ | 100 | 1,939 (1,427-2,634) | 100 |
| 5-FU | 1 | 2,258 (1,540-3,309) | 2.8 (1.9-4.1) | 1,164 (797-1,699) | 60 (41-88) |
| 5-FU | 2 | 2,827 (2,158-3,702) | 3.5 (2.6-4.5) | 952 (715-1,266) | 49 (37-65) |
| 5-FU + KL3-150 | 1 | 2,116 (1,433-3,126) | 2.6 (1.8-3.8) | 885 (557-1,408) | 45.6 (28.7-72.6) |
| 5-FU + KL3-151 | 2 | 127 (87-187) | 0.16 (0.11-0.23) | 263 (179-385) | 13.6 (9.2-19.9) |
| 5-FU + KL3-150 | 2 | 51 (39-66) | 0.06 (0.05-0.08) | 192 (148-250) | 9.9 (7.6-12.9) |
| 5-FU + KL + G-CSF3-152 | 2 | 23.4 (13.0-42.1) | 0.03 (0.02-0.05) | 319 (217-467) | 16.4 (11.2-24.1) |
| Treatment . | No. of Weekly Cycles . | CAFC Survival . | |||
|---|---|---|---|---|---|
| . | . | CAFC-7/HL . | (%) . | CAFC-28/HL . | (%) . |
| Saline | 2 | 81,592 (61,131-108,901)ρ | 100 | 1,939 (1,427-2,634) | 100 |
| 5-FU | 1 | 2,258 (1,540-3,309) | 2.8 (1.9-4.1) | 1,164 (797-1,699) | 60 (41-88) |
| 5-FU | 2 | 2,827 (2,158-3,702) | 3.5 (2.6-4.5) | 952 (715-1,266) | 49 (37-65) |
| 5-FU + KL3-150 | 1 | 2,116 (1,433-3,126) | 2.6 (1.8-3.8) | 885 (557-1,408) | 45.6 (28.7-72.6) |
| 5-FU + KL3-151 | 2 | 127 (87-187) | 0.16 (0.11-0.23) | 263 (179-385) | 13.6 (9.2-19.9) |
| 5-FU + KL3-150 | 2 | 51 (39-66) | 0.06 (0.05-0.08) | 192 (148-250) | 9.9 (7.6-12.9) |
| 5-FU + KL + G-CSF3-152 | 2 | 23.4 (13.0-42.1) | 0.03 (0.02-0.05) | 319 (217-467) | 16.4 (11.2-24.1) |
KL = 50 μg/kg/dose.
KL = 12.5 μg/kg/dose.
KL = 12.5 μg/kg/dose; G-CSF = 100 μg/kg/dose.
ρ Mean with 95% confidence interval range in parentheses.
Comparison of engraftment, RU, and CAFC depletion.All 2-week schedules with KL (50 μg/kg/dose, 12.5 μg/kg/dose, and 12.5 μg/kg/dose + G-CSF ) were effective in depleting CAFC-28 numbers to below 20%. 5-FU + KL and 5-FU + KL + G-CSF also resulted in depletion of repopulating units to less than 20%. These were also the schedules that led to the highest levels of engraftment after BMT of 3 × 107 donor marrow cells (Fig 5). These data show a correlation between the number of residual host primitive stem cells and the level of long-term donor engraftment.
The relationship between CAFC-survival and donor engraftment. The percentage of donor engraftment is shown as a function of the residual CAFC per hind limb. The treatment groups are divided into effective (2-week protocols with KL; 5-FU + KL [high dose], 5-FU + KL [low dose], 5-FU + KL [low dose] + G-CSF ) and noneffective (saline, 5-FU alone [1-week and 2-week], 5-FU + KL [1-week]) protocols based on engraftment data in Table 1.
The relationship between CAFC-survival and donor engraftment. The percentage of donor engraftment is shown as a function of the residual CAFC per hind limb. The treatment groups are divided into effective (2-week protocols with KL; 5-FU + KL [high dose], 5-FU + KL [low dose], 5-FU + KL [low dose] + G-CSF ) and noneffective (saline, 5-FU alone [1-week and 2-week], 5-FU + KL [1-week]) protocols based on engraftment data in Table 1.
DISCUSSION
The data presented in this report show that administration of KL before and after 5-FU leads to stable donor engraftment after transplantation. The correlation between erythroid engraftment and engraftment in other tissues such as BM, spleen, and thymus at 8 months after transplantation suggests that multipotent long-term repopulating stem cells engrafted in these animals. Thus, permanent long-term engraftment can be obtained through cytokine induced stimulation of stem cells into cycle and subsequent killing by 5-FU. This long-term engraftment is stable and can be achieved without the use of TBI or busulfan-related regimens.
It has been reported that BM cells are stimulated when cultured in the presence of cytokines. KL has been shown to synergize with erythropoietin, IL-3, IL-6, IL-12, G-CSF, and granulocyte-macrophage (GM)-CSF to increase the size and numbers of erythroid, granuloid, and macrophage colonies derived from murine16,34,35 or human BM.17,36,37 However, in vitro expansion of BM cells may result in impaired long-term engraftment potential and it therefore remains doubtful whether the most primitive stem cells are expanded in these cultures.38,39 A recent study showed that cytokines such as KL and IL-11 are critical for survival and maintenance of primitive stem cells in culture, but expansion occurs only among the more committed progenitor cell population.40 KL also induces a migration of colony-forming progenitors and long-term repopulating stem cells from the marrow to the spleen and to the PB.18-21 Thus, KL is a very potent stimulator of hematopoiesis at many different levels of differentiation.
G-CSF is often used to accelerate hematopoietic recovery after chemotherapy or radiotherapy41 and has also been shown to be a potent mobilization agent.18,42 The mobilizing effect of KL and the synergy between KL and G-CSF in mobilizing stem cells from the marrow to the PB is probably caused by an increased proliferation of stem cells.43 44 We tried to capitalize on the increased in vivo proliferation of stem cells by using both these agents in combination with 5-FU to prepare the host for BMT. Clinically, KL can be toxic and an effective alternative regimen using lower doses of KL in combination with other cytokines such as G-CSF may make its use more feasible in the clinic.
5-FU is often used experimentally as a pre-enrichment step to purify murine BM stem cells because it preferentially kills cycling cells. Following 5-FU treatment, the number of LTRA per 106 cells increases, but the total number of LTRA per femur does not change significantly.30,45 Thus, the main effect of 5-FU is depletion of proliferating progenitors leading to reduced BM cellularity and this enriches for LTRA.46-48 Others have reported that when a single treatment with 5-FU is administered 24 hours before transplantation of 107 BM cells, very little engraftment is found (3.5%), presumably due to the lack of depletion of the primitive stem cell compartment.45 This result is in agreement with the 6.5% engraftment at 8 months after BMT of 107 cells following one dose of 5-FU reported in this paper.
Evidence was found for a cell dose effect on engraftment, since after similar conditioning, engraftment was higher with a total of 3 × 107 cells than after transplantation of 1 × 107 cells (eg, after two doses of 5-FU, 8-month engraftment was 18.5% with 3 × 107 cells and only 9.8% after 1 × 107 cells). In addition, an interesting difference between 5-FU– and saline-treated animals was observed after BMT of 3 × 107 cells. In 5-FU–treated animals we found an early engraftment of 45%, but this decreased to about 18.5% engraftment at 8 months after BMT. In saline-treated animals, the level of early engraftment was 3.4%; this increased to 17.8% at 8 months, a level comparable to the 5-FU–treated mice. Thus, a dose of 3 × 107 cells appears to result in modest engraftment of primitive stem cells even without conditioning, which in saline-treated mice gradually compete with the endogenous marrow stem cells to result in a low but substantial level of engraftment by 8 months. Two doses of 5-FU depleted the progenitor pool, probably allowing engraftment of transplanted committed progenitors which were eventually lost, leaving the same level of engrafted primitive stem cells (from the large number of donor cells transplanted) as in the saline controls. Therefore, 5-FU alone did not appear to enhance engraftment of primitive stem cells over control animals.
In a previous study, Harrison and Lerner15 reported a reduced long-term repopulation ability of BM after two doses of 5-FU administered 3, 5, or 8 days apart. They used a slightly higher 5-FU dose (150 mg/kg v 125 mg/kg) and found that at 8 days the second 5-FU dose was less effective than when the second dose was given at 3 or 5 days after the first dose. The addition of KL to a protocol with two treatments with 5-FU may change the turnover of LTRA and it therefore requires further investigation to see whether reducing the time between two 5-FU/KL cycles to 3 or 5 days will lead to more stem cell kill to allow for a higher level of long-term engraftment.
Engraftment in the first 3 months after BMT has been ascribed to stem cell subsets residing in the CFU-S compartment as shown from BM separation experiments.45,49,50 However, permanent, durable engraftment requires growth and differentiation of stem cells with LTRA.45,49,50 Preparative regimens that do not deplete host LTRA do not appear to be able to induce significant engraftment of donor LTRA unless very high numbers of BM cells are transplanted.27,51-53 In this study, the 5-FU alone and 5-FU + KL 1-week protocol showed no significant depletion of late CAFC (day 28 or 35) and engraftment after BMT of 3 × 107 cells was therefore not different from saline controls. On the other hand, conditioning of transplantation recipients with 2-week protocols including 5-FU and KL leads to a 80% to 90% depletion of both RU and late CAFC (CAFC-28 and 35) and subsequent engraftment of donor LTRA as demonstrated by 8-month multilineage donor engraftment. The combination of 5-FU and KL also showed an enhanced killing of the early CAFC-7 subset to indicate that KL induced more of these cells into cycle making them more susceptible to 5-FU. The eradication of this stem cell subset is lethal to mice without a BMT.14 The preferential ablation of rapidly dividing cells (more kill of early CAFC [day 7] than of late CAFC [day 28]) may cause side effects on other tissues with rapidly dividing cells such as skin and gut14 which need to be further investigated.
The reduced dose of KL in a 2-week protocol was almost as effective as the normal dose on CAFC-survival, but engraftment levels were not as high. However, when G-CSF was added to this reduced dose similar engraftment levels were reached as the normal dose of KL with 5-FU. The most likely explanation for these results is that one cycle of 5-FU and KL is not effective in depleting LTRA (no significant CAFC-28 killing and engraftment not higher than in saline controls), but induces LTRA to go into cycle, and this makes them more vulnerable to the second cycle of 5-FU and KL. The survival of LTRA is then reduced to 10% to 20%, which allows for long-term engraftment. Although the high BM cell dose of 3 × 107 led to a considerably higher level of engraftment than 1 × 107, 5-FU and KL also induced higher engraftment levels than 5-FU alone when only 1 × 107 were used.
The results with KL and 5-FU are encouraging for the further refinement of non-TBI, nonbusulfan techniques to achieve stable mixed donor-recipient chimerism. Because low levels of engraftment may be sufficient for phenotypic correction of genetic hematopoietic disorders such as thalassemia or chronic granulomatous disease,7 54 the use of alternative regimens with less toxicity than TBI or busulfan should be encouraged if they result in sufficient levels of stable donor chimerism. These techniques may be particularly useful for transplantation of transduced autologous stem cells when options of repeated stem cell infusions following minimally toxic regimens are feasible.
Supported by National Institutes of Health (NIH) RO1 Grant-CA 10941 and NIH P50 Grant-HL54785-01.
Address reprint requests to Ronald van Os, PhD, Joint Center for Radiation Therapy, Department of Radiation Oncology, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215.


![Fig. 5. The relationship between CAFC-survival and donor engraftment. The percentage of donor engraftment is shown as a function of the residual CAFC per hind limb. The treatment groups are divided into effective (2-week protocols with KL; 5-FU + KL [high dose], 5-FU + KL [low dose], 5-FU + KL [low dose] + G-CSF ) and noneffective (saline, 5-FU alone [1-week and 2-week], 5-FU + KL [1-week]) protocols based on engraftment data in Table 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/7/10.1182_blood.v89.7.2376/6/m_bl_0003f5.jpeg?Expires=1767834039&Signature=M8u~LqVsKL1YIUShfJ-rHinVqq9xMYCQUbG49EvwPJzMK8g5ulcQU1z9WgjlkoipTENU2tn7q1wMV7Vin4qoOYBgJTfl7LuQ5VUgCbU-Z-Fklz5xNmIiOQ2GMQBdq7wU~W~zFc8~Qi5a8tqINl95nzht7wY0sYTcY84lr2l~SmnGDMuoCZi8n6QICI9LkxJd9f-W7ULkq8Cqgw~BQyryWsXUm8QHDbEQqthVNezv6LhrA7yHMdpG7GciKPGPpn3cv4TnKSR9N31ulMxd-c-Pt46q1VrltLN6N0TX6wfNeVlLtwb5UrHLIqBmgRv~RiD~iQuln1A8cKAWPL-2XR0KWA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal