Abstract
The infusion of anti-CD3–activated murine T cells plus interleukin-2 (IL-2) exerts antitumor effects against several tumors in murine immunotherapy models. This study compares the therapeutic efficacy of anti-CD3–activated CD4+ or CD8+ T-cell subsets, when given with cyclophosphamide (Cy) and liposome-encapsulated IL-2 (L-IL2) in a murine model. C57BL/6 mice bearing subcutaneous (SC) MC-38 colon adenocarcinoma, 3LL Lewis lung carcinoma, or 38C13 lymphoma for 7 to 14 days were pretreated with low-dose intraperitoneal (IP) Cy before intravenous (IV) injection of anti-CD3–activated T cells or T-cell subsets. Cell administration was followed by IP administration of L-IL2 for 5 days. Mice receiving activated CD4+ T cells showed significantly reduced tumor growth or complete remissions with prolonged disease-free survival in MC-38, 3LL, and 38C13. The timing of Cy doses in relation to adoptive transfer was critical in obtaining the optimal antitumor effect by CD4+ cells. Injecting Cy 4 days before the infusion of CD4+ cells greatly enhanced the antitumor effect of the CD4+ cells and improved survival of the mice compared with other Cy regimens. C57BL/6 mice cured of MC-38 after treatment with CD4+ T cells developed tumor-type immunologic memory as demonstrated by their ability to reject rechallenges with MC-38, but not 3LL. Similarly, mice cured of 3LL tumors rejected rechallenges of 3LL, but not MC-38. The immunologic memory could be transferred with an IV injection of splenocytes from mice cured of MC-38 or 3LL. No cytotoxic T-lymphocyte activity was detected in T cells or T-cell subsets from mice cured of MC-38 or 3LL. Increased IL-2 and interferon-γ (IFN-γ) production was observed from CD4+ subsets in cured animals when stimulated in vitro with the original tumor, but not with an unrelated syngeneic tumor. These results suggest that tumor-specific immunity can be achieved in vivo with anti-CD3–stimulated CD4+ T cells in this cellular therapy model.
THE STIMULATION OF human peripheral blood lymphocytes (PBL) or murine splenocytes with anti-CD3 monoclonal antibodies induces the activation of T cells with antitumor activity, T-cell proliferation, and the production of cytokines.1,2 Treating mice bearing MC-38, P815, or MCA-106 liver or lung metastases with anti-CD3–activated T cells and liposome-encapsulated interleukin-2 (L-IL2) results in a significant decrease in the number of metastases.3-5 Cancer patients treated with anti-CD3–activated T cells and IL-2 showed an impressive proliferation of predominantly CD8+ T cells in vivo (up to 280,000 cells/μL in individual patients).6 Flow cytometry showed upregulation of several T-cell activation markers. An infiltration of tumor deposits by CD8+ cells was observed on biopsy specimens in melanoma patients. However, in contrast to the high therapeutic efficacy seen in mice, the human trial showed infrequent tumor responses.
To determine the T-cell subset responsible for the antitumor activity in the mouse model, we tested the therapeutic efficacy of anti-CD3–stimulated CD4+ or CD8+ T cells given with cyclophosphamide (Cy) and L-IL2 in MC-38 colon adenocarcinoma, 3LL Lewis lung carcinoma (H-2b), or 38C13 lymphoma (H-2k). Treatment with anti-CD3–stimulated CD4+ cells resulted in tumor regressions and cures in a significant proportion of mice. Cured mice developed tumor-specific long-term immunity that was transferable to naive immunocompetent mice, even though the initial anti-CD3 stimulation was not antigen-specific.
MATERIALS AND METHODS
Mice.Inbred female C57BL/6 and C3H/HeNCr-mice (6 to 10 weeks of age) were obtained from the Animal Production Facility, NCI-Frederick Cancer Research and Development Center, Frederick, MD.
Tumor preparation and tumor cell lines.MC-38 and 3LL were maintained by subcutaneous (SC) passage in C57BL/6 mice. 38C13 was maintained in C3H/HeNCr- mice. MC-38 is a weakly immunogenic murine colon adenocarcinoma induced by the SC injection of dimethylhydrazine in C57BL/6 mice.7 The 3LL cell line (3LL-M2) used in this study was a generous gift of Dr Ronald Hornung (NCI-FCRDC, Frederick, MD) and has been described previously.8 The carcinogen-induced 38C13 B-cell lymphoma of C3H origin has been previously described.9 Tumor volumes were calculated with the following formula10: Total Volume = (0.5) (larger diameter) × (smaller diameter).2
Activated lymphocytes.C57BL/6 murine splenocytes were harvested and activated with antimurine CD3 monoclonal antibody (MoAb) (145-2C11, a generous gift from Dr Jeffrey Bluestone, University of Chicago, Chicago, IL) and rhIL-2 (Hoffmann-LaRoche, Inc, Nutley, NJ) at a dose of 100 U/mL overnight before adoptive transfer, as previously described.3 Briefly, mouse spleens were crushed in culture dishes to obtain a single-cell suspension. The splenocytes were resuspended in cold RPMI, and red blood cells (RBCs) were lysed using ACK lysing buffer (Quality Biological, Inc, Gaithersburg, MD). The resulting suspension was filtered through sterile nytex mesh to remove cellular debris, washed twice with phosphate-buffered saline (PBS) (GIBCO-BRL, Grand Island, NY), and counted. T cells were enriched by passing splenocyte suspensions over nylon wool columns using RPMI 1640 plus fetal calf serum (FCS) 10% vol/vol, or T-cell negative selection chromatography columns (Cellect Mouse T Cell Enrichment Immunocolumns, Biotex Inc, Edmonton, Alberta, Canada). Enriched CD4+ or CD8+ subsets for all experiments were collected by passing the enriched T-cell suspensions over negative selection chromatography columns (Cellect Mouse CD4 Enrichment Immunocolumns, Biotex Inc, or Mouse CD4 or CD8 Subset column kits, R & D Systems, Inc, Minneapolis, MN). Purity of cell collections was determined by flow cytometry and consistently showed greater than 90% purity in CD3+ T cells. T cells or T-cell subsets were incubated with anti-CD3 (145-2C11) MoAb supernatant 2% to 3% vol/vol (2 to 3 μg/mL) directly onto the cell pellet for 20 minutes. The activated cells were then placed in tissue culture media (TCM) at 1.5 to 3.0 million cells/mL. TCM consisted of RPMI 1640 supplemented with 25 mmol/L HEPES, 2 mmol/L L-glutamine, 5% FCS, 100 U/mL penicillin, 10 μg/mL streptomycin, 10 mmol/L nonessential amino acids, 100 mmol/L sodium pyruvate (GIBCO, Grand Island, NY) and 25 μmol/L 2-mercaptoethanol (2-ME; Sigma Chemical Co, St Louis, MO) and 100 IU/mL rhIL-2. Cultures carried beyond 24 hours were supplemented every 2 to 3 days with fresh TCM to maintain a cell density of 1.5 × 106 cells/mL. Cell viability and fold increase in cell number was determined by trypan blue exclusion and light microscopy. Experiments using T-cell subsets were consistently greater than 90% CD4+ T cells or 90% CD8+ T cells, respectively.
Experimental Conditions
| Experiment No. . | Tumor Line . | Tumor Size Pretreatment Median (range) μL . | Tumor Age Pretreatment (d) . | Cy Dose (mg/kg)/Day Given* . | No. Anti-CD3–Activated Cells Given . | No. of Treatment Courses . | ||
|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | CD4 . | CD8 . | T Cells . | . |
| 1 | MC38 | 18 (6-32) | 7 | 100/1 | 3.2 × 107 | 3.2 × 107 | 6.5 × 107 | 1 |
| 2 | 3LL | 16 (13-32) | 7 | 150/4 | 5 × 107 | 5 × 107 | — | 1 |
| 3 | 3LL | 23 (13-32) | 16 | 150/4 | 5 × 107† | — | — | 1 |
| MC38 | 4 (4-14) | 8 | 100/4 | 3.1 × 107† | — | — | 2 | |
| 4 | MC38 | 48 (44-75) | 9-11 | 100/2 or 4 | 5.5 × 107 | — | — | 1 |
| Experiment No. . | Tumor Line . | Tumor Size Pretreatment Median (range) μL . | Tumor Age Pretreatment (d) . | Cy Dose (mg/kg)/Day Given* . | No. Anti-CD3–Activated Cells Given . | No. of Treatment Courses . | ||
|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | CD4 . | CD8 . | T Cells . | . |
| 1 | MC38 | 18 (6-32) | 7 | 100/1 | 3.2 × 107 | 3.2 × 107 | 6.5 × 107 | 1 |
| 2 | 3LL | 16 (13-32) | 7 | 150/4 | 5 × 107 | 5 × 107 | — | 1 |
| 3 | 3LL | 23 (13-32) | 16 | 150/4 | 5 × 107† | — | — | 1 |
| MC38 | 4 (4-14) | 8 | 100/4 | 3.1 × 107† | — | — | 2 | |
| 4 | MC38 | 48 (44-75) | 9-11 | 100/2 or 4 | 5.5 × 107 | — | — | 1 |
All mice that received anti-CD3–activated cells were also treated with L-IL-2 (50,000 U IP) for 5 days after cells. Control groups for each experiment are shown in the accompanying figures or are described in Results.
Refers to number of days before cell dose.
Splenocytes were obtained from mice bearing 3LL or MC-38 tumors for 7 days.
Treatment model.All in vivo experiments with established tumors were composed of the following elements: (1) SC tumor inoculation (1 × 106 cells in MC-38 and 2 × 106 in 3LL) into the right hindflank followed by a 7- to 14-day period without treatment; (2) intraperitoneal (IP) Cy dose 1 to 4 days before cell therapy; (3) intravenous (IV) T cell or T-cell subset administration; (4) IP L-IL2 doses (specific conditions for individual experiments are provided in Table 1); and (5) splenocytes were obtained from naive or 7-day tumor-bearing mice. Some experiments used two treatment courses.
Cytotoxicity assays.The chromium and indium release assays used here have been described previously.3 11 Briefly, tumor targets (2 × 106 cells in 0.5 mL media) were incubated with 150 μCi Na512CrO4 (NEN Research Products, Inc, Boston, MA) at 37°C for 60 minutes. The targets were washed three times, resuspended, and counted. Effector cells in chromium-release assays were preincubated with anti-CD3 (10 ng/mL) and IL-2 (100 U/mL) for 4 to 6 days. The effector cells were serially diluted threefold to yield E:T ratios from 42:1 to 3:1. A total of 5,000 targets was added per well. The plates were incubated for 4 hours at 37°C in 5% CO2 . The percentage of specific cytotoxicity was calculated as follows: Experimental Mean cpm − Spontaneous Mean cpm × 100/Maximal Mean cpm − Spontaneous Mean cpm.
Cytotoxicity was also studied using 111Indium-labeled targets and up to 18 hours of incubation time to unmask weak cytotoxic activity.11 Assays were performed as follows: target cells were pretreated with interferon (IFN-γ) (500 to 1,000 U/mL) for 48 to 72 hours to upregulate class I H-2 antigens.11 CD4+ effector cells were either preincubated with irradiated MC-38 for 5 days or activated in vitro with anti-CD3 and IL-2 for 18 hours. Then, 1 × 106 target cells were incubated for 20 minutes with 20 to 50 μCi111 Indium chloride (Amersham Life Science, Arlington Heights, IL) at room temperature. Serial dilutions were performed to obtain E/T ratios of 100:1 to 6.25:1. Plates were incubated at 37°C for 18 hours and cytotoxicity calculated as described above.
Thymidine incorporation assays.For each sample, 1 × 105 anti-CD3–activated cells in 200 μL TCM were placed in triplicate in a 96-well flat bottom plate. A total of 0.5 μCi of 3H-thymidine (6.7 Ci/mmol) (NEN Research Products) in 20 μL TCM was added to each well. The plates were incubated for 18 hours at 37°C. Cells were harvested using a semiautomated cell-harvesting apparatus, and 3H-thymidine incorporation was assessed using a beta counter.
Proliferation of tumor cells with CD4 supernatants.CD4+ T cells were harvested and activated with anti-CD3 (3 μg/mL) and IL-2 (100 U/mL) at a concentration of 1 × 106 cells/mL. Supernatants were collected at 18, 48, 72, and 120 hours and frozen until analysis. The effect on proliferation of MC-38 tumor cells from the supernatants was tested using the Cell Titer 96TM Proliferation Assay (Promega, Madison, WI).
Cytokine production.T-cell subsets from normal or cured mice were cultured between 2 and 5 days with irradiated MC-38 tumor cells at a splenocyte or T cell:tumor cell ratio of 10:1. IL-2 was not added to the TCM during the first 2 days. Cells for each test condition were counted on the day of supernatant harvest. The cell-free supernatant was collected to measure cytokine levels. Cytokines were quantified using the following enzyme-linked immunosorbent assay (ELISA) kits: IL-2, (Genzyme, Cambridge, MA) and IFN-γ (GIBCO-BRL/Life Technologies, Inc, Gaithersburg, MD). Results were indexed to cytokine production per million cells in culture.
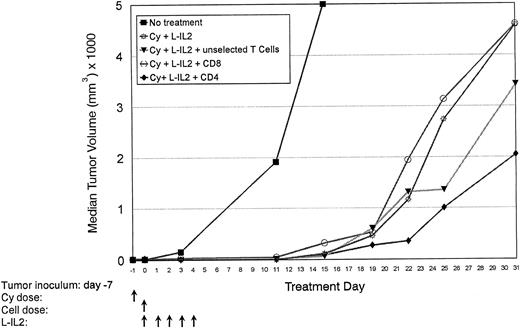
Normal C57BL/6 mice are inoculated with MC-38 SC in the right hindflank (Table 1, experiment 1). After 7 days, mice received T cells (6.5 × 107 cells/injection) or T-cell subsets (3.2 × 107 cells/injection) as indicated. Tumor volumes were measured over 31 days. Median tumor volumes are shown for each treatment group. There were 10 mice per group.
Normal C57BL/6 mice are inoculated with MC-38 SC in the right hindflank (Table 1, experiment 1). After 7 days, mice received T cells (6.5 × 107 cells/injection) or T-cell subsets (3.2 × 107 cells/injection) as indicated. Tumor volumes were measured over 31 days. Median tumor volumes are shown for each treatment group. There were 10 mice per group.
Liposome encapsulated IL-2.L-IL2 was provided by Biomira U.S.A. (Cranbury, NJ), with activity of 1 × 106 IU per 0.5 mL. L-IL2 was reconstituted before use to a concentration of 250,000 U/mL using sterile Hanks' balanced salt solution (HBSS) and given at a dose of 0.2 mL (50,000 U) IP each day for 5 consecutive days.
Cyclophosphamide.Cyclophosphamide monohydrate (Aldrich Chemical Co, Inc, Milwaukee, WI) in crystalline form was reconstituted in HBSS (BioWhittaker, Inc, Walkersville, MD) to the appropriate concentration. Mice received Cy at a dose of 100 mg/kg IP for MC-38 and 150 mg/kg IP for 3LL, unless otherwise indicated.
Statistical methods.Comparisons of tumor growth curves were performed primarily with nonparametric procedures.12-14 For simplicity, probability values obtained by Koziol's distribution-free methods are reported.12,13 Survival times were estimated according to the Kaplan-Meier product-limit method.15 Comparisons among groups of distribution of days to death were performed with the log-rank (Savage) and generalized Wilcoxon tests in stratified Kaplan-Meier time-to-event analyses.16 17 Interpretations of results by the two methods agreed in all cases. Probability values from the log-rank tests are reported.
RESULTS
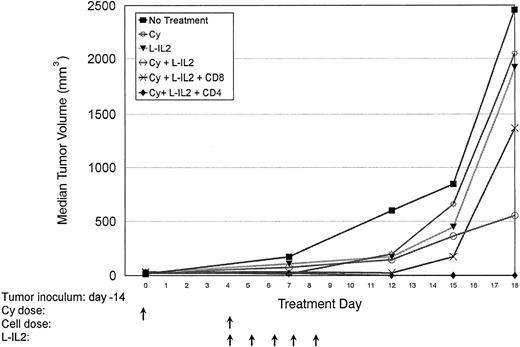
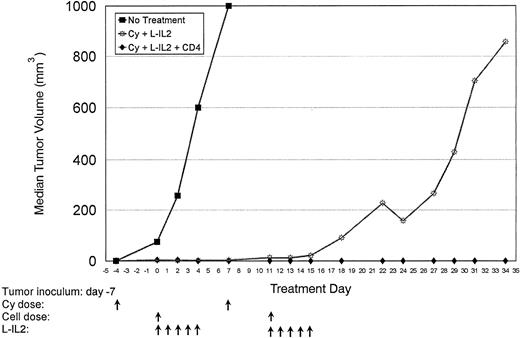
Therapeutic effect of T-cell subsets.Mice-bearing subcutaneous MC-38 (Fig 1, Table 1, experiment 1) or 3LL (Fig 2, Table 1, experiment 2) tumors and treated with CD4+ T cells showed a marked antitumor effect. Repeated experiments in MC-38 confirmed that the infusion of anti-CD3 activated CD4+ T cells + L-IL-2 had a significantly higher therapeutic efficacy compared with CD8+ T cells (P = .0036), unselected T cells (P = .0077) or Cy + L-IL-2 (P = .055). In 3LL, CD4+ cellular therapy caused a significantly greater antitumor effect than CD8+ therapy (P = .0034), or controls using Cy (P = .0079),L-IL-2 (P = .0168) or Cy + L-IL-2 (P = .0112). When two courses of CD4 therapy were given, a cure rate of 60% to 90% was achieved in several experiments with MC-38 or 3LL tumors that had been established for as long as 16 days (Fig 3 and Table 1, experiment 3). No cures were seen with single courses of unseparated T cells or CD8+ T cells, or Cy + L-IL-2. However, multiple courses of treatment with Cy + L-IL-2 with or without unselected T cells cured between 30% to 60% of animals, compared with 80% to 90% with CD4-based therapy. CD4+ T cells obtained from 7-day tumor-bearing donor mice also displayed this antitumor effect. Similar results were obtained in the 38C13 lymphoma model (data not shown).
Normal C57BL/6 mice are inoculated with 3LL SC in the right hindflank (Table 1, experiment 2). CD4+ or CD8+ subsets were given at 5 × 107 cells/injection. Median tumor volumes are shown. A 60% cure rate was observed in the CD4-treated group. No cures were obtained in CD8- or Cy-treated animals. There were five mice per treatment group.
Normal C57BL/6 mice are inoculated with 3LL SC in the right hindflank (Table 1, experiment 2). CD4+ or CD8+ subsets were given at 5 × 107 cells/injection. Median tumor volumes are shown. A 60% cure rate was observed in the CD4-treated group. No cures were obtained in CD8- or Cy-treated animals. There were five mice per treatment group.
Normal C57BL/6 mice were inoculated with MC-38 SC in the right hindflank (Table 1, experiment 3). Two courses of treatment were given as indicated. Cures were achieved in 30% of mice receiving Cy + L-IL-2 therapy, while 90% of mice receiving CD4-based therapy were free of tumor.
Normal C57BL/6 mice were inoculated with MC-38 SC in the right hindflank (Table 1, experiment 3). Two courses of treatment were given as indicated. Cures were achieved in 30% of mice receiving Cy + L-IL-2 therapy, while 90% of mice receiving CD4-based therapy were free of tumor.
Development of tumor-type specific immunologic memory.Cures were defined as those animals having established 7- to 14-day MC-38 or 3LL SC tumor deposits that were measurable before treatment, and were tumor-free after treatment for at least 80 days. Cured animals were rechallenged to determine the presence of immunologic memory. As shown in Table 2 all animals cured from MC-38 rejected subsequent challenges with MC38, but not an unrelated tumor (3LL or B-16 melanoma). Similarly, all animals cured of 3LL rejected subsequent challenges with 3LL, but not MC-38. All control mice were previously untreated and died of progressive tumor, indicating that the injected MC-38, 3LL, and B-16 cell lines were viable.
Rechallenge and Cross-Challenge Experiments
| Test Group . | No. . | Rechallenge . | Tumor Incidence . |
|---|---|---|---|
| . | . | (no. of cells) . | . |
| MC-38 cured | 8 | MC-38 (1 × 106) | 0/8 |
| Naive | 5 | MC-38 (1 × 106) | 5/5 |
| 3LL cured | 4 | 3LL (2 × 106) | 0/4 |
| Naive | 5 | 3LL (2 × 106) | 5/5 |
| Test Group | No. | Cross-Challenge (no. of cells) | Tumor Incidence |
| MC-38 cured | 8 | 3LL (2 × 106) | 8/8 |
| MC-38 cured | 8 | B-16 (1 × 105) | 4/4 |
| 3LL cured | 4 | MC-38 (1 × 106) | 4/4 |
| Test Group . | No. . | Rechallenge . | Tumor Incidence . |
|---|---|---|---|
| . | . | (no. of cells) . | . |
| MC-38 cured | 8 | MC-38 (1 × 106) | 0/8 |
| Naive | 5 | MC-38 (1 × 106) | 5/5 |
| 3LL cured | 4 | 3LL (2 × 106) | 0/4 |
| Naive | 5 | 3LL (2 × 106) | 5/5 |
| Test Group | No. | Cross-Challenge (no. of cells) | Tumor Incidence |
| MC-38 cured | 8 | 3LL (2 × 106) | 8/8 |
| MC-38 cured | 8 | B-16 (1 × 105) | 4/4 |
| 3LL cured | 4 | MC-38 (1 × 106) | 4/4 |
This immunologic memory could be transferred by the IV injection of a single cell suspension of splenocytes from cured mice into naive (ie, nontumor-bearing and untreated) normal adult C57BL/6 mice. Three hours after the splenocyte transfer, tumor lines were injected SC as indicated in Table 3. None of the recipient mice developed tumor over a 30-day period when rechallenged with the same tumor from which the donor mice were cured, but not when challenged with a different tumor. All of the naive control animals died of progressive tumor.
Splenocyte Transfer Experiments
| Experiment No. . | Test Group . | No. . | Source of Splenocytes . | Splenocyte Dose . | Rechallenge Tumor Type . | Dose of Tumor Inoculum . | Outcome/Length of Observation . |
|---|---|---|---|---|---|---|---|
| 1 | Naive | 3 | MC-38 cures | 5 × 107 cells | MC-38 | 1 × 105 | 3/3 tumor free at 30 d |
| Naive | 3 | None | None | MC-38 | 1 × 105 | 3/3 dead of tumor at 30 d | |
| 2 | Naive | 3 | 3LL cures | 5 × 107 cells | 3LL | 1 × 105 | 3/3 tumor free at 30 d |
| Naive | 3 | None | None | 3LL | 1 × 105 | 3/3 dead of tumor at 30 d |
| Experiment No. . | Test Group . | No. . | Source of Splenocytes . | Splenocyte Dose . | Rechallenge Tumor Type . | Dose of Tumor Inoculum . | Outcome/Length of Observation . |
|---|---|---|---|---|---|---|---|
| 1 | Naive | 3 | MC-38 cures | 5 × 107 cells | MC-38 | 1 × 105 | 3/3 tumor free at 30 d |
| Naive | 3 | None | None | MC-38 | 1 × 105 | 3/3 dead of tumor at 30 d | |
| 2 | Naive | 3 | 3LL cures | 5 × 107 cells | 3LL | 1 × 105 | 3/3 tumor free at 30 d |
| Naive | 3 | None | None | 3LL | 1 × 105 | 3/3 dead of tumor at 30 d |
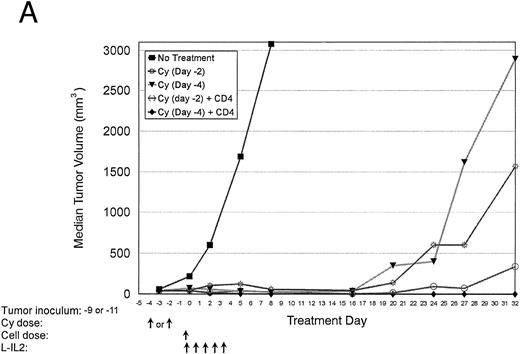
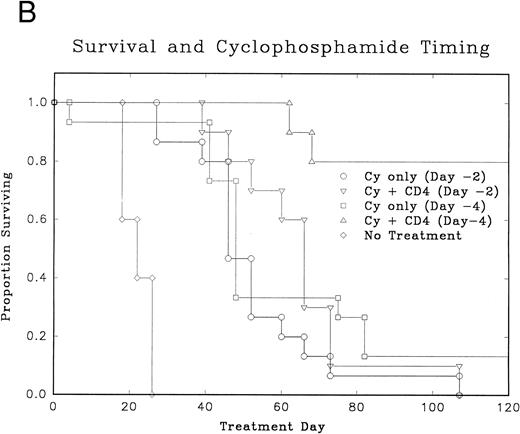
Treatment response and the timing of Cy doses.Pretreatment with low-dose Cy or radiation is routinely used in most adoptive immunotherapy models. Experiments to optimize the timing of Cy administration were done (Table 1, experiment 4). Figure 4A and B shows that the infusion of Cy 4 days before the adoptive transfer of CD4+ cells + L-IL-2 had a significantly better antitumor effect (P = .0006) and improved survival (P = .0003) compared with animals given Cy 2 days before CD4+ cell infusion. Also the administration of Cy 4 days before cellular therapy resulted in 80% tumor cures, not simply delays in tumor growth. The effect did not appear to be related only to debulking of the tumor, as a similar debulking effect was seen with Cy given 2 or 4 days before cellular therapy. Cy alone at 100 mg/kg usually does not cure any mice and rarely induces durable tumor remissions.
Normal C57BL/6 mice were inoculated with MC-38 SC in the right hindflank (Table 1, experiment 4). After 7 days, mice received Cy. Cellular therapy + L-IL-2 was started either 2 or 4 days after Cy. (A) Median tumor volumes for each treatment group. (B) Graph of disease-free survival for each treatment group.
Normal C57BL/6 mice were inoculated with MC-38 SC in the right hindflank (Table 1, experiment 4). After 7 days, mice received Cy. Cellular therapy + L-IL-2 was started either 2 or 4 days after Cy. (A) Median tumor volumes for each treatment group. (B) Graph of disease-free survival for each treatment group.
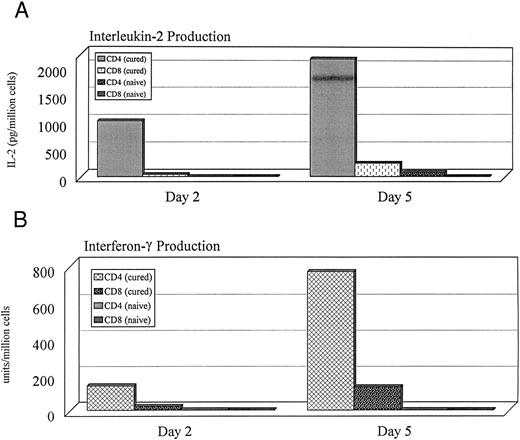
Functional characteristics of T cells from cured animals.T-cell subsets were obtained from naive or cured animals, stimulated with irradiated MC-38 tumor cells, and cultured for 2 to 5 days as described above. Supernatants from these cultures were obtained after 2 and 5 days. CD4+ T cells from cured mice (n = 2) showed significantly higher peak IL-2, and IFN-γ production compared with CD8+ cells from cured animals, or CD4+ and CD8+ T cells from naive animals (Fig 5). Similar cytokine production profiles were seen in three experiments.
IL-2, and IFN-γ production indexed per million cells in culture. Cells were obtained from the spleens of normal and cured animals. CD4+ or CD8+ T-cell subsets were cultured with irradiated MC-38 for 2 or 5 days. Supernatants from these cultures were used to measure cytokine levels. Similar cytokine production profiles were seen in the CD4+ cells of 3 of 3 cured animals studied.
IL-2, and IFN-γ production indexed per million cells in culture. Cells were obtained from the spleens of normal and cured animals. CD4+ or CD8+ T-cell subsets were cultured with irradiated MC-38 for 2 or 5 days. Supernatants from these cultures were used to measure cytokine levels. Similar cytokine production profiles were seen in the CD4+ cells of 3 of 3 cured animals studied.
The incorporation of 3H-thymidine after stimulation with irradiated MC-38 was also greater in CD4+ subsets from cured animals (620 ± 58 cpm) compared with CD4+ subsets from untreated animals (194 ± and 482 ± 124 cpm, respectively). Although CD4+ cells from cured mice showed the ability to proliferate and produce cytokines after exposure to tumor, very low cytotoxicity was detected in total splenocytes or enriched CD4+ or CD8+ T-cell populations (Table 4). Even repeated experiments in a 4- or 18-hour lytic assay with IFN-γ (for class I upregulation) failed to demonstrate any significant cytotoxicity (data not shown). Supernatants were collected from CD4+ T cells isolated from cured animals after activation with anti-CD3 and IL-2 as described in Materials and Methods. MC-38 tumor cells were cultured with the CD4 supernatant to determine if cytokines secreted by the CD4 cells would inhibit tumor growth. The proliferation of MC-38 was not inhibited using pure CD4 supernatant or supernatant diluted 1:2 or 1:4 with RPMI (data not shown).
Cytotoxicity of T Cells or T-Cell Subsets Using 51Chromium Against MC-38 Targets
| Experiment No. 1 . | Experiment No. 2 . | Experiment No. 3 . | Experiment No. 4 . | ||||
|---|---|---|---|---|---|---|---|
| E:T ratio . | % Specific Lysis . | E:T Ratio . | % Specific Lysis . | E:T Ratio . | % Specific Lysis . | E:T Ratio . | % Specific Lysis . |
| 25:1 | 6.6 | 42:1 | 11.2 | 42:1 | 16.1 | 42:1 | 13.2 |
| 12.5:1 | 5.8 | 21:1 | 10.9 | 21:1 | 11.6 | 21:1 | 12.9 |
| 6.25:1 | 5.3 | 10:1 | 11.4 | 10:1 | 13.1 | 10:1 | 12.5 |
| 3:1 | 5.8 | 5:1 | 13.6 | 5:1 | 7.9 | 5:1 | 11.9 |
| Experiment No. 1 . | Experiment No. 2 . | Experiment No. 3 . | Experiment No. 4 . | ||||
|---|---|---|---|---|---|---|---|
| E:T ratio . | % Specific Lysis . | E:T Ratio . | % Specific Lysis . | E:T Ratio . | % Specific Lysis . | E:T Ratio . | % Specific Lysis . |
| 25:1 | 6.6 | 42:1 | 11.2 | 42:1 | 16.1 | 42:1 | 13.2 |
| 12.5:1 | 5.8 | 21:1 | 10.9 | 21:1 | 11.6 | 21:1 | 12.9 |
| 6.25:1 | 5.3 | 10:1 | 11.4 | 10:1 | 13.1 | 10:1 | 12.5 |
| 3:1 | 5.8 | 5:1 | 13.6 | 5:1 | 7.9 | 5:1 | 11.9 |
Effectors in experiment 1: CD4+ T cells from naive mice; effectors in experiment 2: splenocytes from cured mice; effectors in experiment 3: CD4+ T cells from cured mice; effectors in experiment 4: CD8+ T cells from cured mice.
DISCUSSION
Previous studies using whole T-cell preparations were done in mice with MC-38 liver metastases.3 This anatomic location favored the trafficking of the infused cells and required killing mice to count metastases. For the present study, all tumors were implanted SC; therefore, effector cells would have to traffic to a site outside the liver, spleen, or lymph nodes to be effective. Also, this approach allowed easy assessment of antitumor effects and survival after treatment.
The present report demonstrates that anti-CD3–activated CD4+ T cells induce a strong antitumor effect in vivo when injected with L-IL-2. This marked therapeutic effect, confirmed in three different tumor models, not only produces a reduction in tumor size, but results in a significant number of cured animals. The mice that achieved a total regression of the tumor also developed a tumor-specific immunologic response. Although we did not select antigen-specific CD4+ T cells, the results are similar to those previously reported showing that antigen-specific CD4+ T cells can cure tumors in mice18 and facilitate the response of CD8+ T cells.19 In humans, there is also increasing evidence of the presence of antigen-specific CD4+ tumor-infiltrating lymphocytes (TIL) in a variety of tumors including melanomas and breast cancer.20-22
Possible mechanisms for the antitumor effect by CD4+ T cells include the production of high concentrations of cytokines at the site of the tumor and the induction of tumor-specific effector cells. Preliminary data from immunohistochemical studies show a significant infiltration by CD4+ T cells in mice with regressing tumor (Dansky Ullmann et al, manuscript in preparation). However, the precise effector mechanisms are as yet unknown, as no significant in vitro cytotoxic activity against the tumors was detected even in cured mice who had successfully rejected a tumor rechallenge. The development of long-term immunologic memory, as well as the ability to transfer immunity by the infusion of splenocytes, suggests the presence of antigen-specific T cells. It is unlikely that the adoptively transferred polyclonally activated CD4+ T cells inducing the antitumor activity are the same ones that develop the long-term memory. It is possible that primed host cells cannot develop effective antitumor activity because of the weakly immunogenic nature of the tumors, or from T-cell alterations induced by the malignant cells.23 Therefore, the presence of high concentrations of cytokines produced by the activated CD4+ T cells could stimulate these partially activated host cells to become fully responsive and develop immunological memory.
Another interesting observation from this study is the development of tumor-specific memory in the cured animals. The immunity was readily transferred via splenocytes from cured animals. The transfer of the tumor-specific immunity did not require any other manipulation of the recipient animals and specifically did not require Cy pretreatment. Hosts receiving adoptively transferred cells such as lymphokine-activated killer (LAK) or TIL rarely develop specific memory, except when the T cells are cloned antigen-specific cells. The cells that mediate the immunologic memory or transferred immunity in this model are unlikely to be the same anti-CD3–activated CD4+ cells that originated from naive donor mice. However, CD4+ T cells in the cured animals are clearly responsive to stimulation with the tumor cells in vitro. It is possible that the cytokines produced by the transferred CD4+ cells expand host CD4+ or CD8+ T cells that have been primed to tumor antigens. Although we were unable to identify cells with cytotoxic activity in vitro, this does not rule out the participation of cytotoxic T lymphocytes or other cytotoxic immune cells in the in vivo antitumor effect. Another possible explanation is that cytokines secreted by transferred CD4+ T cells induce the infiltration of macrophages or other antigen presenting cells, which prime cytotoxic T cells to tumor antigens. Ongoing in vivo depletion experiments and immunohistochemical studies should elucidate the mechanisms involved in the antitumor response.
Activated CD4+ cells did not induce a significant antitumor response unless Cy pretreatment was given. The use of Cy as an adjuvant in adoptive cellular immunotherapy models has been described for over a decade.24,25 Previous studies showed that immunized Thy-1+ splenocytes induce permanent regression of some murine tumors when tumor bearers were pretreated with Cy.24 The proposed mechanism was that Cy eliminated suppressor lymphocytes. Studies from our laboratory have not shown suppressor cells in mice with MC-38 tumors. Instead, the T cells from mice bearing MC-38 and other tumors had alterations in signal transduction characterized by decreases in intracellular p56lck and the zeta-chain subunit of the T-cell receptor. Abnormalities in nuclear transcription factors were also seen, which correlated with a marked decrease in in vivo antitumor activity.23 These changes appear to be more significant in T cells infiltrating tumor sites.26,27 The dose of Cy used here has a modest antitumor effect, but also produces a marked leukocyte nadir on day 4 after infusion, a finding similar to that reported by Katsanis et al.28 It is possible that Cy eliminates T cells with signaling defects, facilitating the adoptive transfer of more reactive cytokine-secreting lymphocytes. Preliminary experiments support this mechanism. Partial tumor debulking by Cy could be important by exposing immune cells to antigens from dying tumor cells. The debulking effect seems to be only a part of the Cy's action, because Cy given 2 or 4 days before cellular therapy debulks by the same amount, yet the therapeutic outcome is markedly different.
The degree of immunogenicity plays an important role in the antitumor response in most murine models. MC-38 and 3LL are only weakly-to-moderately immunogenic tumors, thus it is significant that CD4+ cells were able to induce cures in models where CD8+ subsets or unselected T cells could not. Activated CD4+ cells, Cy, and L-IL-2 were also tested in C3H mice (H-2k) bearing 38C13 lymphomas, another weakly immunogenic model. Cure rates of 50% were obtained, similar to the antitumor effects in the MC-38 and 3LL models.
Studying the mechanisms for CD4-mediated antigen-specific immunity and tumor responses may yield significant insight into regulatory functions of the immune system. In addition, this approach might be important in immunotherapy, as it could circumvent the difficulties in the detection, isolation, and purification of tumor-specific antigens and antigen-specific T cells. A clinical trial is under way to evaluate the immunologic and antitumor effects of activated CD4+ cells with Cy and IL-2 in humans.
Address reprint requests to Brendan D. Curti, MD, NCI-NMOB, 8901 Wisconsin Ave, Bldg 8, Room 5101, Bethesda, MD 20889.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal