Abstract
The efficacy of a synthetic peptide analog mimicking the CDR3-D1 domain of the CD4 molecule was investigated in murine models of allogeneic bone marrow engraftment after transplantation across major histocompatibility complex (MHC) barriers. A single dose of a CD4-CDR3 peptide analog was administered at the time of marrow transplantation to three different allogeneic mouse strain combinations after appropriate sublethal total body irradiation: (1) B10.BR → C57BL/6J (B6), a full allogeneic MHC difference; (2) (B6xDBA/2)F1 → (B6xCBA)F1 , a haploidentical MHC combination; and (3) B6.C-H2bm12 → B6-Ly5.2, involving only a MHC class II difference. Donor-host chimerism was assessed after 1 and 2 months posttransplantation by flow cytometric analysis of spleen and/or lymph node cells. Peptide-treated animals in all three strain combinations exhibited significantly enhanced donor lymphoid engraftment, which was similarly reflected in the total lymphocyte compartment and its T-cell (CD4+, CD8+) and B-cell subsets. In addition, peptide-treated mice in the haploidentical and MHC class II-mismatched strain combinations exhibited prolonged tolerance of both donor and syngeneic host-type tail skin grafts while rejecting third-party allogeneic grafts, thus supporting the reconstitution of immunocompetence in these chimeras. Lymphocytes from the peptide-treated haploidentical chimeric mice also displayed donor-specific tolerance upon stimulation in a one-way mixed lymphocyte reaction. In a 6-day colony-forming unit–granulocyte-macrophage (CFU-GM) assay to quantitate the level of hematopoietic cell engraftment in both the haploidentical and class II-disparate strain combinations, bone marrow cells from the peptide-treated mice exhibited significant increases in CFU-GM compared with the saline-treated control groups. Finally, early multiple treatments with the peptide after transplantation significantly enhanced donor chimerism in donor-presensitized recipient mice across the MHC class II barrier and proved to be significantly more effective than anti-CD4 monoclonal antibody treatment. These results indicate that the structure-based CD4-CDR3 peptide analog may represent a valuable approach to the inhibition of graft rejection after MHC-mismatched bone marrow transplantation.
T-CELL DEPLETION of the donor bone marrow (BM) inoculum in allogeneic transplantation, alone or in combination with posttransplant immunosuppression, represents an effective approach to reduce the incidence of graft-versus-host disease (GVHD).1-3 However, this regimen is also associated with increased BM graft failure occurring in cases of HLA-matched transplantation but especially noted among recipients of HLA-mismatched marrow.3-7 Rejection of donor BM can be mediated by residual host elements including CD4+ T cells, CD8+ T cells, and natural killer cells, depending on the specific antigenic differences involved.6,8-10 The use of immunosuppressive drugs to inhibit these residual cells and to prevent graft rejection may cause a series of associated side effects and can put the patient at an increased risk of opportunistic infections.11 In considering the complications of T-cell depletion and graft failure in the design of new strategies for BM transplantation (BMT) there is an apparent need to induce specific transplantation tolerance across major histocompatability complex (MHC) barriers of host T cells that survive the conditioning regimen without long-term immunosuppression.
Recently, a family of synthetic peptides has been developed to mimic a distinct molecular surface structure of the CDR3-like region in the D1 Ig-like domain of the murine CD4 molecule.12,13 Previous in vitro studies have shown that these CD4-CDR3 peptide analogs block activation of CD4+ T cells and T-cell lines after T-cell receptor (TCR) engagement, downregulate interleukin-2 (IL-2) production, and inhibit T-cell proliferation in mixed lymphocyte reactions (MLR).14 One such analog designed for in vivo use, designated rD-mPGPtide, has been shown to inhibit significantly the clinical and pathological symptoms of experimental allergic encephalomyelitis (EAE) in the SJL mouse model.13 In addition, this peptide inhibited the development of GVHD and significantly prolonged the survival of irradiated recipients of a haploidentical BM transplant.15 Thus, it was hypothesized that the rD-mPGPtide might also be effective against the alloreactive host cells responsible for marrow graft failure in an MHC-mismatched transplant situation.
The rD-mPGPtide is a 13-amino acid peptide (CELENRKEEPGPC) derived from the p86-94 sequence of the mouse CD4 molecule (CD4-CDR3 D1 domain) with a proline-glycine-proline sequence added at the carboxyl terminus to impose a tertiary structural constraint, followed by a final cysteine residue to allow cyclization. Improved resistance to protease digestion of the peptide analog was achieved by synthesis with D-amino acids and in reverse order to retain the original tertiary structure and side chain presentation of the native molecule.13
In the present study, three murine models of allogeneic BM transplantation across varying degrees of MHC antigenic barriers, involving sublethal total body irradiation conditioning, were used to investigate the effects of the CD4-CDR3 peptide analog on host resistance to donor BM. The results indicated that a single injection of the rD-mPGPtide at the time of transplantation significantly enhanced donor hematopoietic engraftment and established effective donor/host tolerance and subsequent immunocompetence.
MATERIALS AND METHODS
Mice.Mice, B10.BR/SgSnJ (H2k), C57BL/6J (B6) (H2b), B6.C-H2bm12 (bm12), DBA/2 (H2d), (B6xDBA/2)F1 [(B6D2)F1 (H2b/d)], and (B6xCBA)F1 [(B6CB)F1 (H2b/k)] were purchased from the Jackson Laboratory (Bar Harbor, ME). C57BL/6-Ly5.2 (B6-Ly5.2) (H2b) and SJL/J (H2s) mice were purchased from the National Cancer Institute (Bethesda, MD). Male mice used as donors and recipients were between 7 to 12 and 7 to 10 weeks of age, respectively. Mice were kept in a sterile environment in microisolators at all times and were provided with acidified water and autoclaved food.
Media.Phosphate-buffered saline (PBS) solution supplemented with 0.1% bovine serum albumin (BSA; Sigma Chemical Co, St Louis, MO) was used for all in vitro manipulations of the donor BM and lymphocytes. For injection, cells were resuspended in PBS. RPMI 1640 medium (Mediatech, Herndon, VA) supplemented with 10% fetal calf serum (Atlanta Biologicals, Norcross, GA), 2 mmol/L glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin, and 0.05 mmol/L 2-mercaptoethanol (Mediatech) was used for all in vitro assays. Methocult M3230 (Stemcell Technologies, Vancouver, British Columbia, Canada) methylcellulose medium, supplemented with 50 U/mL IL-3 (Biosource International, Camarillo, CA), 2 mmol/L glutamine, 50 U/mL penicillin and 50 μg/mL streptomycin (Mediatech) was used for all in vitro colony assays.
CD4-CDR3 peptide.The rD-mPGPtide (CPGPEEKRNELEC; all D-amino acids), designed as previously described,14 was synthesized on an Applied Biosystems 430A peptide synthesizer (Foster City, CA) using standard F-moc chemistry, refolded to enrich for intramolecular disulfide bonding, and purified by high pressure liquid chromatography on a system using a Waters 600E system controller and Waters 490E programmable multi-wavelength detector (Millipore Corp, Bedford, MA). The peptide was reconstituted in PBS (2.5 mg/mL) for injection into mice.
Irradiation.All recipient mice received appropriate sublethal total body irradiation exposures of 650 to 800 cGy from a Gammacell 137Cs source (116 cGy/min).
Injections.All cell suspensions were administered intravenously (IV) via the tail vein in a final volume of 0.2 mL. PBS or peptide was also administered IV in a volume of 0.2 mL. Anti-CD4 monoclonal antibody (MoAb) was administered intraperitoneally (IP) in a volume of 0.2 mL.
MoAbs.Ascites fluid for anti-Thy1.2 (J1j.10, rat IgM; ATCC TIB184)16 and anti-CD4 (GK1.5, rat IgG2b; ATCC TIB207)17 MoAb were generated in our laboratory from BALB/c nu/nu mice (National Cancer Institute). GK1.5 ascites was tested for in vivo depletion of CD4+ T cells by fluorescence-activated cell sorting (FACS) analysis of spleen and lymph node (LN) cells on days 2, 4, and 6 postinjection using the fluorescein isothiocyanate (FITC)-conjugated rat antimouse-CD4 MoAb (clone RM4-5). Treatment with a 1:400 dilution of the GK1.5 ascites resulted in 100% depletion of CD4+ T cells within both lymphoid populations. Spectrometric analysis of undiluted ascites showed an approximate protein content of 12 mg/mL. A 1:100 dilution of GK1.5 ascites (in 0.2 mL), equivalent to 25 μg of protein, was subsequently used for treatment of experimental groups. Goat antimouse IgG antibody was purchased from Cappel-Organon Teknika (Durham, NC). Guinea pig serum, prepared in our laboratory, was used as a source of complement for all MoAb treatments. Supernatant containing antimouse Fc-γ receptor (2.4G2, rat IgG2b; ATCC HB197)18 MoAb was generated from hybridoma cells purchased from ATCC (Rockville, MD). Surface phenotype was analyzed by dual-color immunofluorescence using the following FITC- and/or R-phycoerythrin (PE)-conjugated antimouse MoAbs: mouse anti-H2Kb (clone AF6-88.5), mouse anti-H2Kd (clone SF1-1.1), mouse anti-H2Kk (clone 36-7-5), rat anti-CD45.1 (anti-Ly5.1, clone A20-1.7), rat anti-Thy1.2 (CD90) (clone 30-H12), rat anti-CD4 (clone RM4-5), rat anti-CD8 α (clone 53-6.7), rat anti-CD45R/B220 (clone RA3-6B2), and, as a negative control, rat IgG2aκ. In addition, mouse anti-CD45.2 MoAb (anti-Ly5.2, clone 104) was biotin-conjugated. All of these MoAbs were purchased from Pharmingen (San Diego, CA), except the FITC-anti-CD45.1 (anti-Ly5.1) MoAb, which was generously provided by Dr B. Blazar (University of Minnesota, Minneapolis, MN).
Cell preparations.BM cells were prepared from the femurs and tibiae of donor mice by flushing with PBS/BSA. To prepare anti-Thy1.2 MoAb-treated (T-cell depleted) BM (ATBM), cells were incubated with J1j.10 MoAb (1:100 dilution) and complement (1:20) for 60 minutes at 37°C and were washed three times. ATBM cells were counted and resuspended at 5 × 107 cells/mL in PBS. This treatment resulted in a donor cell population deficient of Thy-1.2+ cells, as quantitated in immunofluorescence/flow cytometry.
BM engraftment.In an adaptation of the BM engraftment model used by Vallera et al,19 recipient mice were exposed to an appropriate level of sublethal total body irradiation 4 to 6 hours before the injection of donor ATBM cells. Mice then received a 0.2 mL injection of either PBS (IV), rD-mPGPtide (0.5 mg; IV), or anti-CD4 MoAb (1:100 dilution of GK1.5 ascites fluid, 25 μg; IP). Chimerism was analyzed at 1 and 2 months post-BMT and mice were subsequently tested for transplantation tolerance and reconstitution of immunocompetence. For presensitization experiments in the bm12 → B6-Ly5.2 strain combination, recipient B6-Ly5.2 mice received 2 × 107 bm12 spleen cells IP, 14 days before irradiation and transplantation.
Chimerism phenotyping.FITC- and PE-conjugated MoAbs specific for donor or host determinants were used to evaluate the degree of chimerism in the spleens (at 1 month) or spleens and LN (at 2 months) of recipients. For phenotypic analysis of cells from all three mouse strain combinations, the following MoAb were used: (1) (B6D2)F1 (H2b/d) → (B6CB)F1 (H2b/k) mice were typed with PE-anti-H2Kd and FITC anti-H2Kk; (2) bm12 → B6-Ly5.2 mice were typed with FITC-anti-CD45.1 (anti-Ly5.1) and PE-anti-H2Kb; (3) B10.BR → B6 mice were typed with FITC-anti-H2Kk and PE-anti-H2Kb. The MoAbs used in these studies exhibited no cross-reactivity with the allogeneic cells expressing the other MHC haplotypes used in these murine models. MoAbs to T- or B-cell differentiation antigens were used together with MHC-specific MoAbs to determine the origin of the subpopulations detected. For phenotypic analysis, single-cell suspensions (5 × 105 cells/sample) were incubated and washed with PBS/1% BSA and 0.05% NaN3 (FACS buffer). Cell samples in a volume of 100 μL were incubated with 25 μL of anti-Fcγ receptor MoAb for 10 minutes at 4°C to prevent nonspecific Fc binding.18 Optimal concentrations of either PE- or FITC-labeled MoAbs were added to a final volume of 150 μL and incubated for 30 minutes at 4°C in the dark and then washed three times in FACS buffer and fixed overnight at 4°C in PBS/1% paraformaldehyde. Samples were analyzed on a Coulter Epics Profile II (Coulter, Hialeah, FL). Two irrelevant FITC-conjugated rat IgG2aκ and PE-conjugated rat IgG2aκ MoAbs were used to set the negative gates in all experiments. Cells were gated on the basis of their forward and side scatter to exclude red blood cells and debris. A minimum of 104 cells was analyzed for each determination.
Skin grafting.Tail skin grafts were performed according to an adaption of the method of Bailey and Usama.20 Briefly, donor tail skin grafts (0.25 × 0.5 cm) were transplanted onto the ventral side of the tail of the recipient mouse, covered with a glass tube, and held in place with short strips of adhesive tape for 2 days. The tubes were removed and the grafts were monitored every other day for up to 80 days. Graft rejection was defined on the basis of necrosis and hair loss. Surviving grafts exhibited hair growth and full pigmentation. Median survival times (MST) were calculated and statistical analysis was performed by fully factorial MANOVA analysis using SYSTAT 5.2 software (SYSTAT, Inc, Evanston, IL).
In vitro MLR.MLR was used to assay for transplantation tolerance in the haploidentical (B6D2)F1 → (B6CB)F1 strain combination. Chimeric LN cells were depleted of red blood cells by hypotonic lysis in Gey's balanced salt solution containing 0.7% NH4Cl and cultured (2.5 × 105 cells/well) with irradiated (20 Gy) stimulator spleen cells (5 × 105 cells/well) from either allogeneic donor-compatible (DBA/2), third-party (SJL), or host-syngeneic mice in a 96-well round-bottom plate. Cultures were incubated in complete RPMI medium at 37°C and 7% CO2 for 3 to 5 days and 1 μCi [3H]TdR/well was added 8 hours before harvesting onto glass fiber filters (Wallac Oy, Turku, Finland) with a Harvester 96 (TomTec, Orange, CT) and counted in a 1205 Beta-Plate reader (Wallac, Gaithersburg, MD). The results were expressed as the mean CPM ± standard deviation (SD) of quadruplicate cultures and the stimulation indices (SI) were calculated in relation to the syngeneic background control for that group.
Assay for hematopoietic progenitors and phenotypic analysis.The colony-forming unit–granulocyte-macrophage (CFU-GM) assay was performed according to a modification of a method described previously.21 Briefly, spleen cells were collected from three recipient mice per treatment group on day 5 post-BMT, and nucleated cells were pooled and counted. The spleen cells (105) were plated in a 35-mm culture dish (Stemcell Technologies) in 1 mL of a mixture containing Iscove's modified Dulbecco's medium (Mediatech), 0.9% methylcellulose, 30% fetal bovine serum, 1% BSA, 10−4 mol/L 2-mercaptoethanol, 2 mmol/L L-glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin, and 50 U/mL murine recombinant IL-3 (Biosource). Triplicate cultures were maintained at 37°C and 7% CO2 for 6 days. Cell aggregates containing more than 30 cells (CFU-GM) were scored as individual colonies on day 6 of culture. Eight to 10 days after plating, 6 to 9 × 102 cell aggregates were extracted from the methylcellulose by pipette and pooled from each treatment group, yielding approximately 2 × 105 cells. These cells were phenotyped by dual-color flow cytometry with either PE-anti-H2Kd and FITC-anti-H2Kk MoAb for the (B6D2)F1 → (B6CB)F1 strain combination or biotin-anti CD45.2 (anti-Ly5.2), detected with streptavidin R-PE (Caltag Laboratories, Burlingame, CA) and FITC-anti-CD45.1 (anti-Ly5.1) MoAb for the bm12 → B6-Ly5.2 model.
RESULTS
Optimization of BM engraftment in sublethally irradiated recipient mice.Three different allogeneic strain combinations were used in these studies: (1) B10.BR (H2k) → B6 (H2b), a full MHC-mismatch; (2) (B6D2)F1 (H2b/d) → (B6CB)F1 (H2b/k), a haploidentical MHC-mismatch; and (3) bm12 → B6-Ly5.2, a MHC class II-mismatch. Initially, dosage titration experiments were performed to optimize the level of preconditioning irradiation exposure of recipients in each strain combination to allow maximum residual host resistance to donor ATBM cells (107) engraftment. Splenocytes were sampled from these mice 30 days post-BMT and tested by flow cytometric analysis for the degree of donor/host chimerism. As indicated in Fig 1 for the haploidentical group, splenocytes from recipient mice exposed to 700 to 800 cGy irradiation were less than 20% donor chimeric. The donor cell component significantly (P < .001) increased to more than 80% in mice exposed to greater than 850 cGy irradiation. Similar results were obtained in the B10.BR → B6 and bm12 → B6-Ly5.2 strain combinations. However, it was noticed that, for each recipient, the weight of the mice correlated with the maximum level of irradiation exposure still favoring a low donor engraftment. For example, less than 750 cGy induced low donor chimerism in mice greater than 25 g in weight, but 650 cGy was necessary for mice between 22 and 25 g in weight in the B6-Ly5.2 group. Appropriate irradiation levels (ranging between 650 and 800 cGy) were thus selected for each strain combination and adjusted for the weights of the recipient mice in each experiment.
Radiation dose titration of the haploidentical strain combination (B6D2)F1 → (B6CB)F1 . (B6CB)F1 mice (n = 3 to 5; body weight, 25 to 29 g) were irradiated with the indicated γ-ray dose and received a transplant of allogeneic (B6D2)F1 ATBM (107 cells) on day 0. Splenocytes from recipients were analyzed for donor chimerism on day 30 post-BMT by two-color immunofluorescence flow cytometry with FITC-anti-H2Kk and PE-anti-H2Kd MoAb. The data ±SD shown are from one experiment representative of two performed with this strain combination. (▪) Donor; (▨) host.
Radiation dose titration of the haploidentical strain combination (B6D2)F1 → (B6CB)F1 . (B6CB)F1 mice (n = 3 to 5; body weight, 25 to 29 g) were irradiated with the indicated γ-ray dose and received a transplant of allogeneic (B6D2)F1 ATBM (107 cells) on day 0. Splenocytes from recipients were analyzed for donor chimerism on day 30 post-BMT by two-color immunofluorescence flow cytometry with FITC-anti-H2Kk and PE-anti-H2Kd MoAb. The data ±SD shown are from one experiment representative of two performed with this strain combination. (▪) Donor; (▨) host.
Effect of rD-mPGPtide on donor chimerism in BM transplantation across MHC barriers.To investigate the effect of the rD-mPGPtide analog upon BM engraftment across MHC barriers, recipient mice were exposed to sublethal irradiation and transplanted with 107 donor ATBM cells. Immediately after BMT, the mice received injections of either PBS, rD-mPGPtide (0.5 mg; IV), or anti-CD4 MoAb (25 μg; IP) in a volume of 0.2 mL. For the full MHC-disparate strain combination (B10.BR → B6), at 30 days posttransplantation, donor engraftment of mononuclear cells in the spleen reached a level of 19% (Table 1). Both rD-mPGPtide and anti-CD4 MoAb treatments resulted in significantly increased donor cell engraftment (53%, 2.8-fold) compared with that in the PBS control group (P = .001). In the haploidentical MHC strain combination [(B6D2)F1 → B6CB)F1 ], less than 8% and less than 17% donor cell engraftments were observed at days 30 or 60 post-BMT, respectively, but these levels increased by fivefold to ninefold at each of these time points after administration of either rD-mPGPtide or anti-CD4 MoAb. When the MHC disparity was restricted to only class II antigens, as in the bm12 → B6-Ly5.2 group, similar degrees of donor cell engraftment were observed (P < .001 for all groups) after peptide (66%, day 30; 71%, day 60) and anti-CD4 MoAb treatment (62%, day 30; 69%, day 60), in comparison to the PBS control group (9%, day 30; 7%, day 60). Long-term stable donor engraftment was still evident at 150 days post-BMT (data not shown).
Effect of rD-mPGPtide on Donor/Host Chimerism in BM Engraftment Across MHC Barriers
| Strain Combination . | Genetic Difference . | Treatment . | Phenotype, Day 30 (% donor/host) . | Phenotype, Day 60 (% donor/host) . |
|---|---|---|---|---|
| B10.BR → B6 (750 cGy) | Full MHC | PBS | 19/65 | |
| rD-mPGPtide | 53/38 | ND | ||
| Anti-CD4 MoAb | 53/37 | |||
| (B6D2)F1 → (B6CB)F1 (800 cGy) | Haplo-MHC | PBS | 8/87 | 17/76 |
| rD-mPGPtide | 71/21 | 84/13 | ||
| Anti-CD4 MoAb | 75/19 | 86/12 | ||
| bm12 → B6-Ly5.2 (750 cGy) | MHC class II | PBS | 9/73 | 7/85 |
| rD-mPGPtide | 66/26 | 71/20 | ||
| Anti-CD4 MoAb | 62/27 | 69/21 |
| Strain Combination . | Genetic Difference . | Treatment . | Phenotype, Day 30 (% donor/host) . | Phenotype, Day 60 (% donor/host) . |
|---|---|---|---|---|
| B10.BR → B6 (750 cGy) | Full MHC | PBS | 19/65 | |
| rD-mPGPtide | 53/38 | ND | ||
| Anti-CD4 MoAb | 53/37 | |||
| (B6D2)F1 → (B6CB)F1 (800 cGy) | Haplo-MHC | PBS | 8/87 | 17/76 |
| rD-mPGPtide | 71/21 | 84/13 | ||
| Anti-CD4 MoAb | 75/19 | 86/12 | ||
| bm12 → B6-Ly5.2 (750 cGy) | MHC class II | PBS | 9/73 | 7/85 |
| rD-mPGPtide | 66/26 | 71/20 | ||
| Anti-CD4 MoAb | 62/27 | 69/21 |
Data from one experiment representative of three separate experiments are presented as the percentage of positive splenocytes as analyzed by flow cytometry. Chimerism was determined using the following MoAb pairs: FITC-anti-H2Kk (B10.BR) and PE-anti-H2Kb (B6); PE-anti-H2Kb (bm12 and B6-Ly5.2) and FITC-anti-CD45.1 (anti-Ly5.1; B6-Ly5.2); and FITC-anti-H2Kk [(B6CB)F1] and PE-anti-H2Kd [(B6D2)F1]. The mean of samples from five mice per group is presented for each individual treatment. The values in all rD-mPGPtide and anti-CD4 MoAb treatment groups were significantly different from those in the PBS control group, P ≤ .001.
Abbreviation: ND, not done.
Effect of rD-mPGPtide on B- and T-cell reconstitution in haploidentical and class II-disparate strain combinations.The degrees of B- and T-cell engraftment were investigated next to assess whether the peptide treatment had any skewing effect upon the lymphocytic lineage reconstitution. Analysis of splenic lymphocytes in the (B6D2)F1 → (B6CB)F1 combination showed a significant increase of donor-derived T and B cells in mice that had received the peptide treatment. At 30 and 72 days post-ATBM transplantation, the percentage of donor-derived T cells was increased fourfold and 7.2-fold, respectively, in the peptide-treated group as compared with the PBS-treated control mice (Table 2). The donor-derived B-cell population on day 72 was increased ninefold in the rD-mPGPtide–treated recipient mice compared with the control. In addition, LN isolated from the same peptide-treated mice were increased 11.3- and 4.7-fold, respectively in their donor-derived T- and B-cell populations. Similar results were obtained in mice receiving anti-CD4 MoAb treatment. The results of peptide treatment in the MHC class II-disparate strain combination (bm12 → B6-Ly5.2) corresponded with those observed in the haploidentical model. At 30 days post-BMT, the level of the donor-derived splenic T-cell population was 3.1-fold higher in peptide-treated recipient mice, whereas B cells were increased 4.6-fold compared with the untreated control group. Also, on day 60, donor-derived splenic and LN T cells from peptide-treated mice were increased 3.5- and 18.5-fold, respectively. Thus, the peptide treatment resulted in significantly increased donor-derived B-and T-cell reconstitution in the spleen and LN.
Effect of rD-mPGPtide on Reconstitution of Donor T and B Lymphocytes in the Haploidentical and MHC Class II-Disparate Strain Combinations
| Strain Combination . | Treatment . | Phenotype, Day 30; Spleen (% donor/host) . | Phenotype, Day 72; Spleen* (% donor/host) . | Phenotype, Day 72; . | |||
|---|---|---|---|---|---|---|---|
| . | . | T Cells . | B Cells . | T Cells . | B Cells . | LN* (% donor/host) . | |
| . | . | . | . | . | . | T Cells . | B Cells . |
| (B6D2)F1 → (B6CB)F1 (800 cGy) | PBS | 8/47 | 5/26 | 7/59 | 6/39 | 6/28 | |
| rD-mPGPtide | 32/11 | ND | 36/9 | 64/4 | 68/16 | 28/4 | |
| Anti-CD4 MoAb | 29/14 | 32/10 | 61/5 | 61/20 | 27/5 | ||
| bm12 → B6-Ly5.2 (750 cGy) | PBS | 9/22 | 10/44 | 4/25 | 2/57 | ||
| rD-mPGPtide | 28/11 | 41/11 | 14/10 | ND | 37/13 | ND | |
| Anti-CD4 MoAb | 21/9 | 56/6 | 15/8 | 31/14 | |||
| Strain Combination . | Treatment . | Phenotype, Day 30; Spleen (% donor/host) . | Phenotype, Day 72; Spleen* (% donor/host) . | Phenotype, Day 72; . | |||
|---|---|---|---|---|---|---|---|
| . | . | T Cells . | B Cells . | T Cells . | B Cells . | LN* (% donor/host) . | |
| . | . | . | . | . | . | T Cells . | B Cells . |
| (B6D2)F1 → (B6CB)F1 (800 cGy) | PBS | 8/47 | 5/26 | 7/59 | 6/39 | 6/28 | |
| rD-mPGPtide | 32/11 | ND | 36/9 | 64/4 | 68/16 | 28/4 | |
| Anti-CD4 MoAb | 29/14 | 32/10 | 61/5 | 61/20 | 27/5 | ||
| bm12 → B6-Ly5.2 (750 cGy) | PBS | 9/22 | 10/44 | 4/25 | 2/57 | ||
| rD-mPGPtide | 28/11 | 41/11 | 14/10 | ND | 37/13 | ND | |
| Anti-CD4 MoAb | 21/9 | 56/6 | 15/8 | 31/14 | |||
Data from one experiment representative of three separate experiments are presented as the percentage of positive cells, as analyzed by flow cytometry. Chimerism was determined using the following MoAb pairs: PE-anti-Thy1.2 (or B220) and FITC-anti-H2Kk, [(B6D2)F1 → (B6CB)F1 ]; PE-anti-Thy1.2 (or B220) and FITC-anti-CD45.1 (anti-Ly5.1) (bm12 → B6-Ly5.2). The mean of samples from five mice per group is presented for each individual treatment. The values in all rD-mPGPtide and anti-CD4 MoAb treatment groups were significantly different from those in the PBS control group, P ≤ .001.
Abbreviation: ND, not done.
Mice in the bm12 → B6-Ly5.2 strain combination were analyzed on day 60 after transplantation.
Donor cell chimerism in the CD4 and CD8 T-cell subset compartments of peptide-treated recipients.The level of CD4+ and CD8+ T-cell subsets from both spleen and LN of marrow reconstituted recipients was analyzed in mice after rD-mPGPtide treatment. Flow cytometric analysis of the haploidentical strain combination (B6D2)F1 → (B6CB)F1 on days 30 (data not shown) and 72 post-BMT showed significant increases (P < .001) in donor-derived CD4+ as well as CD8+ T cells (Table 3). Donor-derived CD4+ T cells in the spleen and LN from peptide-treated recipient mice were increased sevenfold and 11-fold, respectively, compared with the PBS-treated control group. These levels of increased chimerism were similar to those observed after anti-CD4 MoAb treatment. In addition, the donor CD8+ T cells were increased 2.7- and 10-fold, respectively in the spleen and LN of peptide-treated recipients, compared with PBS-treated groups, in which only 2% to 3% cell engraftment was observed. Similar trends were observed in the MHC class II-disparate strain combination (Table 3), in which donor-derived CD4+ T cells were increased 2.8- and 5.3-fold, respectively, in the spleen and LN from peptide-treated mice (P < .01). The number of donor-derived CD8+ T cells was 3.5-fold higher in the spleen and 4.3-fold higher in the LN compared with the PBS control mice. Overall, a single injection of the CD4-CDR3 peptide analog at the time of BM transplantation caused significant enhancement of donor engraftment, which is later reflected by the high level of reconstitution of peripheral lymphoid organs within all donor-derived lymphoid subsets.
Effect of rD-mPGPtide on Reconstitution of Donor CD4+ and CD8+T Cells in the Haploidentical and MHC Class II-Disparate Strain Combinations
| Strain Combination . | Treatment . | Phenotype of Spleen (% donor/host) . | Phenotype of LN (% donor/host) . | ||
|---|---|---|---|---|---|
| . | . | CD4+ Cells . | CD8+ Cells . | CD4+ Cells . | CD8+ Cells . |
| (B6D2)F1 → (B6CB)F1 (800 cGy) | PBS | 4/22 | 3/10 | 4/35 | 2/18 |
| rD-mPGPtide | 28/10 | 8/2 | 44/16 | 20/2 | |
| Anti-CD4 MoAb | 24/9 | 7/2 | 44/16 | 18/2 | |
| bm12 → B6-Ly5.2 (750 cGy) | PBS | 6/28 | 2/21 | 6/45 | 3/23 |
| rD-mPGPtide | 17/11 | 7/6 | 32/15 | 13/10 | |
| Anti-CD4 MoAb | 15/10 | 5/4 | 28/14 | 10/7 | |
| Strain Combination . | Treatment . | Phenotype of Spleen (% donor/host) . | Phenotype of LN (% donor/host) . | ||
|---|---|---|---|---|---|
| . | . | CD4+ Cells . | CD8+ Cells . | CD4+ Cells . | CD8+ Cells . |
| (B6D2)F1 → (B6CB)F1 (800 cGy) | PBS | 4/22 | 3/10 | 4/35 | 2/18 |
| rD-mPGPtide | 28/10 | 8/2 | 44/16 | 20/2 | |
| Anti-CD4 MoAb | 24/9 | 7/2 | 44/16 | 18/2 | |
| bm12 → B6-Ly5.2 (750 cGy) | PBS | 6/28 | 2/21 | 6/45 | 3/23 |
| rD-mPGPtide | 17/11 | 7/6 | 32/15 | 13/10 | |
| Anti-CD4 MoAb | 15/10 | 5/4 | 28/14 | 10/7 | |
Data from one experiment representative of three separate experiments are presented as the percentage of positive cells, as analyzed by flow cytometry. Chimerism was determined using the following MoAb pairs: PE-anti-H2Kd, FITC-anti-CD4 (or CD8) and FITC-anti-H2Kk, PE-anti-CD4 (or CD8) [(B6D2)F1 → (B6CB)F1 ]; FITC-anti-CD45.1 (anti-Ly5.1) and PE-anti-CD4 (or CD8) (bm → B6-Ly5.2). Mice in the (B6D2)F1 → (B6CB)F1 and the bm12 → B6-Ly5.2 strain combinations were analyzed on days 72 and 60 after transplantation, respectively. The mean of samples from five mice per group is presented for each individual treatment. The values in all rD-mPGPtide and anti-CD4 MoAb treatment groups were significantly different from those in the PBS control group, P ≤ .01.
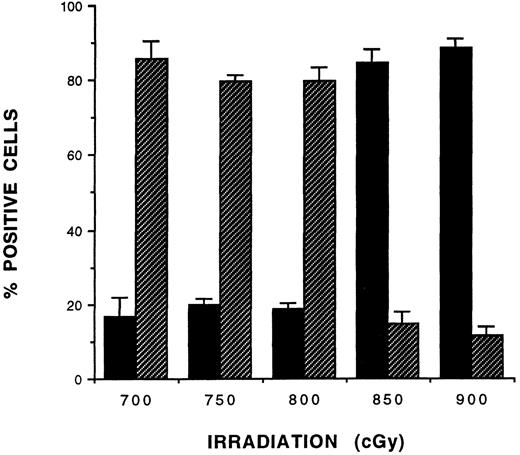
Tolerance induction and immune reconstitution in rD-mPGPtide–treated mice.To determine whether donor-specific tolerance was established in ATBM-transplanted (B6D2)F1 → (B6CB)F1 and bm12 → B6-Ly5.2 mice, all three treatment groups (PBS, rD-mPGPtide, and anti-CD4 MoAb) in each combination were challenged on day 60 with tail skin grafts from either the appropriate donor strain, the host strain, or an unrelated third party strain (SJL, H2s). As shown in Fig 2, for both the haploidentical (Fig 2A) and the class II-disparate (Fig 2C) strain combinations, the mice in the PBS-treated control groups rejected their donor allografts with MST of 13 and 15 days, respectively. In contrast, in both strain combinations, the donor type skin grafts were accepted by the mice treated with either the peptide analog or the anti-CD4 MoAb (MST >50 days; P < .001). Skin grafts from the allogeneic SJL strain were rejected by day 28 in both models by all three treatment groups, with no significant differences between the groups (P > .15; Fig 2B and D). On the other hand, syngeneic skin grafts were completely accepted by all treatment groups in both strain combinations (data not shown). These data indicated that the rD-mPGPtide–treated ATBM recipient mice exhibited tolerance of both donor- or host-type skin grafts but maintained the capacity to reject third-party allografts, supporting that immunocompetence was reconstituted in these chimeras. To further confirm that functional specific tolerance was established in peptide-treated haploidentical BM recipients, (B6CB)F1 mice were killed on day 70 post-BMT and proliferative responses were analyzed in a 4-day MLR, using LN cells from mice in the three treatment groups as responders and irradiated splenocytes from normal DBA/2 (H2d), SLJ (H2s), and host-syngeneic control mice as stimulators (Fig 3). The LN cells of the PBS-treated recipient mice responded to both allogeneic donor-compatible (DBA/2) and third-party (SJL) stimulation. In contrast, cells from either the peptide-treated or anti-CD4 MoAb-treated recipients exhibited significantly lower proliferative responses to DBA/2 stimulation (SI of 1.2 and 0.5, respectively; P < .001 for both groups compared with the untreated control). However, the response to third-party SJL stimulation (SI of 7.4 for the peptide-treated and 10.3 for the MoAb-treated mice) was equivalent to that of the untreated group (SI of 7.3).
Tolerance and immune reconstitution in rD-mPGPtide–treated chimeric mice. On day 60 post-BMT, recipient mice from the haploidentical (A and B) and the MHC class II-disparate (C and D) strain combinations were transplanted with tail skin grafts from the corresponding donor, the host, or an unrelated third party strain (SJL, H2s). Skin graft survival was monitored in four to five mice receiving transplants per group. The data shown are from one experiment representative of two performed for each strain combination.
Tolerance and immune reconstitution in rD-mPGPtide–treated chimeric mice. On day 60 post-BMT, recipient mice from the haploidentical (A and B) and the MHC class II-disparate (C and D) strain combinations were transplanted with tail skin grafts from the corresponding donor, the host, or an unrelated third party strain (SJL, H2s). Skin graft survival was monitored in four to five mice receiving transplants per group. The data shown are from one experiment representative of two performed for each strain combination.
Allogeneic responses of lymphocytes from rD-mPGPtide–treated chimeric mice in MLR. Recipient (B6CB)F1 mice were irradiated (750 cGy) and received transplants of MHC-haploidentical (B6D2)F1 ATBM (107 cells). One hour later, mice were injected with PBS (IV), anti-CD4 MoAb (ascites fluid at 25 μg; IP), or rD-mPGPtide (0.5 mg; IV) at 0.2 mL each. On day 70 post-BMT, LN cells from three mice of each treatment group were isolated and used in a MLR as responder cells (2.5 × 105 cells/well). Irradiated (20 Gy) allogeneic DBA/2 (H2d) and SJL (H2s) spleen cells (5 × 105 cells/well) were used as stimulators. Background counts were determined using irradiated syngeneic stimulator cells (5 × 105 cells/well). Proliferative responses of cells from mice in each of the three experimental groups were measured on days 3 through 5 by [3H]TdR incorporation and the SI was calculated relative to the syngeneic background response. The data shown are from one representative experiment of two performed for day-4 proliferative responses. Values are the mean ±SD of quadruplicate cultures.
Allogeneic responses of lymphocytes from rD-mPGPtide–treated chimeric mice in MLR. Recipient (B6CB)F1 mice were irradiated (750 cGy) and received transplants of MHC-haploidentical (B6D2)F1 ATBM (107 cells). One hour later, mice were injected with PBS (IV), anti-CD4 MoAb (ascites fluid at 25 μg; IP), or rD-mPGPtide (0.5 mg; IV) at 0.2 mL each. On day 70 post-BMT, LN cells from three mice of each treatment group were isolated and used in a MLR as responder cells (2.5 × 105 cells/well). Irradiated (20 Gy) allogeneic DBA/2 (H2d) and SJL (H2s) spleen cells (5 × 105 cells/well) were used as stimulators. Background counts were determined using irradiated syngeneic stimulator cells (5 × 105 cells/well). Proliferative responses of cells from mice in each of the three experimental groups were measured on days 3 through 5 by [3H]TdR incorporation and the SI was calculated relative to the syngeneic background response. The data shown are from one representative experiment of two performed for day-4 proliferative responses. Values are the mean ±SD of quadruplicate cultures.
Hematopoietic progenitor cell engraftment in rD-mPGPtide–treated mice.The ability to form GM colonies within the first week after donor BM transplantation is an important indication of successful engraftment.22 CFU-GM assays were performed with splenocytes pooled from three mice per treatment group on day 5 after transplantation in the haploidentical and the class II-disparate strain combinations (Fig 4). The splenocytes from PBS-treated recipients were used as a baseline control for the development of CFU-GM colonies after 6 days of culture in semisolid methylcellulose medium. Larger numbers of CFU-GM developed from splenocytes isolated from the peptide-treated and anti-CD4 MoAb-treated mice compared with the PBS-treated recipients reproducibly in both strain combinations. In the (B6D2)F1 → (B6CB)F1 combination, CFU-GM from peptide-treated and anti-CD4 MoAb-treated groups were at least double those from the PBS control group, which developed 23 colonies/105 cells. Similarly, in the bm12 → B6-Ly5.2 combination, the number of CFU-GM was increased by at least 45% in both treatment groups over the PBS-treated control group, which developed 271 colonies (much higher CFU-GM numbers were consistently produced in this strain combination than in the haploidentical transplantation recipients).
Effect of rD-mPGPtide on BM engraftment. CFU-GM assays were performed on day 5 post-BMT in the haploidentical (A) and the class II-disparate (B) strain combinations. Spleen cells were pooled from three donor mice per group, plated in triplicate in semisolid methylcellulose media, and cultured for 6 days. Colonies containing more than 30 (CFU-GM) cells were scored as positive. The results are from one experiment representative of three separate experiments. Values are the percent increase CFU-GM from anti-CD4 MoAb and rD-mPGPtide–treated mice over the PBS control, which provided the baseline level.
Effect of rD-mPGPtide on BM engraftment. CFU-GM assays were performed on day 5 post-BMT in the haploidentical (A) and the class II-disparate (B) strain combinations. Spleen cells were pooled from three donor mice per group, plated in triplicate in semisolid methylcellulose media, and cultured for 6 days. Colonies containing more than 30 (CFU-GM) cells were scored as positive. The results are from one experiment representative of three separate experiments. Values are the percent increase CFU-GM from anti-CD4 MoAb and rD-mPGPtide–treated mice over the PBS control, which provided the baseline level.
The CFU-GM progenitor cells were further analyzed for donor/host origin by dual-color flow cytometry (Table 4). Cells pooled from the cultured colonies derived from untreated ATBM recipients, in both the haploidentical and MHC class II-disparate strain combinations, were more than 90% of host origin and all positive for the myeloid differentiation antigen Gr-1 (Ly-6G; data not shown). In contrast, the CFU-GM originating from the peptide-treated recipients showed a drastic increase in cells derived from the respective (B6D2)F1 or bm12 donor strains. A similar observation was made for the anti-CD4 MoAb-treated animals. The combined results of this CFU-GM study supported the general enhancement of donor hematopoietic cell engraftment in the early stages after transplantation and treatment with either rD-mPGPtide or anti-CD4 MoAb.
Effect of rD-mPGPtide on Progenitor Cell Origin in the Haploidentical and MHC Class II-Disparate Strain Combinations
| Strain Combination . | Treatment . | Phenotype of CFU-GM (% donor/host) . |
|---|---|---|
| (B6D2)F1 → (B6CB)F1 (750 cGy) | PBS | 9.7/91.3 |
| rD-mPGPtide | 97.1/0.2 | |
| Anti-CD4 MoAb | 97.8/0.5 | |
| bm12 → B6-Ly5.2 (650 cGy) | PBS | 0.8/92.0 |
| rD-mPGPtide | 85.7/2.3 | |
| Anti-CD4 MoAb | 90.2/1.5 |
| Strain Combination . | Treatment . | Phenotype of CFU-GM (% donor/host) . |
|---|---|---|
| (B6D2)F1 → (B6CB)F1 (750 cGy) | PBS | 9.7/91.3 |
| rD-mPGPtide | 97.1/0.2 | |
| Anti-CD4 MoAb | 97.8/0.5 | |
| bm12 → B6-Ly5.2 (650 cGy) | PBS | 0.8/92.0 |
| rD-mPGPtide | 85.7/2.3 | |
| Anti-CD4 MoAb | 90.2/1.5 |
Progenitor cell origin was determined by dual-color flow cytometry using the following MoAb pairs: PE-anti-H2Kd and FITC-anti-H2Kk [(B6D2)F1 → (B6CB)F1 ]; SA-PE-biotin-anti-CD45.2 (anti-Ly5.2) and FITC-anti-CD45.1 (anti-Ly5.1) (bm12 → B6-Ly5.2). CFU-GM originating from the bm12 → B6-Ly5.2 and the (B6D2)F1 → (B6CB)F1 strain combinations were phenotyped on days 8 and 10, respectively, after in vitro plating. The data are from a representative of two separate experiments and the values presented are the percentage of positive-staining cells derived from host or donor origin. The data in all rD-mPGPtide and anti-CD4 MoAb treatment groups were significantly different from those in the PBS control group, P ≤ .001.
Effect of rD-mPGPtide on donor chimerism across MHC barriers in presensitized recipients.In clinical BM transplantation, prior blood transfusions may presensitize the patients to donor histocompatibility antigens and thereby increase the risk of marrow graft rejection.23 To assess the capacity of the rD-mPGPtide to inhibit host resistance to engraftment in this more aggressive type of situation, a presensitized class II-disparate strain combination was used. B6-Ly5.2 mice were immunized IP 14 days before transplantation with 2 × 107 bm12 spleen cells. On the day of transplantation, the recipient B6-Ly5.2 mice were conditioned with 700 cGy irradiation and injected with 107 bm12 ATBM cells. Preliminary experiments with a single treatment of PBS, rD-mPGPtide, or anti-CD4 MoAb on the day of transplantation (previously found to be effective in nonsensitized recipients, see above) resulted in only minimal enhancement of donor chimerism in the spleen, as measured on day 30 (rD-mPGPtide treatment resulted in only 20% increase in donor engraftment and anti-CD4 MoAb had no effect). In an attempt to maximize the effects of the peptide, in subsequent presensitization experiments recipient mice received rD-mPGPtide (0.5 mg; IV) on each day on days 0 through 3 post-BMT. As shown in Fig 5, this treatment schedule resulted in an approximately fivefold increase in donor chimerism over the PBS-treated control mice (donor chimerism increased from 15% to 71% in the spleen at day 30; P < .001). Interestingly, this effect was observed only in mice that received the rD-mPGPtide treatment, whereas a marginal nonsignificant (P > .05) increase (9%) in donor chimerism over the control level (25%) was observed in mice treated with multiple dosages of anti-CD4 MoAb (days 0 through 3). Thus, in the case of presensitized recipient mice, treatment with the rD-mPGPtide proved to be more effective than anti-CD4 MoAb (with the protocol used) in overcoming the host memory CD4+ T-cell response and enhancing donor engraftment.
Effect of rD-mPGPtide on donor chimerism in presensitized recipients. B6-Ly5.2 mice were presensitized (IP) with 2 × 107 bm12 spleen cells 14 days before irradiation (700 cGy; body weight, 24 to 25 g) and transplanted with 107 bm12 ATBM cells. One hour later, mice were treated with PBS (IV), anti-CD4 MoAb (ascites fluid 1:100 dilution; IP), or rD-mPGPtide (0.5 mg; IV), each at 0.2 mL. Recipient mice were injected again daily with the same reagents through day 3. Donor chimerism was analyzed 30 days after transplantation by two-color immunofluorescence flow cytometry using PE-anti-H2Kb and FITC-anti-CD45.1 (anti-Ly5.1) MoAb. The mean percentages of positive donor/host cells of four mice per group ±SD are presented for each individual treatment and are from one experiment representative of two performed with similar results. (▪) Donor; (▨) host.
Effect of rD-mPGPtide on donor chimerism in presensitized recipients. B6-Ly5.2 mice were presensitized (IP) with 2 × 107 bm12 spleen cells 14 days before irradiation (700 cGy; body weight, 24 to 25 g) and transplanted with 107 bm12 ATBM cells. One hour later, mice were treated with PBS (IV), anti-CD4 MoAb (ascites fluid 1:100 dilution; IP), or rD-mPGPtide (0.5 mg; IV), each at 0.2 mL. Recipient mice were injected again daily with the same reagents through day 3. Donor chimerism was analyzed 30 days after transplantation by two-color immunofluorescence flow cytometry using PE-anti-H2Kb and FITC-anti-CD45.1 (anti-Ly5.1) MoAb. The mean percentages of positive donor/host cells of four mice per group ±SD are presented for each individual treatment and are from one experiment representative of two performed with similar results. (▪) Donor; (▨) host.
DISCUSSION
The results presented here demonstrate that a single injection of a CD4-CDR3 peptide analog (rD-mPGPtide) at the time of transplantation significantly enhances donor marrow engraftment across MHC barriers. The levels of donor chimerism achieved after peptide treatment in nonsensitized recipients were equivalent to those achieved with anti-CD4 MoAb treatment and were similarly reflected in B- and T-cell (CD4+ and CD8+) subsets. Treatment with the rD-mPGPtide significantly increased donor-derived CFU-GM colony formation at early stages post-BMT, indicating greater efficiency of BM engraftment. Peptide-treated mice exhibited donor-specific tolerance in vitro and did not reject donor-type skin grafts, while maintaining the capacity to reject third-party allografts. Perhaps the most encouraging finding is that the peptide was also capable of inhibiting the activity of presensitized CD4+ host T cells (Fig 5).
Several laboratories have reported the inability of anti-CD4 MoAb treatments to inhibit memory T-cell responses.24,25 In addition, clinical trials for treatment of autoimmune patients with several different anti-CD4 MoAb have resulted in significantly decreased numbers of peripheral blood CD4+ T cells, increasing the potential risk of developing opportunistic infections.26-29 Murine antibodies have also been found to induce antimouse Ig responses that limit the effectiveness and prolonged application of these treatments.26,27,30 In contrast, the CD4-CDR3 peptide offers several advantages over the use of anti-CD4 MoAb. In addition to the finding, reported here, of its effect on presensitized CD4+ T cells, the peptide does not seem to be immunogenic and has failed to generate antibody responses upon repeated injection in mice (unpublished result). In other recent studies in the EAE SJL model, it has also been established that in vivo treatment with the peptide does not deplete the entire CD4+ T-cell subset but involves functional impairment of activated antigen-specific T cells.31 There was no significant diminution in any of the lymphoid cellular compartments, including the CD4+ T-cell subset within 1 to 14 days after peptide administration. In addition, LN T cells are fully functional, as evidenced by their capacity to respond to both recall antigens and third-party alloantigens. The half-life retention of the peptide in the serum of mice is approximately 25 minutes and responsiveness to antigen stimulation is significantly inhibited for up to 6 hours after administration but has virtually no effect on T-cell responses by 12 hours. This short window of efficacy might account for the observation that multiple treatments over the first 3 days after transplantation were much more effective than a single dosage in the inhibition of presensitized host T-cell rejection of the marrow graft (Fig 5).
It is not yet clear how the CD4-CDR3 peptide inhibits T-cell activation and/or expansion. Earlier in vitro studies showed that the inhibitory activity of CD4-CDR3 peptide analogs could be localized to effects on the helper T cell itself.14 It was hypothesized that these analogs acted by uncoupling a CD4 association with another surface molecule critical to the generation of an appropriate immune response. Based on recent investigations, the CDR3 region of CD4-D1 is now thought to play a critical role in CD4 homodimerization.32 Dimerization of CD4 may be required for stable interaction with the nonpolymorphic sites in the α2 and β2 domains of MHC class II molecules presenting antigen to the TCR of the CD4+ T cell.33-35 In turn, this stable interaction then allows for CD4-mediated signal transduction via the noncovalently associated protein tyrosine kinase p56lck.36-38 Disruption of dimerization by the peptide would thus interfere with the proper signal cascade of the T cell initiated upon TCR engagement by MHC-presented antigen. The consequences of this disruption would hypothetically lead either to programmed cell death,38 anergy of the antigen-specific T cell,40,41 or possibly a switch in cytokine production.42 43
The issue of pretransplant blood transfusion enhancement of marrow graft rejection was originally a primary concern for patients with aplastic anemia.23,44 However, the subsequent use of T-cell depletion of BM inocula in HLA-identical transplantation increased the incidence of graft failure in all clinical situations.5,45 The incidence of graft rejection was even higher for HLA-mismatched marrow donors.46-48 Although several risk factors, including the sex and age of donors and the level of immunosuppressive conditioning of recipients, are important to the incidence of graft failure,45 it is evident that residual host immunologic components are a predominant problem.46 Rejection may be mediated by either humoral49 or cellular mechanisms,3 and both processes can be enhanced by presensitizing transfusions. In regard to the cells responsible for marrow graft rejection, CD8+ T cells seem to be heavily involved, in humans, in the case of HLA-identical transplants.50 In HLA-mismatched situations, both CD4+ T cells directed to MHC class II antigens and CD8+ T cells directed to MHC class I antigens can be involved.9,10 This distinctive role of the T-cell subsets in graft failure has also been clearly shown experimentally in MHC-mismatched strain combinations,51 including the bm12 → B6-Ly5.2 model used in this study.19 In light of the potential importance of host presensitization to graft rejection mediated by either T-cell subset, it is significant that the CD4-CDR3 peptide analog was able to substantially block the response of the primed host to MHC class II-disparate donor marrow (Fig 5). Experiments to determine whether the peptide can enhance engraftment also in presensitized MHC haploidentical, MHC class I-mismatched, or MHC-matched, multiple minor histocompatibility-mismatched strain combinations are currently in progress.
In a related investigation, the rD-mPGPtide has been shown to have an inhibitory effect on the development of GVHD across a MHC-haploidentical barrier.15 Experiments performed both in vitro and in vivo showed the potential of the CD4-CDR3 analog to affect immune responses to MHC alloantigens. Peptide treatment of allogeneic marrow transplanted mice undergoing GVHD induced significant prolongation of their MST and resulted in notably reduced tissue destruction in GVHD target organs. This enhanced survival was evident for GVHD mediated by either unseparated T cells or purified CD4+ T cells. Even a single injection of rD-mPGPtide at the time of transplantation was effective in delaying the onset of disease mediated by CD4+ T cells.
In the current study, the CD4-CDR3 peptide appears to be an effective agent for the prevention of marrow graft rejection due to MHC class II incompatibilities. It also seems effective across full MHC barriers involving class I differences, although this activity may only occur if the host cells are nonsensitized. We hypothesize that the peptide primarily affects the host alloreactive CD4+ T cells that are being activated (or reactivated in the case of presensitized cells) early after transplantation, rendering them incapable of inducing marrow graft rejection. Noting the short window of efficacy of the CD4-CDR3 peptide in vivo, it is a most intriguing possibility that, if administered only within 1 week of transplantation, the peptide could specifically inhibit both GVHD-reactive and host-versus-graft–reactive CD4+ T cells, while leaving the remaining nonalloreactive CD4+ T-cell populations intact for subsequent development of responses to opportunistic infections or potential leukemic relapse. Further studies are planned to clarify these issues.
The current results support the approach of using structure-base designed peptide analogs to inhibit the responses of CD4+ alloreactive T cells. In conjunction with the ongoing murine studies, several peptides have also been designed from the human CD4 template, including the equivalent of those for the CDR3 loop region. These human peptides have inhibitory effects in human MLR assays52 and may therefore be good candidates for advancing this peptide approach toward clinical application.
ACKNOWLEDGMENT
We are grateful for the expert technical assistance of D. Dicker for flow cytometric analysis and the members of the KCI Peptide Synthesis Facility for their expertise in producing the peptide. We also acknowledge Dr B. Jameson for his role in designing the rD-mPGPtide and helping to initiate this project and Dr B. Perussia for critical reading of the manuscript.
Supported in part by National Institutes of Health Grants No. HL-55593 and CA-40358 and by funds provided from the Translational Research Committee of the Kimmel Cancer Institute.
Address reprint requests to Robert Korngold, PhD, Kimmel Cancer Institute, Jefferson Medical College, 233 S 10th St, Philadelphia, PA 19107.


![Fig. 3. Allogeneic responses of lymphocytes from rD-mPGPtide–treated chimeric mice in MLR. Recipient (B6CB)F1 mice were irradiated (750 cGy) and received transplants of MHC-haploidentical (B6D2)F1 ATBM (107 cells). One hour later, mice were injected with PBS (IV), anti-CD4 MoAb (ascites fluid at 25 μg; IP), or rD-mPGPtide (0.5 mg; IV) at 0.2 mL each. On day 70 post-BMT, LN cells from three mice of each treatment group were isolated and used in a MLR as responder cells (2.5 × 105 cells/well). Irradiated (20 Gy) allogeneic DBA/2 (H2d) and SJL (H2s) spleen cells (5 × 105 cells/well) were used as stimulators. Background counts were determined using irradiated syngeneic stimulator cells (5 × 105 cells/well). Proliferative responses of cells from mice in each of the three experimental groups were measured on days 3 through 5 by [3H]TdR incorporation and the SI was calculated relative to the syngeneic background response. The data shown are from one representative experiment of two performed for day-4 proliferative responses. Values are the mean ±SD of quadruplicate cultures.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2880/4/m_bl_0031f3.jpeg?Expires=1767745631&Signature=dwBqFaA9paqu3KCD1Iv8LaNgvQIGhwigP4yaC6UX7thCFJPt5xQjxOiG8qwj33u0kQgFY5xWkmj7LMw54M5I32gTIBrX1THF4jEN7qFPaJJo16pwDGd1cyEWJaopOViNMNTs85UCdf03z3e3Fa8Ioh~iJnuf7zIoEg1ZLtWPuXqLgOBRhYVkiIW0mk9fkgInYUtyyXlP~U7rnvmwQ7Ol~L3SGgZziowY~Vs3WD2o0ktmmjTGwLVjybqAAK8a1noKdVY7CE4v68mtBExeBApDdaBCnyLyvBo8kji4U4poad8TKNRA4Hq8qxbi8tJpGJT9ksTjArwSMlFVJcRj8kn6mA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal