Abstract
Nonrandom chromosomal abnormalities are found in most human malignancies, particularly leukemias and lymphomas. A characteristic t(1; 19) (q23; p13.3) chromosomal translocation is detected in 5% of childhood acute lymphoblastic leukemia (ALL) cases. This translocation results in the formation of a fusion gene, which leads to the expression of an oncogenic E2A/pbx1 protein. Breakpoints in the E2A gene almost invariably occur within a single intron, and the identical portion of PBX1 is joined consistently to exon 13 of E2A in fusion mRNA. In this article, we report the development of monoclonal antibodies against E2A/pbx1 fusion protein using a specific peptide that corresponds to the junction region of the protein. The obtained antibodies recognize specifically the chimeric E2A/pbx1 fusion protein and lack cross-reactivities with E2A and pbx1. Immunohistochemical staining and flow cytometric studies show that these antibodies can distinguish t(1; 19)-positive from t(1; 19)-negative leukemic cells. These results indicate that the obtained E2A/pbx1-specific monoclonal antibodies might prove to be valuable diagnostic reagents and important tools for elucidating the mechanisms involved in oncogenesis and progression of t(1; 19)-positive childhood ALL.
THE t(1; 19) (q23; p13.3) chromosomal translocation creates a fusion protein named E2A/pbx1.1,2 This protein is detected in 25% of childhood pre-B acute lymphoblastic leukemia (ALL) cases3,4 and also has been reported to be present in some cases of adult ALL.5
In normal B cells, the E2A gene encodes three different transcription factors E12, E47, and ITF-1/E-25, which derive from alternative splicing of the E2A transcript.6,7 These proteins belong to the basic helix-loop-helix (bHLH) family of transcription factors. It has been shown that this family of proteins forms homo- and heterodimers by interaction through their respective bHLH motifs. These dimeric complexes form tissue-specific transcriptional regulatory factors involved in various developmental pathways.8-11 PBX1, on the other hand, belongs to a homeobox gene family whose members are PBX1, PBX2, and PBX3. PBX1 is expressed in all tissues except B- and T-cell lineages. The homeodomain of pbx1 binds to the sequence ATCAATCAA.12-14 There is a high degree of sequence homology between the pbx1 homeodomain and that of yeast repressor a2. In addition, pbx1 and the Drosophila homeodomain protein Exd are closely related. Therefore, it has been suggested that pbx1 may function as a transcription regulator through interactions with other homeodomain proteins.
The E2A/pbx1 fusion protein consists of an N-terminal portion of 483 amino acids derived from E2A fused with 342 or 259 amino acids from the C-terminus of pbx1.1,2 The bHLH motif including the DNA binding domain of E2A is deleted as a result of the fusion, but the activation domain of E2A is retained in the fusion protein. Little is known about the oncogenic mechanism conferred by the E2A/pbx1 chimeric protein. It has been shown that a reporter gene construct containing the pbx1 homeodomain region that contains the DNA binding motif is transactivated by E2A/pbx1 but not by pbx1 alone.12 This leads to the postulation that the spatially and temporally incorrect activation of pbx1-responsive genes contributes to the pre-B ALL phenotype.12-14 However, transgenic mice studies show that the E2A region is essential and that the pbx1 homeodomain is dispensable for the development of malignant lymphomas.15 This finding suggests that oncogenesis might be caused by interactions between cellular proteins and E2A/pbx1 instead of homeodomain–DNA interactions. Leukemic patients carrying the t(1; 19) translocation and expressing the E2A/pbx1 fusion protein are associated with a poorer prognosis. It is not known how this genetic abnormality confers such aggressive behavior. Over 85% of patients with ALL with t(1; 19) expressing E2A/pbx1 transcripts exhibit the same breakpoint.16 17 Monoclonal antibodies (MoAbs) specific for the neoepitope formed by the junction region of the E2A/pbx1 chimeric protein might be clinically valuable diagnostic tools for delineating the mechanisms involved in oncogenesis and the aggressive behavior associated with poor prognosis. In the present study, we report the development and characterization of MoAbs that are highly specific for the junction region of the E2A/pbx1 chimeric protein.
MATERIALS AND METHODS
Preparation of immunogen and recombinant proteins.Recombinant E2A/pbx1 junction protein: The BstU1 fragment (nucleotides 1386-1883) from pSK-E2A/pbx1 (a kind gift from Dr Cornelis Murre, University of California, San Diego) was inserted into the Sma I site of pGEX4T-3 (Pharmacia, Uppsala, Sweden). The recombinant E2A/pbx1 junction protein was expressed as a GST fusion protein in Escherichia coli DH5α cells and affinity purified on glutathione-agarose beads as described previously.18
Keyhole limpet hemocyanin (KLH)-conjugated peptide: An E2A/pbx1-specific synthetic peptide (SYSVLSIRGAQEEC), peptide 14, was conjugated to maleimide-activated KLH (Pierce, Rockford, IL) according to the manufacturer's instructions.
Recombinant pbx1 protein: pET21-pbx1 containing the entire coding region of PBX1 was transformed into BL21 (DE3) E coli cells. The recombinant (His)6 -tagged pbx1 protein was expressed after isopropylthio-b-galactoside (IPTG) induction and purified using Ni-NTA beads (Qiagen, Chatsworth, CA) according to the manufacturer's instructions.
Clinical, Cytogenetic, and Antibody Staining Data of the 14 Leukemia Patients
| Case . | Sex . | Leukocyte Count . | Blast %* . | FAB . | Immunophenotype . | Cytogenetics . | Reactivity With . |
|---|---|---|---|---|---|---|---|
| . | . | (×109/L) . | . | . | . | . | G289-781† . |
| 1 | M | 5.1 | 99 | L1 | Pre-B ALL | der(19)t(1; 19)(q23; p13) | + |
| 2 | M | 12.0 | 66 | L1 | Early pre-B ALL | 57,XY,[no t(1; 19)] | − |
| 3 | F | 36.1 | 95 | L1 | Pre-B ALL | 56,XX,[no t(1; 19)] | − |
| 4 | M | 74.1 | 95 | L1 | Pre-B ALL | t(1; 19)(q23; p13) | + |
| 5 | F | 248.1 | 95 | L1 | Pre-B ALL | der(19)t(1; 19)(q23; p13) | + |
| 6 | F | 159.2 | 98 | L1 | Pre-B ALL | der(19)t(1; 19)(q23; p13) | + |
| 7 | F | 19.7 | 97 | L1 | Pre-B ALL | der(19)t(1; 19)(q23; p13) | + |
| 8 | F | 193.0 | 98 | L1 | Pre-B ALL | der(19)t(1; 19)(q23; p13) | + |
| 9 | M | 402.0 | 94 | L1 | T-ALL | 46,XY,[no t(1; 19)] | − |
| 10 | F | 395.5 | 99 | L1 | Early pre-B ALL | 46,XX,[no t(1; 19)] | − |
| 11‡ | M | 19.0 | 12 | Myeloid | 46,XY, t(9; 22)(q34; q11) | − | |
| 12 | F | 97.3 | 91 | L2 | Early pre-B ALL | 39,XX,[no t(1; 19)] | − |
| 13 | F | 531.3 | 98 | L1 | Pre-B | 46,XX, t(4; 11)(q21; q23) | − |
| 14 | M | 496.0 | 97 | L2 | T-ALL | 46,XY, t(11; 19)(q23; p13.3) | − |
| Case . | Sex . | Leukocyte Count . | Blast %* . | FAB . | Immunophenotype . | Cytogenetics . | Reactivity With . |
|---|---|---|---|---|---|---|---|
| . | . | (×109/L) . | . | . | . | . | G289-781† . |
| 1 | M | 5.1 | 99 | L1 | Pre-B ALL | der(19)t(1; 19)(q23; p13) | + |
| 2 | M | 12.0 | 66 | L1 | Early pre-B ALL | 57,XY,[no t(1; 19)] | − |
| 3 | F | 36.1 | 95 | L1 | Pre-B ALL | 56,XX,[no t(1; 19)] | − |
| 4 | M | 74.1 | 95 | L1 | Pre-B ALL | t(1; 19)(q23; p13) | + |
| 5 | F | 248.1 | 95 | L1 | Pre-B ALL | der(19)t(1; 19)(q23; p13) | + |
| 6 | F | 159.2 | 98 | L1 | Pre-B ALL | der(19)t(1; 19)(q23; p13) | + |
| 7 | F | 19.7 | 97 | L1 | Pre-B ALL | der(19)t(1; 19)(q23; p13) | + |
| 8 | F | 193.0 | 98 | L1 | Pre-B ALL | der(19)t(1; 19)(q23; p13) | + |
| 9 | M | 402.0 | 94 | L1 | T-ALL | 46,XY,[no t(1; 19)] | − |
| 10 | F | 395.5 | 99 | L1 | Early pre-B ALL | 46,XX,[no t(1; 19)] | − |
| 11‡ | M | 19.0 | 12 | Myeloid | 46,XY, t(9; 22)(q34; q11) | − | |
| 12 | F | 97.3 | 91 | L2 | Early pre-B ALL | 39,XX,[no t(1; 19)] | − |
| 13 | F | 531.3 | 98 | L1 | Pre-B | 46,XX, t(4; 11)(q21; q23) | − |
| 14 | M | 496.0 | 97 | L2 | T-ALL | 46,XY, t(11; 19)(q23; p13.3) | − |
Abbreviation: FAB, French-American-British Cooperative Group Classification.
Bone marrow blast percentage.
Results of antibody reactivity by flow cytometry, immunohistochemistry, and Western blotting.
Case of chronic myelocytic leukemia.
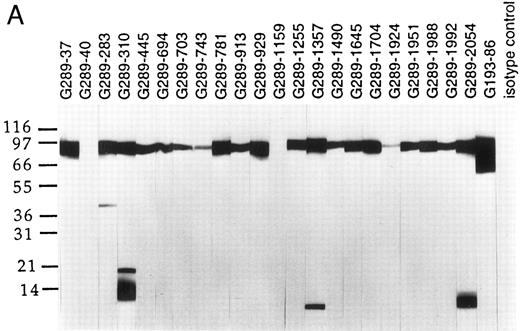
Western blot analyses of G289 MoAb clones. Total cell lysates from the E2A/pbx1 carrying line 697 (A) and the Burkitt's lymphoma cell line Namalwa (B) were resolved on 4% to 20% SDS-PAGE and transferred to Immobilon-P membrane. Individual membrane strips were probed with various G289 clones, an E2A-specific G193-86 clone, and an isotype control MoAb using enhanced chemoluminescence.
Western blot analyses of G289 MoAb clones. Total cell lysates from the E2A/pbx1 carrying line 697 (A) and the Burkitt's lymphoma cell line Namalwa (B) were resolved on 4% to 20% SDS-PAGE and transferred to Immobilon-P membrane. Individual membrane strips were probed with various G289 clones, an E2A-specific G193-86 clone, and an isotype control MoAb using enhanced chemoluminescence.
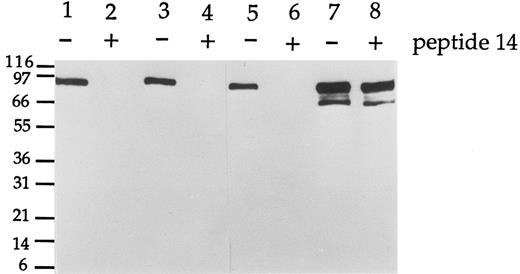
Peptide 14 blocks the reactivity of G289 MoAbs against E2A/pbx1. Total cell lysate of the 697 line was resolved by SDS-PAGE, transferred to Immobilon-P membrane, and incubated with antibodies G289-781 (lanes 1 and 2), G289-929 (lanes 3 and 4), and G289-1951 (lanes 5 and 6) with (+) or without (−) the peptide 14 added. An E2A-specific antibody G193-86 was used (lanes 7 and 8) as control. Each lane represented the equivalent of 2 × 105 cells. The E2A/pbx1 and E2A signals were detected by enhanced chemiluminescence.
Peptide 14 blocks the reactivity of G289 MoAbs against E2A/pbx1. Total cell lysate of the 697 line was resolved by SDS-PAGE, transferred to Immobilon-P membrane, and incubated with antibodies G289-781 (lanes 1 and 2), G289-929 (lanes 3 and 4), and G289-1951 (lanes 5 and 6) with (+) or without (−) the peptide 14 added. An E2A-specific antibody G193-86 was used (lanes 7 and 8) as control. Each lane represented the equivalent of 2 × 105 cells. The E2A/pbx1 and E2A signals were detected by enhanced chemiluminescence.
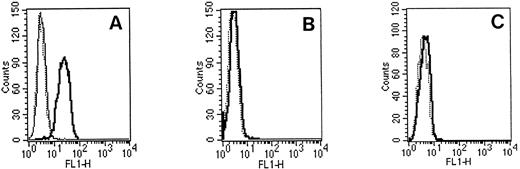
Flow cytometric studies using G289-781 MoAb. 697 cells (A) or Namalwa cells (B) were fixed, permeabilized, and incubated with an isotype control antibody (⋅⋅⋅) or G289-781 MoAb without (━) or with (─) peptide 14. Then cells were stained with FITC-conjugated goat antimouse Ig. (C) Cell surface staining of 697 cells using G289-781 MoAb (━) and an isotype control antibody (⋅⋅⋅).
Flow cytometric studies using G289-781 MoAb. 697 cells (A) or Namalwa cells (B) were fixed, permeabilized, and incubated with an isotype control antibody (⋅⋅⋅) or G289-781 MoAb without (━) or with (─) peptide 14. Then cells were stained with FITC-conjugated goat antimouse Ig. (C) Cell surface staining of 697 cells using G289-781 MoAb (━) and an isotype control antibody (⋅⋅⋅).
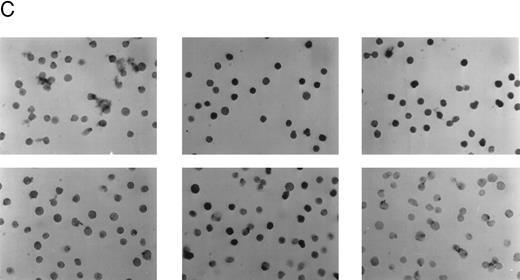
Detection of the E2A/pbx1 chimeric protein in patient bone marrow specimens using the G289-781 MoAb. (A) Western blot analysis of specimens from four representative patients (lanes 1 to 4), 697 cell lysate (lane 5), and Namalwa cell lysate (lane 6) using the G289-781 MoAb. (B) Flow cytometric analyses of the same patient specimens with an isotype control antibody (- - - -) or G289-781 MoAb (——). (C) Representative immunohistochemical staining of bone marrow specimens from patient 1 (top) and patient 3 (bottom) using an isotype-matched negative control antibody (left), an E2A-specific positive control antibody (G98-271.1, center), and the E2A/pbx1-specific G289-781 MoAb (right).
Detection of the E2A/pbx1 chimeric protein in patient bone marrow specimens using the G289-781 MoAb. (A) Western blot analysis of specimens from four representative patients (lanes 1 to 4), 697 cell lysate (lane 5), and Namalwa cell lysate (lane 6) using the G289-781 MoAb. (B) Flow cytometric analyses of the same patient specimens with an isotype control antibody (- - - -) or G289-781 MoAb (——). (C) Representative immunohistochemical staining of bone marrow specimens from patient 1 (top) and patient 3 (bottom) using an isotype-matched negative control antibody (left), an E2A-specific positive control antibody (G98-271.1, center), and the E2A/pbx1-specific G289-781 MoAb (right).
Immunization and development of MoAbs.Balb/c mice were immunized with antigen on days 1, 7, 14, 21, and 28 and fused 3 days after the final immunization. The KLH-conjugated peptide 14 was used for the first three immunizations, and recombinant E2A/pbx1 junction protein together with conjugated-peptide 14 was used for the last two immunizations. Splenocytes from immunized mice were then fused with F0 myeloma cells. Hybridoma supernatants were initially screened against KLH-conjugated peptide 14 by enzyme-linked immunosorbent assay (ELISA). Hybridomas secreting antibodies that recognize conjugated peptide 14 were further screened by dot blot analysis for specific reactivity against the peptide sequence corresponding to the junction of the chimeric protein. Selected hybridoma supernatants were purified via Sepharose G chromatography (Pharmacia) according to the manufacturer's instructions.
Western blotting.The Namalwa Burkitt's lymphoma cell line and the t(1; 19)-carrying pre-B cell line 697 were cultured in Dulbecco's modified Eagle's medium containing 10% fetal calf serum. Cells in log phase were harvested and washed with phosphate-buffered saline (PBS). Cell lysates in sample loading buffer were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto Immobilon-P membranes (Millipore, Bedford, MA). Western blotting was performed via the standard method using hybridoma supernatants and horseradish peroxidase (HRPO)-conjugated secondary antibodies and was developed by enhanced chemiluminescence (Pierce). For peptide blocking, 2 μg/mL of purified MoAb was preincubated for 1 hour at room temperature with 50 μg/mL of peptide 14 before using them in Western blot analysis.
Immunohistochemical staining.Cells were attached to precoated slides (BioRad, Hercules, CA), fixed with 2% formaldehyde, washed with PBS, and permeabilized with 0.1% saponin plus 1% hydrogen peroxide in PBS. The cells were then blocked in 1% bovine serum albumin (BSA) plus 0.1% saponin and incubated overnight with the primary antibody. Biotinylated donkey antimouse immunoglobulin (Ig; Jackson Immunoresearch Labs, West Grove, PA) was used as a secondary antibody. The staining was visualized using HRPO-conjugated streptavidin (Dako, Carpentera, CA) and 3,3′-diaminobenzidine as chromogen.
Flow cytometric analysis.Approximately 5.5 × 105 cells were fixed with 4% paraformaldehyde, permeablized with methanol, and blocked with 1% BSA. Cells were then incubated with 1 μg of E2A/pbx1 junction-specific MoAb for 30 minutes. An IgG1 isotype antibody was used as a negative control antibody. Cells were stained with FITC-conjugated goat antimouse Ig (2.5 μg/mL). For peptide 14 blocking experiments, 1 μg of junction-specific antibody was preincubated with 0.1 μg peptide 14 for 30 minutes before its incubation with cells. Cellular fluorescence was measured using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA).
Clinical samples.Bone marrow samples of childhood leukemia were obtained from the tissue bank of St. Jude Children's Research Hospital. The leukemic blast features of fourteen cases are summarized in Table 1.
RESULTS
Because initial attempts to raise MoAbs specific to the junction region using the conjugated peptide as the sole immunogen were not successful, we subsequently used purified recombinant GST fusion protein containing the E2A/pbx1 junction region as antigen for the last two immunizations to enhance the possibility of developing specific antibodies. This E2A/pbx1 junction fragment contained 20 amino acid residues from the E2A protein and 144 amino acid residues from pbx1. Of 1,000 hybridomas tested, 134 were positive by ELISA against the KLH-peptide 14. Each of these 134 clones was subcloned by limiting dilution and further tested for reactivity with pbx1 and recombinant E2A/pbx1 junction protein by dot blotting. All of the 134 clones recognized the E2A/pbx1 junction protein. However, only antibodies from 22 clones failed to react with pbx1. These antibodies were further screened by Western blotting using cell lysate from the pre-B cell line 697, which contains the t(1; 19) translocation and expresses the E2A/pbx1 protein. As a negative control, lysate from Namalwa cells, an EBV-transformed pre-B cell line that lacks expression of the E2A/pbx1 chimeric protein, was used. The results are shown in Fig 1. The E2A-specific MoAb G193-86 (PharMingen, San Diego, CA) was used as a reference reagent. This antibody recognized a band of approximately 74 kD corresponding to the E2A protein in Namalwa cell lysate. In addition to the 74-kD band, G193-86 detected an additional band of approximately 85 kD corresponding to E2A/pbx1 chimeric protein in 697 cell lysate. Twenty of the 22 MoAbs recognized the E2A/pbx1 in 697 cells by Western blotting. Five of the 20 antibodies also cross-reacted with several additional unidentified protein bands in the 697 lysate. None of these reagents could recognize E2A in Namalwa cells or Jurkat T cells (data not shown), which do not express the E2A/pbx1 chimeric protein. All the 22 hybridoma clones proved to be specific for E2A/pbx1 and did not recognize E2A or pbx1 individually. Peptide 14 completely blocked the reactivity of those MoAbs to the E2A/pbx1 fusion protein in 697 cell lysate, as demonstrated in Fig 2. Peptide 14 could not block the E2A-specific reactivity of antibody G193-86.
To examine the specificity of these junction-specific MoAbs at a cellular level, the selected MoAbs were further characterized by flow cytometric analysis. Over 95% of 697 cells were stained by the G289-781 MoAb (Fig 3A) compared with fewer than 5% Namalwa cells (Fig 3B), a result similar to that obtained for the isotype control. The fluorescence of stained cells was intracellular because there was no staining observed by junction-specific antibody when cells were not fixed and permeabilized before the addition of antibody (Fig 3C). Furthermore, preincubation of 100 ng of peptide 14 with the MoAb completely blocked 697 cell staining (Fig 3A). These combined results indicate that the 697 cell staining was E2A/pbx1 specific. The significant difference in staining between E2A/pbx1-positive and -negative cells implies that these antibodies can be used for flow cytometric studies. At equal concentration, the G289-781 clone showed the strongest fluorescence staining in comparison with the other analyzed clones.
To test the diagnostic potential of the G289-781 MoAb, bone marrow specimens from 14 patients with leukemia were examined, without prior knowledge of the diagnosis based on cytogenetic analysis or immunophenotyping. Our data showed that only 6 of 14 bone marrow specimens were positive for the E2A/pbx1 chimeric protein by Western blotting, flow cytometry, and immunohistochemistry. These results were found to be in exact agreement with the diagnosis based on cytogenetic analysis (Table 1). Figure 4A and B shows results of the Western blotting and flow cytometric analysis of representative bone marrow specimens from four patients using the G289-781 MoAb. Figure 4C shows immunohistochemical staining of representative t(1; 19)-positive and t(1; 19)-negative bone marrow specimens. The staining result is consistent with published data that E2A/pbx1 is a nuclear protein.19 20
DISCUSSION
In childhood ALL, the t(1; 19) (q23; p13.3) chromosomal translocation results in the fusion of the E2A and PBX1 genes, leading to expression of a fusion transcript. The translated chimeric protein is correlated with oncogenic properties, presumably due to its aberrant DNA binding and transactivation functions. However, results of transgenic mice studies indicate that the DNA binding domain of E2A/pbx1 is not essential for induction of malignant lymphomas, implying that the mechanism of action of the chimeric protein resulting from t(1; 19) translocation is more complex. The availability of E2A/pbx1-specific MoAbs would therefore provide a valuable tool to elucidate the oncogenic mechanisms.
In the present study, we have developed MoAbs that are specific for the junction of the E2A/pbx1 chimeric protein. These antibodies do not interact with E2A or pbx1 alone. An especially important property of these antibodies is their ability to distinguish t(1; 19)-positive from t(1; 19)-negative cells by recognizing the aberrant gene product directly by immunohistochemical staining and flow cytometric analysis.
We have used these antibodies to demonstrate their specificity for cells containing the t(1; 19) translocation by immunoperoxidase staining and immunofluorescence for microscopy and flow cytometry, respectively. These reagents will be diagnostically useful for the early detection of t(1; 19) positive leukemias. In fact, we have used G289-781 on 14 clinical samples and demonstrated that the antibody was able to distinguish t(1; 19)-positive leukemia from those lacking the translocation. A few cases of t(1; 19)-positive leukemias have been reported in which the fusion region of E2A and pbx1 differs.21,22 Izraeli et al. reported that three of 21 cases in their study have an E2A/pbx1 transcript with a variant junction region containing an in-frame insertion of 27 base pairs.17 We are presently developing MoAbs that are specific for this junction variant. These junction-specific antibodies will provide helpful information for clinical diagnosis, subclassification of ALL, and predicting prognosis of patients harboring these genetic abnormalities. They also may be useful for the identification of high-risk patients who might benefit from intensified initial chemotherapeutic regimens, for the detection of minimal residual disease, and for monitoring early relapse and response to therapy.
In summary, we report the development and characterization of MoAbs that are highly specific for the common form of the E2A/pbx1 chimeric protein found in most t(1; 19)-containing ALLs. These MoAbs do not react with either E2A or pbx1 protein alone. The use of a large number of clinical specimens from patients with leukemia will allow a detailed evaluation of their clinical application as well as their utility in understanding the pathogenic mechanisms of the E2A/pbx1 chimeric protein for this type of leukemia.
Supported by NIH SBIR Grant 1R43CA66341-01 and by Grants No. NIH CA-21765 and CA-20180 to F.B.
Address reprint requests to Stefan Gruenwald, PharMingen Inc, 10975 Torreyana Road, San Diego, CA 92121.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal