Abstract
Sickle cell disease (SCD) is characterized by repeated vaso-occlusive events, which result in substantial morbidity. Abnormal adhesion of sickle red blood cells (RBC) to the vascular endothelium is postulated to play a role in the pathogenesis of vaso-occlusion. Two adhesion receptors, very late activation antigen-4 (VLA-4) and CD36, are found in unusually high numbers on sickle cell reticulocytes and do mediate adhesion of sickle RBC to endothelium. Hydroxyurea (HU) therapy results in fewer vaso-occlusive episodes, and we postulated that HU-related modulation of VLA-4 and CD36 receptors may contribute to its clinical benefit. Using flow cytometry, eight patients were followed from the onset of HU treatment through a mean treatment length of 200 ± 49 days. Mean corpuscular volume and percent fetal hemoglobin (Hb F ) increased from 87% ± 6% to 98% ± 9% and 6.6% ± 3.9% to 12.7% ± 5.6%, respectively. The percentage of reticulocytes expressing VLA-4 decreased from 29.0% ± 5.9% to 14.9% ± 2.3% (P = .0003). Two thirds of the total decrease in VLA-4 expression occurred after 10 weeks of HU and plateaued by 20 weeks. Changes in VLA-4 expression occurred before substantial increases in Hb F. The percentage of reticulocytes expressing CD36 decreased from 55.3% ± 6.4% to 42.6% (P = .0046). Changes in adhesion receptor expression were not caused by a decrease in reticulocytosis with HU therapy. This report is the first to associate a decrease in adhesion receptor expression with a therapy known to reduce the clinical severity of SCD.
VASO-OCCLUSION IS the hallmark of sickle cell disease (SCD) and results in the pain and organ dysfunction that characterize this disease. While the polymerization of sickle hemoglobin (Hb S) is central to the process of vaso-occlusion,1 it is likely that other factors such as plasma proteins and cytokines,2-6 endothelial abnormalities,2,3 abnormal vasomotor tone,6-8 and adherence of sickle red blood cells (RBC) to the vascular endothelium2,3,9 10 are involved.
The abnormal adherence of sickle RBC to vascular endothelium has been demonstrated using a variety of in vitro models. It is postulated that adherence of sickle RBC to the vascular endothelium could prolong the transit time of RBC through the hypoxic postcapillary system enhancing polymerization, sickle cell formation, and subsequent vaso-occlusion.2,11 Additionally, adhesion of sickle RBC to the endothelium could obstruct blood flow and facilitate occlusion of vessels by sickled RBC.7,10 12
Several mechanisms for increased adherence have been postulated and two of the most well-studied models involve adhesion receptors very late activation antigen-4 (VLA-4 or α4β1 integrin) and CD36 (glycoprotein IV).2,3,7 VLA-4 is a member of the integrin family of cell surface adhesion receptors.13,14 Integrins are the major receptors by which cells attach to the extracellular matrix and mediate cell-cell interactions. VLA-4 is expressed on a variety of cell types including early erythroid progenitors where it is required for normal erythroid development.15-17 Expression of VLA-4 is lost during RBC maturation and circulating RBC do not normally express this receptor.2,11,17 Recently, Swerlick et al2 and Joneckis et al3 independently demonstrated that VLA-4 is expressed in unusually high numbers on circulating sickle reticulocytes. The presence of VLA-4 on sickle reticulocytes is consistent with previous findings demonstrating that the reticulocyte fraction of RBC exhibit the greatest degree of adherence to vascular endothelium.7,10,11,18 Ligands for VLA-4 include vascular cell adhesion molecule 1 (VCAM-1) and the extracellular matrix protein fibronectin.19 VCAM-1 is expressed on vascular endothelium and is exposed to circulating sickle reticulocytes expressing VLA-4 thereby providing a mechanism by which sickle RBC may adhere to the endothelium. VCAM-1 expression on the endothelium is upregulated by cytokines such as tumor necrosis factor (TNF ) and interleukin-1 (IL-1).3,19,20 Sickle reticulocytes demonstrate increased adherence when endothelial cells have been incubated with TNF and antibodies to VCAM-1 and the α4 integrin inhibit adherence.2,5 11 Taken together, these data suggest a role for VLA-4 interacting with VCAM-1 in sickle cell vaso-occlusion.
CD36 is a nonintegrin adhesion receptor that binds to a variety of extracellular matrix proteins including thrombospondin.21 The CD36 receptor mediates adhesion to αvβ3 receptors on vascular endothelium via thrombospondin.20 CD36 expression on erythroid progenitors is lost as the RBC matures.21,22 Like VLA-4, CD36 has an abnormally high expression on circulating sickle reticulocytes.3 Previous in vitro studies have documented that thrombospondin mediates adherence of CD36+ sickle reticulocytes to endothelial cells and that thrombospondin derived from activated platelets promotes sickle cell adherence, as well.23-25 CD36 and its interactions with thrombospondin, therefore, are another potential mechanism by which adhesion receptors may contribute to sickle cell vaso-occlusion.
Hydroxyurea (HU) is a cancer chemotherapy agent that decreases the frequency of SCD crises.26 The clinical benefits of HU are largely attributed to its ability to increase fetal hemoglobin (Hb F )27 and higher levels of Hb F result in clinically milder SCD.28 Despite the link between Hb F levels and clinical course, there is evidence that HU may provide clinical benefit by mechanisms other than increasing Hb F, such as decreasing cellular dehydration and increasing deformability of sickle cells.29 30 Because of the postulated role of adhesion receptors in vaso-occlusion and the reduction of vaso-occlusive events with HU treatment, we sought to determine if treatment with HU modulates adhesion receptor expression.
MATERIALS AND METHODS
Patients.Eight severely affected patients, 7 with hemoglobin SS and 1 with hemoglobin S-β0 thalassemia, were followed from the onset of therapy with HU. Investigational review board approval and informed written consent was obtained from all patients before beginning HU therapy. There were 7 males and 1 female. Patients' mean age was 9 ± 5 years (median, 9 years; range, 2 to 15 years). HU therapy was started at 15 mg/kg/d and, in the absence of toxicity, increased by 5 mg/kg/d every 8 weeks. Patients had complete blood counts every 2 weeks throughout their treatment with HU and flow cytometric analysis for adhesion receptor expression every 2 to 4 weeks. Five patients had baseline flow cytometry values before starting HU and the remaining 3 were first tested within a month of starting HU. Eight patients not on HU were also assessed for adhesion receptor expression on two occasions when they did not complain of illness. Complete blood counts including RBC indices and reticulocyte counts were performed. Hb F levels were measured by alkaline denaturation.31
Flow cytometric analysis.Peripheral venous blood anticoagulated with EDTA was obtained by venipuncture. The blood was diluted 1/10 in phosphate-buffered saline (PBS) containing bovine serum albumin (BSA) and azide (PBS-BSA-Az; 150 mmol/L NaCl, 10 mmol/L sodium phosphate, pH 7.30, 1% BSA, and 0.1% sodium azide). A total of 10 μL of the diluted preparation was washed in 1 mL PBS-BSA-Az, and the supernatant was carefully aspirated so as not to remove any of the red blood cell pellet. The cell pellet was then resuspended by gentle agitation and 50 μL of primary antibody diluted to a concentration of 10 μg/mL were added. The three primary antibodies consisted of purified mouse monoclonal IgG. Clone HP 2/1 anti-CD49d (α4 integrin subunit) was from Immunotech, Inc (Westbrook, ME). Clone 4B4 anti-CD29 (β1 integrin subunit) was purchased from Coulter Diagnostics (Hialeah, FL). Clone OKM5 anti-CD36 was generously provided by Dr Thomas Mercolino (Ortho Diagnostics, Raritan, NJ). The cell suspensions were incubated for 45 minutes at 4°C, washed twice with 1 mL of PBS-BSA-Az and resuspended in 10 μL of PBS-BSA-Az. A total of 10 μL of a 1/50 dilution of R-phycoerythrin-conjugated goat antimouse IgG (Jackson ImmunoResearch, West Grove, PA) was then added and the mixtures incubated for an additional 45 minutes at 4°C in the dark. The cells were washed twice with PBS-BSA-Az, resuspended, and incubated with either 1 mL of PBS or 1 mL of thiazole orange (Retic-COUNT, Becton Dickinson Immunocytometry Systems, San Jose, CA) for 30 minutes at room temperature in the dark. Controls consisted of cells incubated with PBS alone, with thiazole orange alone, or with R-phycoerythrin-conjugated goat antimouse IgG alone. The samples then were analyzed for two-color immunofluorescence on a FACScan (Becton Dickinson). The instrument was gated on red cells using the forward scatter (FSC) versus side scatter (SSC) plot and 50,000 events in this gate were collected for subsequent analysis. Fluorescence compensation settings were established using cells incubated with primary antibody and R-phycoerythrin-conjugated goat antimouse IgG for FL1-FL2 compensation and cells incubated with thiazole orange alone for FL2-FL1 compensation. CELLQuest software (Becton Dickinson) was used for two-color analysis and determination of cells staining positive with thiazole orange (ie, reticulocytes) and for CD49d, CD29, or CD36. Relative reticulocyte RNA content was assessed by comparing the mean fluorescence intensity of RBC staining with thiazole orange.
Statistical methods.The paired t-test and Signed Rank Test were used to establish statistical significance for all measurements relating to treatment with HU.
RESULTS
Complete blood count and Hb F changes.Patients were treated with HU for an average of 200 ± 49 days (median, 190 days; range, 147 to 286 days). The mean HU dose at last evaluation was 23.4 ± 5.7 mg/kg/d (median, 25 mg/kg/d; range, 14.3 to 30 mg/kg/d). Hemoglobin concentration increased from a mean of 7.9 ± 0.6 g/dL to 8.8 ± 1.2 g/dL (P = .016) on HU therapy. Reticulocyte percentage decreased from 12.0% ± 4.9% before HU therapy to 9.5% ± 4.9% on HU (P = .017). Absolute number of reticulocytes decreased from 320 × 103/dL to 209 × 103/dL (P = .019). Mean RBC number did not change significantly (2.80 × 106/dL pre-HU to 2.64 × 106/dL on HU, P = .19). Mean corpuscular volume (MCV) increased significantly from an average of 87 ± 6 pretreatment to 98 ± 9 at last evaluation (P = .008). Similarly, Hb F levels increased from 6.6% ± 3.9% to 12.7% ± 5.6% (P = .004). When changes in MCV and Hb F were analyzed (Fig 1), both parameters showed a nearly linear increase with time, with MCV increasing more rapidly.
MCV (A) and Hb F (B) levels in study patients treated with HU. Time = length of time the patient had been receiving HU. Data points represent the mean (±standard deviation [SD]) value for all patients evaluated at the indicated time points. Numbers in parentheses represent the number of patients sampled at each time point.
MCV (A) and Hb F (B) levels in study patients treated with HU. Time = length of time the patient had been receiving HU. Data points represent the mean (±standard deviation [SD]) value for all patients evaluated at the indicated time points. Numbers in parentheses represent the number of patients sampled at each time point.
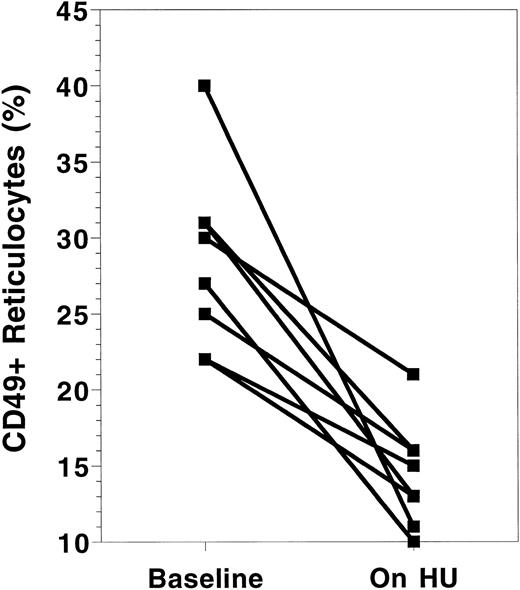
VLA-4 expression.Both components of VLA-4, CD49d (α4 ) and CD29 (β1 ), were measured using flow cytometry. Their results were virtually identical and, therefore, only CD49d results are reported here. The absolute number and percentage of reticulocytes expressing CD49d decreased with HU treatment (Table 1). All patients demonstrated a decrease in CD49d expression (Fig 2). The percentage of reticulocytes expressing CD49d decreased from an average of 29.0% ± 5.9% at baseline to 14.9% ± 2.3% (P < .001) on HU. Similarly, the absolute number of reticulocytes with CD49d decreased from 129 ± 39 × 103/μL to 48 ± 18 × 103/μL. The total number and percentage of all RBC expressing CD49d was also calculated to determine if HU resulted in a reduction in circulating RBC expressing VLA-4. Again, a significant reduction in both the percentage and total number of RBC expressing CD49d was found.
Effects of HU on Expression of CD49d ( α4) and CD36 on Sickle Cell Erythrocytes
| . | . | Baseline . | HU . | P . |
|---|---|---|---|---|
| CD49d | % Retic+ | 29.0 ± 5.9 | 14.9 ± 2.3 | .0003 |
| (α4) | ||||
| # Retic+ | 129 ± 39 | 48 ± 18 | .0008 | |
| (× 103/μL) | ||||
| % RBC+ | 5.2 ± 1.8 | 2.3 ± 1.0 | .0078 | |
| # RBC+ | 139 ± 36 | 58 ± 15 | .0011 | |
| (× 103/μL) | ||||
| CD36 | % Retic+ | 55.3 ± 6.4 | 42.6 ± 8.9 | .0046 |
| (GPIV) | ||||
| # Retic+ | 245 ± 60 | 141 ± 67 | .0054 | |
| (× 103/μL) | ||||
| % RBC+ | 15.8 ± 3.8 | 14.2 ± 5.0 | .4349 | |
| # RBC+ | 441 ± 133 | 357 ± 74 | .1718 | |
| (× 103/μL) |
| . | . | Baseline . | HU . | P . |
|---|---|---|---|---|
| CD49d | % Retic+ | 29.0 ± 5.9 | 14.9 ± 2.3 | .0003 |
| (α4) | ||||
| # Retic+ | 129 ± 39 | 48 ± 18 | .0008 | |
| (× 103/μL) | ||||
| % RBC+ | 5.2 ± 1.8 | 2.3 ± 1.0 | .0078 | |
| # RBC+ | 139 ± 36 | 58 ± 15 | .0011 | |
| (× 103/μL) | ||||
| CD36 | % Retic+ | 55.3 ± 6.4 | 42.6 ± 8.9 | .0046 |
| (GPIV) | ||||
| # Retic+ | 245 ± 60 | 141 ± 67 | .0054 | |
| (× 103/μL) | ||||
| % RBC+ | 15.8 ± 3.8 | 14.2 ± 5.0 | .4349 | |
| # RBC+ | 441 ± 133 | 357 ± 74 | .1718 | |
| (× 103/μL) |
Baseline = value at the time of the first measurement of adhesion receptor expression. HU = value at the time of the last measurement of adhesion receptor expression while on HU.
Abbreviations: Retic, reticulocyte; GPIV, glycoprotein IV.
Changes in CD49d receptor expression on sickle cell reticulocytes before and while on HU therapy. Baseline = value at the time of the first measurement of adhesion receptor expression. On HU = value at the time of the last measurement of adhesion receptor expression while on HU therapy. Each paired data point represents one patient.
Changes in CD49d receptor expression on sickle cell reticulocytes before and while on HU therapy. Baseline = value at the time of the first measurement of adhesion receptor expression. On HU = value at the time of the last measurement of adhesion receptor expression while on HU therapy. Each paired data point represents one patient.
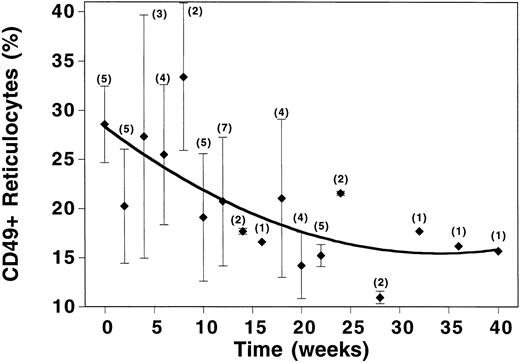
The time course over which the changes in CD49d occurred was examined by plotting average values for percentage of reticulocytes expressing CD49d versus weeks on therapy (Fig 3). Changes in CD49d expression began from the onset of therapy and continued to decrease linearly until the tenth week of treatment. After 10 weeks of HU therapy, two-thirds of the drop in CD49d expression had already occurred. During this same time period, MCV had increased from 87 ± 6 to 91 ± 7, but Hb F levels had only risen from 6.6% ± 3.9% to 8.4% ± 2.4%. By 20 weeks of therapy, CD49d levels had plateaued, yet MCV and Hb F continued to rise (Figs 1 and 3).
Time course of changes in CD49d expression in SCD patients treated with HU. Time = length of time the patient had been receiving HU. Data points represent the mean (±SD) value for all patients evaluated at the indicated time points. Numbers in parentheses represent the number of patients sampled at each time point.
Time course of changes in CD49d expression in SCD patients treated with HU. Time = length of time the patient had been receiving HU. Data points represent the mean (±SD) value for all patients evaluated at the indicated time points. Numbers in parentheses represent the number of patients sampled at each time point.
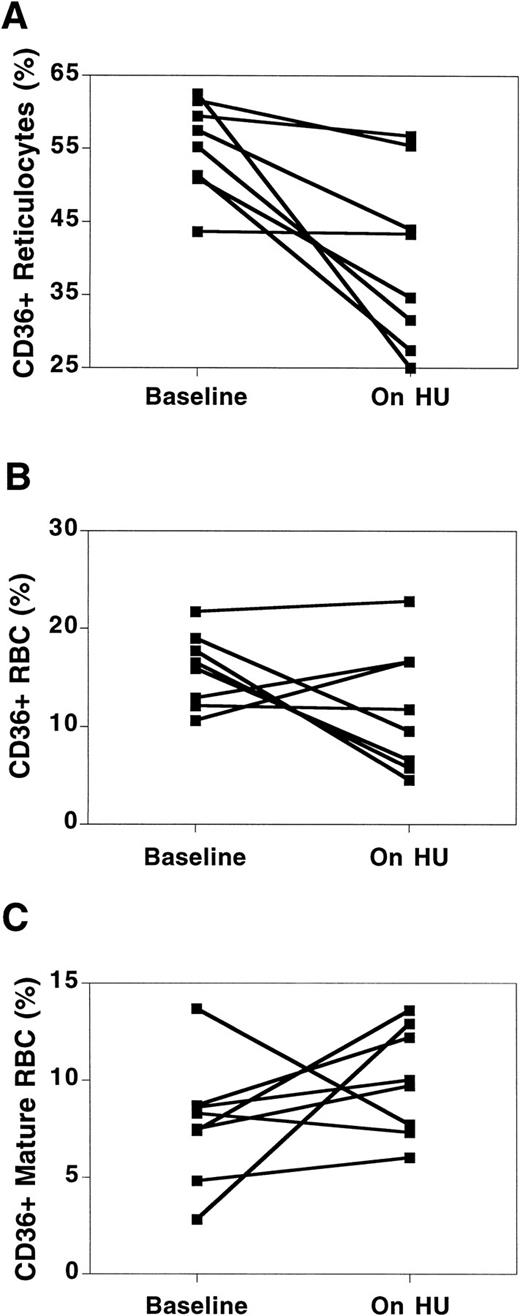
CD36 expression.CD36 expression on reticulocytes decreased in all patients, although the degree of change was not nearly as marked as with VLA-4 (Table 1, Fig 4A). HU did not produce a predictable change in total RBC stained for CD36, as some patients showed no change or an actual increase (Fig 4B). The difference in CD36 expression between reticulocytes and total RBC was due to low levels of expression of CD36 on mature RBC (nonreticulocytes). Before HU, a mean of 7.7% ± 3.2% of mature RBC expressed CD36, while on HU therapy, the percentage of mature RBC expressing CD36 had increased to 9.7% ± 2.6%. While the change in CD36 expression on mature RBC was not statistically significant (likely due to the small sample size), the change was consistent, with 6 of the 8 patients demonstrating increases in CD36 on mature RBC (Fig 4C).
Changes in CD36 receptor expression on sickle cell RBC before and while on HU therapy. Changes in CD36 expression are shown for the reticulocyte fraction (A), total RBC (B), and mature RBC (nonreticulocyte) fraction (C). Baseline = value at the time of the first measurement of adhesion receptor expression. On HU = value at the time of the last measurement of adhesion receptor expression while on HU therapy. Each paired data point represents one patient.
Changes in CD36 receptor expression on sickle cell RBC before and while on HU therapy. Changes in CD36 expression are shown for the reticulocyte fraction (A), total RBC (B), and mature RBC (nonreticulocyte) fraction (C). Baseline = value at the time of the first measurement of adhesion receptor expression. On HU = value at the time of the last measurement of adhesion receptor expression while on HU therapy. Each paired data point represents one patient.
Adhesion receptor expression in SCD patients not on HU.CD49d and CD36 expression were measured on the eight patients on two separate occasions when the patients were in the steady state (no evident illness). The mean value for the first measurement was 21.9% ± 10.7% versus a mean of 23.8% ± 10.1% for the second measurement. Similarly, percentages of CD36+ reticulocytes were 46.8% ± 12.4% versus 47.0% ± 14.6% for the two measurements. This confirmed that changes in receptor expression with HU treatment were due to HU therapy and not experimental or natural RBC variation within individuals over time.
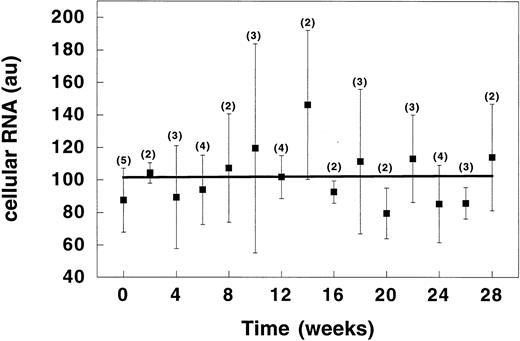
Reticulocyte RNA studies.By comparing the mean fluorescence intensity of RBC staining with thiazole orange, relative reticulocyte RNA content was assessed throughout HU therapy (Fig 5). RNA content in sickle cell reticulocytes did not change while the patients were receiving HU. Assuming a direct inverse relation between RNA content and cell age, these results suggest that, despite a reduction in reticulocytosis, the mean age of reticulocytes did not change while on HU therapy.
Time course of changes in RNA content of sickle reticulocytes during therapy with HU. Time = length of time the patient had been receiving HU. Data points represent the mean (±SD) value for all patients evaluated at the indicated time points. Numbers in parentheses represent the number of patients sampled at each time point. au, arbitrary units.
Time course of changes in RNA content of sickle reticulocytes during therapy with HU. Time = length of time the patient had been receiving HU. Data points represent the mean (±SD) value for all patients evaluated at the indicated time points. Numbers in parentheses represent the number of patients sampled at each time point. au, arbitrary units.
DISCUSSION
Hebbel et al9 32 first described abnormal adherence of sickle cells to the vascular endothelium and then correlated the degree of adherence with clinical severity. Since these first observations, in vitro investigations have attempted to elucidate the mechanisms by which sickle cells adhere to the endothelium. Although various mechanisms for increased adherence of sickle cells have been explored, no studies have correlated in vitro data with the clinical status of the patient. Our results are the first report relating well-defined mechanisms of cellular adhesion with a therapeutic intervention known to alter the clinical severity of sickle cell disease.
This report documents that VLA-4 and CD36 expression are both affected by treatment with HU. VLA-4 expression on reticulocytes demonstrated the most significant change with levels dropping by nearly half. VLA-4 has multiple ligands and such a reduction would be expected to affect its interactions with VCAM-1 and fibronectin in association with the vascular endothelium. A recent report indicates that the most tenacious adherence of sickle RBC to endothelium is found with pathways involving VLA-4.33 In addition, when both VLA-4–mediated pathways (VCAM-1 and fibronectin) are invoked simultaneously, adherence is greater than with either pathway alone. Because fibronectin and VCAM-1 are both upregulated by infection, VLA-4–mediated adhesion also provides a plausible mechanism to explain the association of vaso-occlusion with inflammation.2 34
The time course of changes in VLA-4 expression demonstrate an immediate response to HU therapy. Two thirds of the changes seen with HU treatment were in the first 10 weeks, and a plateau was reached by 20 weeks of therapy. The rapid decrease in VLA-4 expression well before substantial changes in Hb F suggest that VLA-4 expression was not influenced by the Hb F level. The Multicenter Study of Hydroxyurea documented that clinical differences in patients treated with hydroxyurea were first noted after 2 months of treatment and were clearly evident by 4 months.26 The reduction in VLA-4 expression, therefore, paralleled the expected time course of clinical benefit for patients treated with HU. Whether further dose increases after 20 weeks of therapy would have resulted in a greater reduction in adhesion receptor expression is unknown.
The expression of CD36 on reticulocytes also decreased following treatment with HU, but the degree of change was not as great as with VLA-4. However, the effect of HU on CD36 expression on the total number of RBC was unpredictable and often increased. An increase in CD36 expression on mature RBC accounted for this increase and potentially reflected an increased RBC survival associated with HU treatment.35 Reticulocytes are the most adherent fraction of RBC and the impact of increased CD36 expression in mature RBC on adhesion is unknown. Given that almost twice as many reticulocytes express CD36 as express VLA-4, it is interesting that HU did not produce an equal or greater reduction in CD36 expression. This suggests that the mechanism by which HU affects the expression of the different adhesion receptors differs for each type of receptor. How this impacts on clinical changes is also unknown, but a recent study noted a significant decrease in sickle RBC adhesion to immobilized CD36 ligands, thrombospondin, and laminin in patients treated with HU.36 CD36 expression on reticulocytes is greater than that of VLA-4, but changes in CD36 may not need to be as great to affect adhesion in vivo.
VLA-4 and CD36 expression are necessary for normal erythroid differentiation in the bone marrow, and both are normally lost before the cell matures and enters the systemic circulation.2,11,17 Why SCD patients have persistent expression of adhesion receptors is unknown, but it is postulated that increased erythropoiesis results in the premature release of reticulocytes expressing VLA-4 and CD36.2,3 Supporting this hypothesis is the finding that the youngest reticulocytes have the greatest expression of VLA-4.2,3,11 This mechanism does not entirely explain why SCD patients have higher levels of adhesion receptors, however, because other disease states with similarly elevated reticulocyte counts do not demonstrate the same degree of abnormal expression of these molecules.2
HU therapy decreases the reticulocyte count and, therefore, could be postulated to reduce expression of adhesion receptors by eliminating the stimulus for the early release of RBC expressing VLA-4 and CD36. In our analysis, we corrected for the observed decrease in reticulocyte count due to HU by separately analyzing the percentage of reticulocytes that expressed adhesion receptors. A reduction in reticulocytosis would be expected to result in a decrease in the youngest reticulocytes, those with the greatest expression of VLA-4 and CD36. A shift toward more mature reticulocytes might then result in fewer cells with adhesion receptor expression. Using RNA staining as a measure of reticulocyte age, we found that mean fluorescence for RNA was unchanged while on HU treatment, suggesting that the mean age of the reticulocytes did not increase. These findings indicate that HU did not result in an older population of reticulocytes and suggest that HU seems to exert its effect on adhesion receptor expression independent of its effects on reticulocyte age.
In summary, our results indicate that treatment with HU results in a significant reduction of VLA-4 and CD36 on sickle cell reticulocytes. These findings are the first to relate a reduction in adhesion receptor expression with a therapy known to reduce the severity of SCD and provides the first in vivo evidence suggesting that a treatment intervention aimed at decreasing adhesion receptor expression may result in fewer vaso-occlusive events. The mechanism by which HU affects adhesion receptor expression is unknown, but does not seem to result solely from its effects on erythropoiesis or Hb F levels. This report suggests that Hb F may not be the only mechanism by which HU results in clinical benefit for the patient with SCD. Lastly, these findings could have clinical relevance, as inhibition of adhesion receptor expression or activity could be a novel new therapeutic approach for the patient with SCD.
ACKNOWLEDGMENT
We are indebted to Jolene Edwards for editorial assistance and Laura Vichinsky for data organization and analysis.
Supported in part by National Institutes of Health Grants No. HL-27059, HL-55213, and HL-20985.
Address reprint requests to Lori A. Styles, MD, Department of Hematology/Oncology, Children's Hospital Oakland, 747 52nd St, Oakland, CA 94609.

![Fig. 1. MCV (A) and Hb F (B) levels in study patients treated with HU. Time = length of time the patient had been receiving HU. Data points represent the mean (±standard deviation [SD]) value for all patients evaluated at the indicated time points. Numbers in parentheses represent the number of patients sampled at each time point.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/7/10.1182_blood.v89.7.2554/6/m_bl_0036f1.jpeg?Expires=1767754394&Signature=arn06AmXn~QeKVcQnjiR4AzOkyhZFLUw21LFr-tOMyj3a~ASSe7OK495QmV9sHnkRkYDU6x2s-GPpqF05nTfRHntcSTEOA91RX2Zjc2WLawsPHPhyXBZq-kKreDUYIXLkGkXasND96HlZm~B97rWXdl~Om9ILjcfBExUmJUEaBwITyIvxnGYVcnL~u82lEeoQlziP45VUhDQwTc~6MITzbei7CKLqZLaV4ayMW-e6eQPyJXj2pyNcPumhNaQQeOvcl7BP2Z6xDWLtsYEevLm2KkVZBwFxc~s8ZfoMQThWiX2iIx7nCoxHYBtx4JtB9zVqDdYiWdekmez7RASaJmtCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal