Abstract
Thrombopoietin has an essential role in megakaryopoiesis and thrombopoiesis. To investigate the signaling processes induced by thrombopoietin, we have employed human platelets and recently demonstrated that thrombopoietin induces rapid tyrosine phosphorylation of Jak-2, Tyk2, Shc, Stat3, Stat5, p120c-cbl and other proteins in human platelets. Because the apparent molecular weight of a major tyrosine phosphorylated protein in platelets stimulated by thrombopoietin is approximately 85 to 95 kD, we examined the possibility that this could be Vav, a 95-kD proto-oncogene product. Specific antisera against Vav recognized the same 95 kD protein in lysates of Jurkat cells, which are known to express Vav, and platelets, indicating that platelets have Vav. Thrombopoietin induced rapid tyrosine phosphorylation of Vav in platelets without an elevation in cytosolic free calcium concentration or activation of protein kinase C. Vav was also tyrosine phosphorylated upon treatment of platelets with thrombin, collagen, or U46619, which activate phospholipase C, leading to an increased ionized calcium concentration and activation of protein kinase C. Ionomycin or phorbol 12-myristate 13-acetate (PMA) also induces tyrosine phosphorylation of Vav, suggesting that an increase in ionized calcium concentration or activation of protein kinase C may lead to phosphorylation of Vav. Thrombopoietin also induced tyrosine phosphorylation of Vav in FDCP-2 cells, genetically engineered to express human c-Mpl (FDCP-hMpl5). However, neither ionomycin nor PMA induced an increase in tyrosine phosphorylation of Vav in FDCP-hMpl5 cells, suggesting that the calcium and protein kinase C pathways of Vav phosphorylation may be unique to platelets. Further, Vav became incorporated into the Triton X-100 insoluble 10,000g sedimentable residue in an aggregation-dependent manner, suggesting that it may have a regulatory role in platelet cytoskeletal processes. Vav was constitutively associated with a 28-kD adapter protein, Grb2, which is also incorporated into the cytoskeleton in an aggregation-dependent fashion. Lastly, we found that Vav is cleaved when there is activation of calpain, a protease that may have a role in postaggregation signaling processes. Our data suggest that thrombopoietin and other agonists may induce tyrosine phosphorylation of Vav by different mechanisms and Vav may also be involved in signaling during platelet aggregation by its redistribution to the cytoskeleton.
THROMBOPOIETIN IS A MAJOR humoral regulator of megakaryopoiesis/thrombopoiesis.1 To understand the molecular basis for the function of thrombopoietin, we have employed human platelets to investigate signaling events following the binding of thrombopoietin to its receptor, c-Mpl.2-5 We previously reported that treatment of human platelets with thrombopoietin led to tyrosine phosphorylation of several intracellular proteins with relative molecular weights of 130 to 120, 95 to 85, and 52 kD.2 Using specific antisera, we have identified Jak-2, Tyk2, Shc, Stat3, Stat5, and p120c-cbl as six of the proteins that were inducibly tyrosine phosphorylated in platelets in response to thrombopoietin (concentration, 1 to 100 ng/mL).2-4 We also found that treatment of platelets with thrombopoietin, in a similar dose range, primes aggregation induced by various agonists such as epinephrine and adenine diphosphate (ADP) or by shear stress.5 These studies and others indicate that c-Mpl on the surface of platelets is functional.6-9 Since protein tyrosine phosphorylation is one of the earliest biochemical events detectable following treatment of cells with thrombopoietin, the identification of proteins that become tyrosine phosphorylated in platelets treated with thrombopoietin may contribute to our understanding of events required for priming of platelet functions and signaling by thrombopoietin.
The focus of this report is the proto-oncogene product Vav, a 95-kD protein primarily expressed in hematopoietic cells.10-20 Whether Vav is essential in hematopoiesis is a controversial issue.17-20 However, a recent report suggests that Vav may be indispensable for adult erythropoiesis.21 It is known that multiple cytokines and hematopoietic growth factors induce tyrosine phosphorylation of Vav.10-16 Further, several reports indicate that Vav has a major role in signaling through the T- and B-cell antigen receptors in lymphoid cells.22-24
Given the presence of functional thrombopoietin receptors on platelets and the existence of 95 to 85 kD proteins as the major tyrosine phosphorylated proteins following stimulation of platelets with thrombopoietin,2 we examined whether platelets may have Vav protein, and if it becomes tyrosine phosphorylated after treatment with thrombopoietin. We show that thrombopoietin induces an increase in tyrosine phosphorylation of Vav in platelets, without an elevation of cytosolic ionized calcium concentration ([Ca2+]i) or activation of protein kinase C. Thrombin, U46619, collagen, or phorbol 12-myristate 13 acetate (PMA), all of which are known to activate protein kinase C,25 also induce an increase in tyrosine phosphorylation of Vav. Thrombin induces redistribution of Vav into the Triton X-100 insoluble residue in an aggregation dependent fashion. Finally, similar to many proteins incorporated into the Triton X-100 insoluble residue following platelet aggregation, such as integrin β3, p60c-src , talin, and actin binding protein, we found that Vav is an endogenous substrate for calpain, suggesting that Vav may have a role in postaggregation events.26-34
MATERIALS AND METHODS
Materials.Prostaglandin E1 (PGE1 ), A23187, ionomycin, Arg-Gly-Asp-Ser (RGDS) peptide, dibucaine, dimethyl sulfoxide (DMSO), aspirin, apyrase (type VIII), N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), sodium dodecyl sulfate (SDS), 2-mercaptoethanol, sodium orthovanadate, chicken egg albumin, protein A-Sepharose, antimouse IgG-conjugated agarose, Triton X-100 and Tris (hydroxymethyl) aminomethane (Tris) were purchased from Sigma (St Louis, MO). BAPTA-acetoxymetylester (AM) was from Molecular Probes (Eugene, OR). Ro 31-8220 was from Calbiochem (La Jolla, CA). Polyvinylidene difluoride (PVDF ) membranes (pore size, 0.45 mm) were from Millipore Corporation (Bedford, MA). SDS-polyacrylamide gel electrophoresis (PAGE) molecular standards, 32Pi, and enhanced chemiluminesence (ECL) reagents including secondary antibodies were from Amersham (Arlington Heights, IL). Calpeptin was a generous gift from Dr Junichi Kambayashi (Osaka University, Osaka, Japan).32 Anticalpain and GPIIbα (AP4) monoclonal antibodies in ascites form were kind gifts from Dr Thomas J. Kunicki (Scripps Research Institute, La Jolla, CA).33 The anticalpain antibody mostly recognizes the 80 kD subunit of μ-calpain in platelets.33 Antiphosphotyrosine murine monoclonal antibody (4G10) was used as described.35,36 Jurkat cell lysate, a positive control for Vav and an anti-Grb2 monoclonal antibody were from Transduction Laboratories (Lexington, KY). An anti-Jak–2 rabbit polyclonal antibody was from UBI (Lake Placid, NY). Anti-Vav was from Santa Cruz (Santa Cruz, CA). Iscove's Modified Dulbeco's Medium (IMDM) was from GIBCO BRL (Gaithersburg, MD). Purified human thrombin was generously provided by Green Cross Co. Ltd (Osaka, Japan). Recombinant thrombopoietin was provided by Kirin Brewery Co, Ltd (Maebashi, Japan). Horse tendon collagen was from Hormon Chemie (Munich, Germany).
Platelet preparation.Human blood from healthy volunteers was drawn by venipuncture into 1/10 volume of 3.8 % (wt/vol) trisodium citrate and gently mixed. Platelet rich plasma (PRP) was prepared by centrifuging the whole blood at 200g for 20 minutes and aspirating PRP. PRP was incubated with aspirin (2 mmol/L) for 30 minutes at room temperature. PGE1 (1 μmol/L) was added from a stock solution in absolute ethanol (1 mmol/L). The PRP was spun at 800g to form a soft platelet pellet. The pellet was resuspended in 1 mL of a modified HEPES-Tyrode buffer (129 mmol/L NaCl, 8.9 mmol/L NaHCO3 , 0.8 mmol/L KH2PO4 , 0.8 mmol/L MgCl2 , 5.6 mmol/L dextrose, and 10 mmol/L HEPES, pH 7.4) also containing apyrase (2 U/mL) and washed twice. Platelets were resuspended in the same buffer at a concentration of 3 × 108 cells/mL with apyrase (2 U/mL) containing 1 mmol/L CaCl2 at 37°C.
Cell lines.A thrombopoietin-dependent cell line (FDCP-hMpl5) was previously established by transforming FDCP-2 cells with an expression plasmid containing the full-length human c-mpl cDNA driven by the long terminal repeat of Moloney murine sarcoma virus.3,37,38 Before treatment of the cells with reagents, they were incubated in IMDM containing 10% fetal calf serum (FCS) without exogenous interleukin (IL)-3 for 6 hours. After two washes, they were incubated in phosphate buffered saline (PBS; pH 7.4).
Gel electrophoresis and Western blotting to detect tyrosine phosphorylated proteins or Vav.Platelet stimulation was terminated by the addition of an equal volume of two times concentrated Laemmli's sample buffer (10% glycerol, 1% SDS, 5% 2-mercaptoethanol, 50 mmol/L Tris-HCl (pH6.8), and 0.002% bromophenol blue),39 10 mmol/L EGTA and 1 mmol/L sodium orthovanadate. After boiling at 95°C for 5 minutes, one-dimensional SDS-electrophoresis was performed on 10% or 7.5% to 15% polyacrylamide gels as described.39 Separated proteins were electrophoretically transferred from the gel onto PVDF membranes in a buffer containing Tris (25 mmol/L), glycine (192 mmol/L), and 20% methanol at 0.2A for 12 hours at room temperature. To block residual protein binding sites, membranes were incubated in TBST (TBS: Tris-buffered-saline, 10 mmol/L Tris, 150 mmol/L NaCl, pH 7.6 with 0.1% Tween 20) with 10% chicken egg albumin. The blots were washed with TBST and incubated overnight with primary antibodies at a final concentration of 1.0 μg/mL in TBST. The primary antibody was removed and the blots were washed four times in TBST and incubated with horseradish peroxidase-conjugated second antibody diluted 1:3000 in TBST. Blots were then washed four times in TBST. Antibody reactions were detected with chemiluminescence according to the manufacturer's instructions.
Immunoprecipitation.Cell stimulation was terminated by the addition of an equal amount of lysis buffer (15 mmol/L HEPES, 150 mmol/L NaCl, 1 mmol/L PMSF, 10 mmol/L EGTA, 1 mmol/L sodium orthovanadate, 0.8 μg/mL leupeptin, 2% Triton X-100 (V/W), pH 7.4). After 20 minutes on ice, the lysates were centrifuged at 10,000g (at 4°C) for 20 minutes. The supernatant was removed and incubated with preimmune serum and Protein A-Sepharose (40 μL of 50% slurry) for 1 hour. The desired polyclonal or monoclonal antibodies were then added and incubated for 2 to 3 hours on ice. Protein A-Sepharose or antimouse IgG-conjugated agarose (40 μL of 50% slurry) was added and incubated for several hours. The immune complexes were washed with 1 mL of cold washing buffer (15 mmol/L HEPES, 150 mmol/L NaCl, 1 mmol/L PMSF, 10 mmol/L EGTA, 1 mmol/L sodium orthovanadate, 0.8 μg/mL leupeptin, 1% Triton X-100 [vol/wt], pH 7.4) three times and then resuspended in Laemmli's sample buffer.
Isolation of operationally defined platelet cytoskeleton. The Triton X-100 insoluble cytoskeleton was isolated as described with the following modification.36 Briefly, an equal amount of lysis buffer (15 mmol/L HEPES, 150 mmol/L NaCl, 1 mmol/L PMSF, 10 mmol/L EGTA, 1 mmol/L sodium orthovanadate, 0.8 μg/mL leupeptin, 2% Triton X-100 [vol/wt], pH 7.4) was added to platelet suspensions to solubilize platelets. After 5 minutes on ice, the lysates were centrifuged at 10,000g. The resulting pellet was washed twice in wash buffer (145 mmol/L NaCl, 0.1 mmol/L MgCl2 , 0.5 mmol/L PMSF, 5 mmol/L EGTA, 1 mmol/L sodium orthovanadate, 0.4 μg/mL leupeptin, 1% [vol/wt] Triton-X 100, pH 7.4). For one-dimensional SDS electrophoresis, the Triton X-100 insoluble residue was solubilized in SDS sample buffer. The supernatant was diluted with an equal volume of two times concentrated SDS sample buffer.
Loading of platelets with 32Pi and analysis of phosphorylated proteins.Detection of the phosphorylation of myosin light chain and pleckstrin was performed as described with the following modifications.40 The soft platelet pellets, prepared as described above, were resuspended in the modified HEPES-Tyrode buffer without KH2PO4 , but with apyrase (2 U/mL) and incubated for 1 hour at room temperature. Washed platelets were prepared as described above. The platelet lysate was prepared and analyzed on 7.5% to 15% SDS-PAGE.39 The gels were dried and autoradiographed with X-Omat films (Eastman Kodak, Rochester, NY).
RESULTS
Specific antisera against Vav were used to determine whether Vav is present in human platelets. These antisera recognize a 95-kD band in lysates from human platelets that comigrates with a 95-kD band in lysates from Jurkat cells, which express Vav, indicating that platelets have Vav (data not shown).41-43 As in other hematopoietic cells, we found that Vav is constitutively associated with Grb2, an SH2/SH3 adapter protein (data not shown).11 44
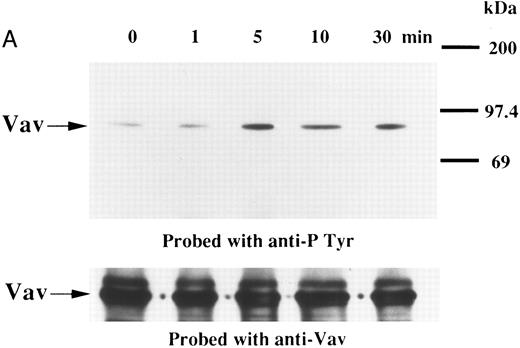
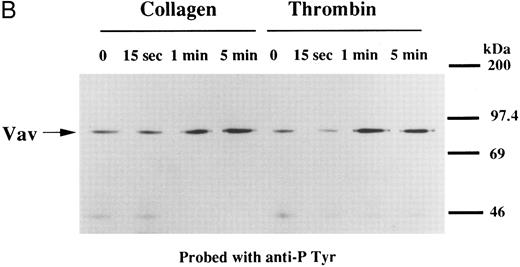
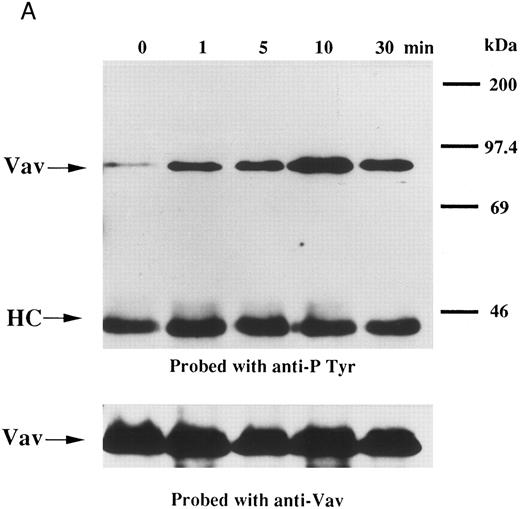
We examined whether Vav becomes tyrosine phosphorylated in platelets treated with thrombopoietin. Platelets treated with thrombopoietin (100 ng/mL) for various times were lysed in a buffer containing 1% Triton X-100 and immunoprecipitated with specific Vav antisera. The same amount of a 95-kD protein recognized by Vav antisera was immunoprecipitated from all cellular lysates (Fig 1A, lower panel). Following treatment with thrombopoietin, this same protein was increasingly tyrosine phosphorylated over the low basal level (Fig 1A, upper panel). Thus, platelets have Vav, which becomes tyrosine phosphorylated following thrombopoietin treatment of human platelets. Thrombin (1 U/mL), collagen (10 μg/mL), or U46619 (1 μmol/L), a thromboxane mimetic, also induce tyrosine phosphorylaion of Vav in platelets (Fig 1B and C). It should be noted that we routinely add RGDS peptide (200 μg/mL) to platelet suspensions and minimized stirring in the presence of agonists to diminish the effects of fibrinogen binding to α IIbβ3 and subsequent aggregation, which profoundly influence platelet protein tyrosine phosphorylation.34 45
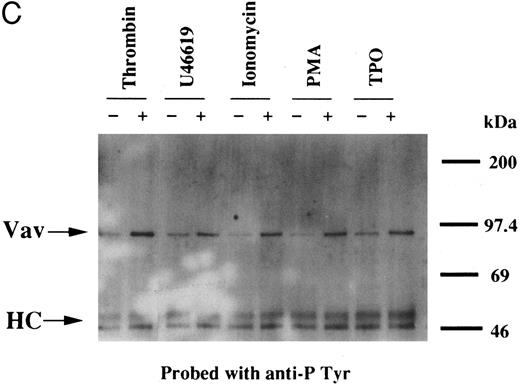
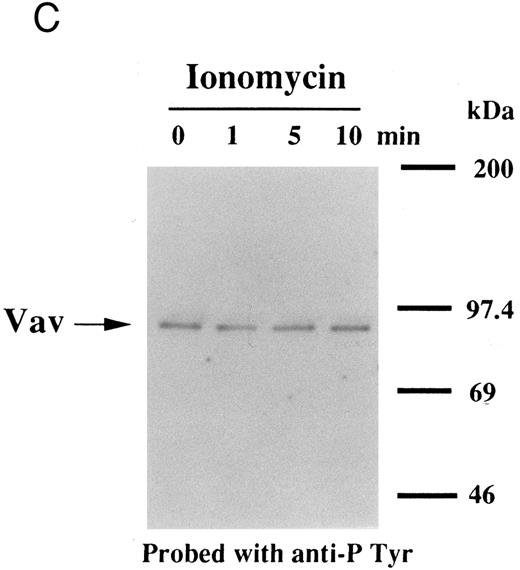
(A) Tyrosine phosphorylation of Vav in platelets stimulated by thrombopoietin (100 ng/mL). Platelets were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after exposure to thrombopoietin (100 ng/mL) for various times as indicated. Vav was immunoprecipitated with specific anti-Vav antisera. Immune complexes were resuspended in SDS-sample buffer. Proteins were separated by 10% SDS-PAGE and transferred onto PVDF membranes. Immunoblots were probed with antiphosphotyrosine antibodies and bands were visualized by chemiluminescence (upper panel). The same PVDF membrane used in the upper panel was stripped and reprobed for Vav (lower panel). (B) Collagen- and thrombin-induced tyrosine phosphorylation of Vav in platelets. Platelet suspensions were incubated in the presence of RGDS peptide (200 μg/mL) for 5 minutes. Collagen (10 μg/mL) or thrombin (1 U/mL) was added to the suspension for various times as indicated. Platelets were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after the addition of collagen or thrombin. Tyrosine phosphorylation of Vav was detected as in (A). (C) Tyrosine phosphorylation of Vav induced by various agonists. Platelet suspensions were incubated in the presence of RGDS peptide (200 μg/mL) for 5 minutes. Thrombin (1 U/mL), U46619 (1 μmol/L), ionomycin (20 nmol/L), PMA (100 nmol/L), or thrombopoietin (TPO, 100 ng/mL) was added to the suspension for 2 minutes. Platelets were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 and tyrosine phosphorylation of Vav was detected as in (A).
(A) Tyrosine phosphorylation of Vav in platelets stimulated by thrombopoietin (100 ng/mL). Platelets were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after exposure to thrombopoietin (100 ng/mL) for various times as indicated. Vav was immunoprecipitated with specific anti-Vav antisera. Immune complexes were resuspended in SDS-sample buffer. Proteins were separated by 10% SDS-PAGE and transferred onto PVDF membranes. Immunoblots were probed with antiphosphotyrosine antibodies and bands were visualized by chemiluminescence (upper panel). The same PVDF membrane used in the upper panel was stripped and reprobed for Vav (lower panel). (B) Collagen- and thrombin-induced tyrosine phosphorylation of Vav in platelets. Platelet suspensions were incubated in the presence of RGDS peptide (200 μg/mL) for 5 minutes. Collagen (10 μg/mL) or thrombin (1 U/mL) was added to the suspension for various times as indicated. Platelets were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after the addition of collagen or thrombin. Tyrosine phosphorylation of Vav was detected as in (A). (C) Tyrosine phosphorylation of Vav induced by various agonists. Platelet suspensions were incubated in the presence of RGDS peptide (200 μg/mL) for 5 minutes. Thrombin (1 U/mL), U46619 (1 μmol/L), ionomycin (20 nmol/L), PMA (100 nmol/L), or thrombopoietin (TPO, 100 ng/mL) was added to the suspension for 2 minutes. Platelets were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 and tyrosine phosphorylation of Vav was detected as in (A).
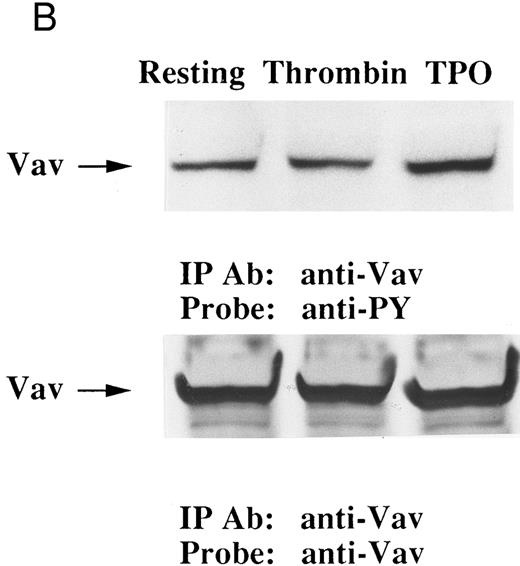
Thrombin, collagen, and U46619 induce activation of phospholipase C, leading to elevation of [Ca2+]i and activation of protein kinase C, independent of fibrinogen binding to α IIbβ3.25,46 Therefore, we examined whether PMA, an activator of protein kinase C, or calcium ionophore induces tyrosine phosphorylation of Vav. Both PMA (100 nmol/L) and ionomycin (20 nmol/L) induced tyrosine phosphorylation of Vav in the presence of RGDS peptide, suggesting that tyrosine phosphorylation of Vav may be mediated by both activated protein kinase C and elevation of [Ca2+]i (Fig 1C). On the other hand, thrombopoietin (100 ng/mL) did not induce elevation of [Ca2+]i in platelets (data not shown and Oda et al5 ) or phosphorylation of pleckstrin (a substrate for major protein kinase) and myosin light chain (Fig 2A), while thrombin (1 U/mL) did. This data indicates that thrombopoietin induces tyrosine phosphorylation of Vav independent of the elevation of [Ca2+]i and activation of protein kinase C. We also examined the effects of BAPTA-AM plus Ro 31-8220 on tyrosine phopshorylation induced by thrombin or thrombopoietin. Treatment of platelets with BAPTA-AM (40 μmol/L, a chelator of intracellular calcium) plus Ro 31-8220 (10 μmol/L, a protein kinase C inhibitor) in the presence of EGTA (1 mmol/L) effectively inhibited thrombin (1 U/mL)-induced tyrosine phosphorylation of Vav, while there was an increased tyrosine phosphorylation induced by thrombopoietin in the presence of these reagents (Fig 2B).
(A) Thrombopoietin does not induce phosphorylation of pleckstrin or myosin light chain. 32Pi-loaded platelets were stimulated with either thrombopoietin (100 ng/mL) or thrombin (1 U/mL). Whole cell lysates were separated by SDS-PAGE (7.5% to 15% gel). Gels were dried and autoradiographed. Lanes 1 and 6, resting platelets; lanes 2 to 5, 15 seconds, 1 minute, 5 minutes, 10 minutes after addition of thrombopoietin (100 ng/mL); lanes 7 to 9, 15 seconds, 1 minute, 2 minutes after addition of thrombin (1 U/mL). The upper arrow indicates the relative position of pleckstrin; the lower arrow, myosin light chain. (B) Effects of BAPTA-AM and Ro 31-8220 on tyrosine phosphorylation of Vav induced by thrombin or thrombopoietin. Platelets were preincubated with BAPTA-AM (40 μmol/L) and Ro-31-8220 (10 μmol/L) in the presence of 1 mmol/L EGTA for 15 minutes. After the incubation, thrombin (1 U/mL, lane 2) or thrombopoietin (100 ng/mL, lane 3) was added to the platelet suspension. After 10 minutes, platelets were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100. Tyrosine phosphorylation of Vav was detected as in Fig 1 (upper panel). The same PVDF membrane used in the upper panel was stripped and reprobed for Vav (lower panel).
(A) Thrombopoietin does not induce phosphorylation of pleckstrin or myosin light chain. 32Pi-loaded platelets were stimulated with either thrombopoietin (100 ng/mL) or thrombin (1 U/mL). Whole cell lysates were separated by SDS-PAGE (7.5% to 15% gel). Gels were dried and autoradiographed. Lanes 1 and 6, resting platelets; lanes 2 to 5, 15 seconds, 1 minute, 5 minutes, 10 minutes after addition of thrombopoietin (100 ng/mL); lanes 7 to 9, 15 seconds, 1 minute, 2 minutes after addition of thrombin (1 U/mL). The upper arrow indicates the relative position of pleckstrin; the lower arrow, myosin light chain. (B) Effects of BAPTA-AM and Ro 31-8220 on tyrosine phosphorylation of Vav induced by thrombin or thrombopoietin. Platelets were preincubated with BAPTA-AM (40 μmol/L) and Ro-31-8220 (10 μmol/L) in the presence of 1 mmol/L EGTA for 15 minutes. After the incubation, thrombin (1 U/mL, lane 2) or thrombopoietin (100 ng/mL, lane 3) was added to the platelet suspension. After 10 minutes, platelets were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100. Tyrosine phosphorylation of Vav was detected as in Fig 1 (upper panel). The same PVDF membrane used in the upper panel was stripped and reprobed for Vav (lower panel).
Interestingly, previous reports indicated that neither phorbol esters nor calcium ionophores induced tyrosine phosphorylation of Vav in other cell types,47,48 raising the possibility that the later pathway may be unique to platelets. To evaluate the possibility of cell type specificity of Vav tyrosine phosphorylation, we examined Vav tyrosine phosphorylation in FDCP-2 cells,3 expressing human c-Mpl (FDCP-hMPL5). FDCP-hMPL5 cells were treated with thrombopoietin (100 ng/mL) for various times and lysed in a buffer containing 1% Triton X-100. The same amount of a 95-kD protein recognized by Vav was immunoprecipitated from the resting and thrombopoietin-stimulated cells (Fig 3A, lower panel). This same protein became tyrosine phosphorylated after treatment with thrombopoietin (100 ng/mL) (Fig 3A, upper panel). On the other hand, neither PMA (100 nmol/L) nor ionomycin (20 nmol/L) induced tyrosine phosphorylation of Vav in FDCP-hMPL5 cells (Fig 3B and C).
Tyrosine phosphorylation of Vav in FDCP-hMpl5 cells. (A) Thrombopoietin-induced tyrosine phosphorylation of Vav in FDCP-hMpl5 cells. FDCP-hMpl5 cells (107 cells/mL in PBS, pH 7.4) were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after exposure to thrombopoietin (100 ng/mL) for various times as indicated. Vav was immunoprecipitated with specific Vav antisera. Immune complexes were resuspended in SDS-sample buffer. Tyrosine phosphorylation of Vav was detected as described in the legend to Fig 1. (B and C) PMA or ionomycin failed to induce tyrosine phosphorylation of Vav. FDCP-hMpl5 lysates were made as described in (A), except PMA (100 nmol/L) (B) or ionomycin (20 nmol/L) (C) was used as a stimulant where indicated.
Tyrosine phosphorylation of Vav in FDCP-hMpl5 cells. (A) Thrombopoietin-induced tyrosine phosphorylation of Vav in FDCP-hMpl5 cells. FDCP-hMpl5 cells (107 cells/mL in PBS, pH 7.4) were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after exposure to thrombopoietin (100 ng/mL) for various times as indicated. Vav was immunoprecipitated with specific Vav antisera. Immune complexes were resuspended in SDS-sample buffer. Tyrosine phosphorylation of Vav was detected as described in the legend to Fig 1. (B and C) PMA or ionomycin failed to induce tyrosine phosphorylation of Vav. FDCP-hMpl5 lysates were made as described in (A), except PMA (100 nmol/L) (B) or ionomycin (20 nmol/L) (C) was used as a stimulant where indicated.
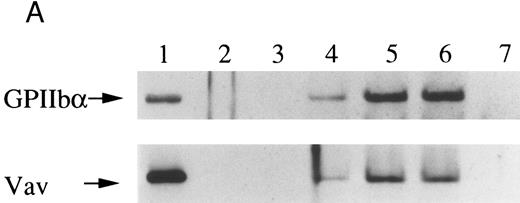
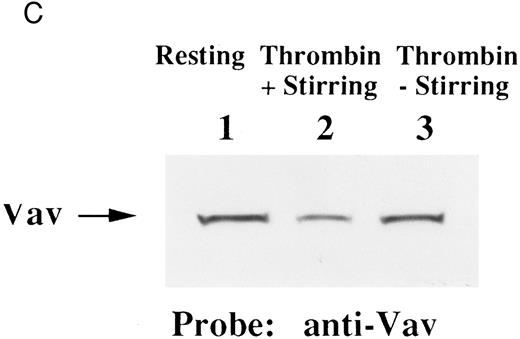
Finally, we examined the possibility that Vav may have a role in signaling following platelet aggregation. It has been shown that many SH2/SH3 signaling molecules including p60c-src become incorporated into the integrin rich cytoskeleton in a manner dependent on platelet aggregation.34,36,45 We tested the possibility that Vav may also be incorporated into the cytoskeleton. In resting platelets, most of the Vav was present in the supernatant fraction, but Vav became associated with the Triton X-100 insoluble residue after stimulation of platelets with thrombin with stirring to induce platelet aggregation (Fig 4A). This association was inhibited by omitting stirring to minimize platelet aggregation (Fig 4A, lane 7), as reported for p60c-src and α IIbβ3.26-32 Because activation of calpain is known to accompany platelet aggregation, we examined whether Vav is a substrate for calpain and, if so, it is cleaved during platelet aggregation. Activation of μ-calpain is known to be accompanied by cleavage of the enzyme, although it is unclear whether cleavage is necessary for activation. When platelets were treated with A23187 and dibucaine, which induce calpain activation in platelets in the presence of extracellular calcium, μ-calpain was cleaved and there were significant losses of Vav immunoreactivity (Fig 4B, lanes 2 and 5). Inhibition of calpain activation by EGTA or calpeptin resulted in an inhibition of cleavage of μ-calpain (the 80-kD subunit) and Vav (Fig 4B, lanes 3, 4, and 7). Activation of calpain by dibucaine does not require extracellular calcium, whereas A23187 does. Thus, μ-calpain and Vav cleavage induced by dibucaine was not inhibited by EGTA, but A23187-induced cleavage was (compare lanes 3 and 6, Fig 4B). Thus, only when calpain was activated and cleaved, was Vav cleaved. This data suggests that Vav may be a substrate for calpain, although we cannot rule out the possibility that activation of calpain may result in activation of other proteases that are responsible for the cleavage of Vav. When platelets were activated with thrombin (1 U/mL) with stirring to induce platelet aggregation, a significant loss of Vav immunoreactivity was also observed (compare lanes 1 and 2, Fig 4C). Omitting stirring to minimize aggregation resulted in an inhibition of the loss of Vav immunoreactivity (Fig 4C, lane 3). It should be noted that EGTA and EDTA were added before lysis of platelets to prevent artificial cleavage.33 As expected, thrombopoietin did not elicit translocation of Vav to the cytoskeleton (data not shown), probably due to the fact that it does not induce platelet aggregation by itself.3,5 6-9
Association of Vav and GPIIb α with the Triton X-100 insoluble residue (A) and its cleavage in platelets (B and C). (A) Platelets were lysed with Triton X-100-EGTA buffer before or after stimulation with thrombin (1 U/mL) with or without stirring. Lysates were separated into soluble and insoluble residue. Proteins from each fractions were separated by 10% SDS-PAGE and immunoblotted with Vav antisera (lower panel) or an anti-GPIIbα monoclonal antibody (AP4) (upper panel). Lane 1, Triton X-100 soluble residue of resting cells (7.5 × 106 cells); lane 2, Triton X-100 insoluble residue of resting cells (3.0 × 107 cells); lanes 3 to 6, Triton X-100 insoluble residue from 3.0 × 107 cells, 15 seconds, 1 minute, 5 minutes, and 10 minutes after exposure to thrombin (1 U/mL) with stirring; lane 7, Triton X-100 insoluble residue of cells (3.0 × 107 cells) stimulated for 10 minutes with thrombin without stirring. (B) Cleavage of calpain (upper panel) and Vav (lower panel). Cleavage of the 80-kD subunit of μ-calpain in human platelets. Platelets were incubated with either calpeptin or DMSO (vehicle of calpeptin, final 0.1%) for 5 minutes. Platelets were then treated with A23187 (1 μmol/L, for 5 minutes), or dibucaine (1 mmol/L, for 15 minutes) in the presence of 1 mmol/L CaCl2 or 5 mmol/L EGTA. Whole platelet lysates (1.5 × 107 cells/lane) were analyzed by 10% SDS-PAGE. Separated proteins were electrophoretically transferred from the gel onto nitrocellulose membranes. Calpain (upper panel) or Vav (lower panel) was detected by immunoblotting with specific monoclonal antibodies. The arrow indicates the relative position of the intact 80-kD subunit of μ-calpain (upper panel) or Vav (lower panel). Lane 1, control, DMSO (final 0.2%) + 1 mmol/L CaCl2; lane 2, A23187 (1 μmol/L, for 5 minutes) + 1 mmol/L CaCl2; lane 3, A23187 (1 μmol/L, for 5 minutes) + 5 mmol/L EGTA; lane 4, A23187 (1 μmol/L, for 5 minutes) + 1 mmol/L CaCl2 + calpeptin (20 μmol/L); lane 5, dibucaine (1 mmol/L, for 15 minutes) + 1 mmol/L CaCl2; lane 6, dibucaine (1 mmol/L, for 15 minutes) + 5 mmol/L EGTA; lane 7, dibucaine (1 mmol/L, for 15 minutes) + 1 mmol/L CaCl2 + calpeptin (20 μmol/L). (C) Cleavage of Vav during platelet aggregation. Platelets were stimulated with thrombin (1 U/mL) for 30 minutes with or with stirring. After the addition of EGTA (final, 5 mmol/L) and EDTA (final, 5 mmol/L), platelets were lysed by boiling in SDS sample buffer. Vav was detected by immunoblotting as described as above. Lane 1, resting; lane 2, thrombin stimulation for 30 minutes with stirring; lane 3, thrombin stimulation for 30 minutes without stirring.
Association of Vav and GPIIb α with the Triton X-100 insoluble residue (A) and its cleavage in platelets (B and C). (A) Platelets were lysed with Triton X-100-EGTA buffer before or after stimulation with thrombin (1 U/mL) with or without stirring. Lysates were separated into soluble and insoluble residue. Proteins from each fractions were separated by 10% SDS-PAGE and immunoblotted with Vav antisera (lower panel) or an anti-GPIIbα monoclonal antibody (AP4) (upper panel). Lane 1, Triton X-100 soluble residue of resting cells (7.5 × 106 cells); lane 2, Triton X-100 insoluble residue of resting cells (3.0 × 107 cells); lanes 3 to 6, Triton X-100 insoluble residue from 3.0 × 107 cells, 15 seconds, 1 minute, 5 minutes, and 10 minutes after exposure to thrombin (1 U/mL) with stirring; lane 7, Triton X-100 insoluble residue of cells (3.0 × 107 cells) stimulated for 10 minutes with thrombin without stirring. (B) Cleavage of calpain (upper panel) and Vav (lower panel). Cleavage of the 80-kD subunit of μ-calpain in human platelets. Platelets were incubated with either calpeptin or DMSO (vehicle of calpeptin, final 0.1%) for 5 minutes. Platelets were then treated with A23187 (1 μmol/L, for 5 minutes), or dibucaine (1 mmol/L, for 15 minutes) in the presence of 1 mmol/L CaCl2 or 5 mmol/L EGTA. Whole platelet lysates (1.5 × 107 cells/lane) were analyzed by 10% SDS-PAGE. Separated proteins were electrophoretically transferred from the gel onto nitrocellulose membranes. Calpain (upper panel) or Vav (lower panel) was detected by immunoblotting with specific monoclonal antibodies. The arrow indicates the relative position of the intact 80-kD subunit of μ-calpain (upper panel) or Vav (lower panel). Lane 1, control, DMSO (final 0.2%) + 1 mmol/L CaCl2; lane 2, A23187 (1 μmol/L, for 5 minutes) + 1 mmol/L CaCl2; lane 3, A23187 (1 μmol/L, for 5 minutes) + 5 mmol/L EGTA; lane 4, A23187 (1 μmol/L, for 5 minutes) + 1 mmol/L CaCl2 + calpeptin (20 μmol/L); lane 5, dibucaine (1 mmol/L, for 15 minutes) + 1 mmol/L CaCl2; lane 6, dibucaine (1 mmol/L, for 15 minutes) + 5 mmol/L EGTA; lane 7, dibucaine (1 mmol/L, for 15 minutes) + 1 mmol/L CaCl2 + calpeptin (20 μmol/L). (C) Cleavage of Vav during platelet aggregation. Platelets were stimulated with thrombin (1 U/mL) for 30 minutes with or with stirring. After the addition of EGTA (final, 5 mmol/L) and EDTA (final, 5 mmol/L), platelets were lysed by boiling in SDS sample buffer. Vav was detected by immunoblotting as described as above. Lane 1, resting; lane 2, thrombin stimulation for 30 minutes with stirring; lane 3, thrombin stimulation for 30 minutes without stirring.
DISCUSSION
c-vav is the cellular homologue of the v-vav oncogene.49-52 The c-vav product, p95vav (Vav) is predominantly expressed in hematopoietic cells and is inducibly tyrosine phosphorylated in cells treated with a wide variety of growth factors. Consistent with previous reports using cell lines,53-56 we have demonstrated that thrombopoietin induces tyrosine phosphorylation of Vav in FDCP-hMpl5 cells that express human c-Mpl, a receptor for thrombopoietin. However, we extended these studies by showing that Vav is present in platelets and that it is inducibly tyrosine phosphorylated following thrombopoietin treatment. This data suggests that Vav may have a role in signaling by thrombopoietin. We have also found that Vav may have a role in postaggregation signaling events in platelets and in contrast to Vav phosphorylation induced by a variety of other agents, thrombopoietin induces tyrosine phosphorylation of Vav without activation of protein kinase C.
Although the precise role of Vav in signal transduction is unknown, this proto-oncogene product is known to be involved in signaling of T- and B-cell, FcγIIIa, c-kit, granulocyte macrophage colony-stimulating factor (GM-CSF ), IL-3, IL-2, and erythropoietin receptors.10-16,22-24,47,48,56 The widespread involvement of Vav in various signal transduction systems may imply that Vav is involved in the regulation of cellular growth. However, the inducible tyrosine phosphorylation of Vav in postmitotic cells such as platelets suggests that tyrosine phosphorylation of Vav may have functions other than promotion of growth. Neutrophils, which are also postmitotic cells, express Vav, supporting this idea.12 Recently, we and others found that thrombopoietin primed platelet aggregation induced by various agonists.3,5,6-9 In this study, we have shown that tyrosine phosphorylation of Vav is an early event in signaling following thrombopoietin stimulation (Figs 1A and 3). Thus, it is possible that tyrosine phosphorylation of Vav may have a role in events that prime platelets for aggregation, such as cell-to-cell or cell-to-matrix adhesion. Recently, it was reported that thrombopoietin enhanced erk2 phosphorylation induced by thrombin, although thrombopoietin alone did not induce significant erk2 phosphorylation.9 Because tyrosine phosphorylation of Vav may contribute to the activation of ras-erk pathways,57 it is possible that Vav may contribute to the priming of erk2 phosphorylation. If so, erk2 activation may lead to phosphorylation and activation of phospholipase A2,58,59 contributing to the “priming” effects of platelet activation by thrombopoietin.
Thrombin, U46619 or collagen also induce tyrosine phosphorylation of Vav in platelets (Fig 1). Cichowski et al60 have recently reported similar findings for thrombin or collagen inducing tyrosine phosphorylation of Vav in platelets. Although they did not find tyrosine phosphorylation of Vav when platelets were stimulated by U46619, this may have been due to differences in platelet preparations. Similarly, we observed a somewhat slower increase in tyrosine phosphorylation induced by thrombin or collagen. These differences may also have been due to differences in platelet preparations. We have extended these observations by attempting to determine whether a common signaling pathway, induced by thrombin, collagen, and U46619 results in tyrosine phosphorylation of Vav. Since these three agonists are known to induce activation of phospholipase C, eliciting activation of protein kinase C and elevation of [Ca2+]i,25 we also examined the effects of PMA and ionomycin and found that both reagents induced tyrosine phosphorylation of Vav in platelets (Fig 1). This suggests that tyrosine phosphorylation of Vav can be induced by activation of protein kinase C or elevation of [Ca2+]i in platelets. However, PMA and ionomycin did not induce increased tyrosine phosphorylation of Vav in FDCP-hMpl5 cells, while thrombopoietin did (Fig 3). This is particularly interesting as there are reports indicating that calcium ionophore or phorbol ester do not induce tyrosine phosphorylation of Vav in other cell types including B lymphocytes.49,50 Thus, protein kinase C- or calcium-mediated tyrosine phosphorylation of Vav may be unique to platelets. Moreover, tyrosine phosphorylation of Vav, induced by thrombopoietin, occurs without activation of protein kinase C and elevation of [Ca2+]i. We conclude that protein kinase C/calcium and thrombopoietin activate separate pathways or induce a similar signaling event that leads to Vav tyrosine phosphorylation. This notion was supported by the inhibitory effects of BAPTA-AM and Ro-31-8220 on tyrosine phosphorylation induced by thrombin, but not by thrombopoietin (Fig 2B). The combination of these reagents is known to strongly inhibit activation of protein kinase C and elevation of [Ca2+]i,61 although we do not claim that these reagents specifically chelate intracellular calcium ion and inhibit protein kinase C.
One of the candidates for mediating tyrosine phosphorylation of Vav is Jak-2, as thrombopoietin, thrombin, calcium ionophore, or phorbol ester have all been reported to induce Jak-2 phosphorylation.2,6-9,46 However, the degree of Jak-2 phosphorylation induced by thrombin was much weaker than that induced by thrombopoietin, while both reagents elicited similar degrees of phosphorylation of Vav (Fig 1 and data not shown). Thus, activation of Jak-2 may not readily explain tyrosine phosphorylation of Vav in platelets stimulated by thrombin.
Previously, a role for the tyrosine kinase, p72syk, in the tyrosine phosphorylation of Vav in macrophages was postulated.56 Thrombin, thromboxane mimetics, and collagen are known to induce tyrosine phosphorylation and activation of p72syk, even when platelet aggregation is inhibited by RGDS peptide.62-65 Thus, it is possible that p72syk is involved in tyrosine phosphorylation of Vav induced by these agonists. On the other hand, we found that p72syk was not significantly tyrosine phosphorylated in platelets stimulated by thrombopoietin (data not shown). This data, combined with protein kinase C activation data, demonstrate that thrombin and thrombopoietin treatment of platelets results in differential activation of several signaling pathways and is most consistent with thrombin and thrombopoietin stimulation having separate pathways for tyrosine phosphorylation of Vav.
Finally, Vav may have a role in postaggregation signaling as evidenced by the redistribution of Vav to the cytoskeleton following thrombin stimulated platelet aggregation. This is similar to what has been described for p60c-src , p59c-fyn , c-Cbl, and α IIbβ3 integrin.4,34,36,45 The amino terminal portion of Vav contains a region that is similar to some actin binding proteins, such as calponin and α-actinin, which may allow binding of Vav to filamentous actin.52,66 Since the deletion of the amino terminal portion of Vav leads to the acquisition of oncogenic potential of Vav,52 the redistribution to the cytoskeleton may be essential for the normal functions of Vav as opposed to the oncogenic function of Vav. Previously, Vav was shown to be associated with Grb2 in hematopoietic cells.11,44 However, the physiological significance of the association is unknown. Since Vav is also constitutively associated with Grb2, an SH2/SH3 adapter protein, in platelets, Vav and Grb2 may recruit more signaling molecules into the cytoskeleton. This hypothesis was directly supported by our previous finding that Grb2 also redistributes to the platelet cytoskeleton in an aggregation-dependent fashion.4
In conclusion, our data suggest that there are two pathways leading to tyrosine phosphorylation of Vav in platelets. One of them, triggered by thrombopoietin, does not seem to involve phospholipase C activation and could be mediated by Jak2. The other pathway may be downstream of activation of phospholipase C with modest Jak-2 phosphorylation. The latter pathway could involve p72syk. Our data also suggests that Vav may have a role in aggregation events, as we found that Vav redistributes to the cytoskeleton during platelet aggregation and is an endogenous substrate for calpain.
ACKNOWLEDGMENT
We thank Drs Junichi Kambayashi, Thomas J. Kunicki, Amgen, Inc, and Green Cross Co for kindly providing reagents. We also thank Dr Akihiko Shimosaka for his continual encouragement and helpful comments.
Y.M. and A.O. contributed equally to this study and should be regarded as cofirst authors.
Supported in part by Grants in Aid from The Ministry of Education, Science and Technology of Japan (Y.I. and A.O.), The Ryoichi Naito Foundation for Medical Research (A.O.), and Research Grants for Life Sciences and Medicine, Keio University Medical Science Fund (A.O.).
Address reprint requests to Atsushi Oda, MD, PhD, Department of Internal Medicine, School of Medicine, Keio University, 35 Shinanomachi, Shinjuku-ku, Tokyo 160, Japan.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal