Abstract
We demonstrated previously that OX40 and its ligand, gp34, directly mediate adhesion of activated normal CD4+ T cells, as well as human T-cell leukemia virus type I (HTLV-I)–transformed T cells to vascular endothelial cells. In the present study, we examined expression of OX40 on fresh leukemic cells from patients with adult T-cell leukemia (ATL) and its possible involvement in cell adhesion. Flow cytometric analysis showed that peripheral blood mononuclear cells (PBMC) or lymph node tumor cells from 15 of 17 cases expressed significant levels of OX40 without stimulation. On the other hand, gp34 was not expressed on these cells, although its expression is also known to be associated with HTLV-I-infection. In Western blot analysis, a 50-kD protein band was detected by anti-OX40 monoclonal antibody (MoAb) in two ATL cases examined, as well as phytohemagglutinin (PHA) blasts and Hut102, an HTLV-I–infected T-cell line, but not in resting PBMC or Jurkat. Expression of OX40 mRNA was shown by reverse transcriptase-polymerase chain reaction in all ATL cases tested, PHA-blasts, and Hut102, but not in resting PBMC or Jurkat. We could not detect expression of HTLV-I viral mRNA in any of the cases tested. Cell adhesion assay was performed and in at least three cases, fresh ATL cells exhibited adhesion to human umbilical vein endothelial cells that could be considerably inhibited by either anti-OX40 MoAb or anti-gp34 MoAb. Immunohistochemical staining of skin biopsy specimens indicated that infiltrating mononuclear cells express OX40 in vivo. Taken together, these data indicate that leukemic cells from most, but not all, ATL patients constitutively express OX40, which may play a role in leukemic cell infiltration in addition to cell adhesion in vivo.
ADULT T-CELL LEUKEMIA (ATL)1 is a leukemia/lymphoma of mature T cells with characteristic hematologic features and endemic incidence that is caused by human T-cell leukemia/lymphoma virus type I (HTLV-I).2 One of the frequent manifestations of ATL is infiltration of leukemic cells into various organs such as lymph node, liver, spleen, lung, skin, and intestinal tract. Although its pattern and magnitude vary from one case to another, leukemic cell infiltration poses serious clinical problems for patients, which affects the disease profile and prognosis. Recent studies have indicated that homing and localization of circulating lymphocytes are virtually determined by expression of specified adhesion molecules on lymphocytes, as well as on vascular endothelial cells.3-5 A number of adhesion molecules have been identified, molecularly cloned, and demonstrated to be able to reconstitute cell adhesion. It is believed that infiltration or metastasis of lymphoid neoplastic cells use a similar set of adhesion molecules that are either normally present or induced upon malignant transformation.
We reported previously that fresh leukemic cells from ATL patients adhered to human umbilical vein endothelial cells (HUVEC) in vitro predominantly by very late antigen-4 (VLA-4) or E-selectin ligand (cutaneous lymphocyte antigen recognized by HECA452 monoclonal antibody [MoAb]), although the mode of adhesion was quite heterogeneous among patients examined.6 It was noted at the same time that a considerable proportion of the adhesion between ATL cells and HUVEC remained unaffected in the presence of a combination of MoAbs against known adhesion molecules, suggesting that unknown pathway(s) might be involved in adhesion of these cells. We have recently established MoAbs that can inhibit this unknown type of adhesion between an ATL-derived T-cell line and HUVEC. The antigen molecule has been shown to be a 50-kD glycoprotein and expressed on activated normal T cells, as well as some HTLV-I–infected T-cell lines. We have succeeded in cDNA cloning of the antigen molecule to unexpectedly find that it is identical to human OX40.7
OX40 was first described as a cell surface antigen expressed on rat CD4+ T cells.8 Molecular cloning of rat OX40 showed that it is a member of tumor necrosis factor (TNF ) receptor/nerve growth factor (NGF) receptor family.9 During the past several years, cDNAs encoding mouse and human OX40 have been isolated.10,11 The members of this family including CD40, Fas (APO-1), CD30, CD27, and 4-1BB/ILA are type I membrane molecules and contain three to four repeats of cysteine rich sequence in their extracellular domain.12 It has been reported that OX40 is predominantly expressed on activated CD4+ T cells11 and interaction of OX40 with its ligand, gp34,13-15 generates costimulatory signals resulting in enhanced T-cell proliferation in the presence of mitogens.16,17 We have demonstrated that gp34 is constitutively expressed on endothelial cells and that the OX40/gp34 system directly mediates adhesion of activated normal CD4+ T cells or a certain HTLV-I–infected T-cell line cells to HUVEC.7 Because gp34 and OX40 are categorized as a membrane-bound cytokine and its receptor, this means that a pair of cytokines and cytokine receptors can directly mediate cell adhesion.
In the present study, we examined expression of OX40 on fresh ATL cells and their binding capacity to HUVEC via the OX40/gp34 system in selected cases. Data are presented here indicating that leukemic cells from most, but not all, ATL patients express significant levels of OX40 without any stimulation and that the OX40/gp34 system contributes to cell adhesion of leukemic cells to HUVEC in at least certain cases. The pathophysiological relevance of OX40 expression by ATL leukemic cells in tissue infiltration, as well as in vivo growth of ATL cells, is discussed.
MATERIALS AND METHODS
Patients.Samples from 17 patients with ATL were analyzed. The diagnosis was made on the basis of clinical features, hematologic characteristics, serum antibodies to HTLV-I antigens and, in cases examined, HTLV-I provirus integration. So far, nine cases have been tested and demonstrated to be positive for HTLV-I proviral integration in chromosomal DNA. The sex, age, and clinical subtype of the patients are listed in Table 1.
Results of Flow Cytometric Analysis of Samples From 17 Patients With ATL
| Case . | Age/Sex . | Type . | Material . | % Positive Cells . | ||||
|---|---|---|---|---|---|---|---|---|
| . | . | . | . | CD3 . | CD4 . | Tac . | OX40 . | gp34 . |
| 1 | 38/F | Acute | PB | 92.3 | 85.5 | 83.6 | 64.5 | NE |
| 2 | 65/M | Acute | PB | 74.1 | 86.3 | 87.4 | 39.6 | NE |
| 3 | 56/M | Acute | PB | 81.3 | 84.9 | 80.9 | 0 | 0.7 |
| 4 | 61/M | Acute | PB | 75.6 | 85.7 | 76.9 | 64.8 | 0 |
| 5 | 39/F | Acute | PB | 84.9 | 88.4 | 85.1 | 46.2 | 1.1 |
| 6 | 72/F | Acute | LN | 87.6 | 94.8 | 87.6 | 83.4 | 0.6 |
| 7 | 71/M | Acute | PB | 81.2 | 61.7 | 80.4 | 47.7 | 2.1 |
| 8 | 51/F | Acute | PB | 65.8 | 78.2 | 68.3 | 26.4 | 0.4 |
| 9 | 72/M | Chronic | PB | 97.9 | 96.3 | 94.7 | 85.3 | NE |
| 10 | 40/M | Chronic | PB | 68.2 | 80.9 | 74.0 | 0 | 0 |
| 11 | 49/F | Chronic | PB | 71.0 | 67.5 | 45.9 | 40.5 | 0 |
| 12 | 46/F | Chronic | PB | 86.3 | 84.1 | 56.8 | 70.9 | 1.9 |
| 13 | 37/M | Chronic | PB | 98.6 | 94.8 | 46.1 | 81.0 | 1.6 |
| 14 | 54/M | Chronic | LN | 73.3 | 61.8 | 20.4 | 46.1 | 0 |
| 15 | 56/M | Chronic | PB | 85.8 | 85.6 | 38.1 | 85.9 | 2.4 |
| 16 | 54/M | Lymphomatous | ST | 90.1 | 85.2 | 55.9 | 26.1 | 3.5 |
| 17 | 65/F | Lymphomatous | LN | 94.6 | 90.6 | 34.5 | 46.7 | 0.9 |
| Case . | Age/Sex . | Type . | Material . | % Positive Cells . | ||||
|---|---|---|---|---|---|---|---|---|
| . | . | . | . | CD3 . | CD4 . | Tac . | OX40 . | gp34 . |
| 1 | 38/F | Acute | PB | 92.3 | 85.5 | 83.6 | 64.5 | NE |
| 2 | 65/M | Acute | PB | 74.1 | 86.3 | 87.4 | 39.6 | NE |
| 3 | 56/M | Acute | PB | 81.3 | 84.9 | 80.9 | 0 | 0.7 |
| 4 | 61/M | Acute | PB | 75.6 | 85.7 | 76.9 | 64.8 | 0 |
| 5 | 39/F | Acute | PB | 84.9 | 88.4 | 85.1 | 46.2 | 1.1 |
| 6 | 72/F | Acute | LN | 87.6 | 94.8 | 87.6 | 83.4 | 0.6 |
| 7 | 71/M | Acute | PB | 81.2 | 61.7 | 80.4 | 47.7 | 2.1 |
| 8 | 51/F | Acute | PB | 65.8 | 78.2 | 68.3 | 26.4 | 0.4 |
| 9 | 72/M | Chronic | PB | 97.9 | 96.3 | 94.7 | 85.3 | NE |
| 10 | 40/M | Chronic | PB | 68.2 | 80.9 | 74.0 | 0 | 0 |
| 11 | 49/F | Chronic | PB | 71.0 | 67.5 | 45.9 | 40.5 | 0 |
| 12 | 46/F | Chronic | PB | 86.3 | 84.1 | 56.8 | 70.9 | 1.9 |
| 13 | 37/M | Chronic | PB | 98.6 | 94.8 | 46.1 | 81.0 | 1.6 |
| 14 | 54/M | Chronic | LN | 73.3 | 61.8 | 20.4 | 46.1 | 0 |
| 15 | 56/M | Chronic | PB | 85.8 | 85.6 | 38.1 | 85.9 | 2.4 |
| 16 | 54/M | Lymphomatous | ST | 90.1 | 85.2 | 55.9 | 26.1 | 3.5 |
| 17 | 65/F | Lymphomatous | LN | 94.6 | 90.6 | 34.5 | 46.7 | 0.9 |
The percentage of positive cells was determined by immunofluorescence staining and flow cytometric analysis.
Abbreviations: NE, not examined; PB, peripheral blood; LN, lymph node; ST, subcutaneous tumor.
Preparation of fresh ATL leukemic cells.Peripheral blood mononuclear cells (PBMC) were separated from heparinized venous blood by Ficoll-Paque density gradient centrifugation. In the case of lymphomatous type patients, a piece of a lymph node (a subcutaneous tumor in case no. 16) resected for diagnosis was minced and pressed through a steel mesh to make a single cell suspension. PBMC or lymph node cells (LN), which contained more than 60% malignant cells by morphology or flow cytometry were used fresh or stored in liquid nitrogen. Only the frozen samples with good (more than 85%) viability of recovered cells were used.
MoAbs.Fluorescein isothiocyanate (FITC) conjugated MoAbs, Leu-2 (anti-CD8a), Leu-3 (anti-CD4), and Leu-4 (anti-CD3) were purchased from Becton Dickinson (San Jose, CA). MoAbs 131, 315 (both anti-OX40), and anti-Tac (anti–interleukin-2 receptor α) were purified from ascitic fluids as described.7 Purified 315 and anti-Tac were biotinylated using NHS-LC–Biotin II (Pierce Chemical, Rockford, IL). FITC-conjugated anti-Tac was made as described.18 TS1/18(anti-CD18, the β chain of lymphocyte functional antigen-1 [LFA-1]), W6/32 (anti-major histocompatibility complex [MHC] class I, control IgG1). HP2/1(anti-CD49d, the α chain of VLA-4) was purchased from Immunotech S. A. (Marseille, France). 7A9 (anti-E–selectin) was kindly provided by Dr W. Newman (Otsuka America Pharmaceutical Inc, Rockville, MD). Anti-gp34 MoAb, 5A8 was described elsewhere.14
Immunofluorescence staining and flow cytometric analysis.Cells were stained by direct or indirect immunofluorescence and analyzed using a FACScan (Becton Dickinson Immunocytometry Systems, San Jose, CA) as described previously.19 Indirect staining with nonconjugated and biotinylated MoAbs was performed using FITC-conjugated (Fab′)2 fraction of goat antimouse IgG + IgM (Biosource International, Camarillo, CA) and phycoerythrin (PE)-conjugated streptavidin (Becton Dickinson), respectively.
Western blotting.Western blotting was done as described.7 In brief, cell lysates from 5 × 106 cells were mixed with sodium datecyl sulfate (SDS) sample buffer, boiled for 5 minutes, and electrophoresed through 7.5% to 10% polyacrylamide gel. Samples were electrotransferred onto Immobilon-P (PVDF ) (Millipore, Marlborough, MA) membranes. The membranes were incubated serially with blocking buffer and with diluted MoAb solutions, washed, and then incubated with rabbit peroxidase-conjugated antimouse IgG (Amersham, Qualex, CA). After washing, the binding of MoAb was visualized by an ECL detection kit (Amersham).
Reverse transcriptase-polymerase chain reaction (RT-PCR).Total cellular RNA was isolated by the acid-guanidium thiocyanate-phenol–chloroform method. Single strand cDNA was synthesized from 1 μg total RNA using avian myeloblastosis virus RT and oligo(dT)12-18 primer (Pharmacia, Uppsala, Sweden) in a volume of 20 μL, as previously described. The cDNA preparation was then diluted to 100 μL. Two microliters of cDNA was amplified in a volume of 20 μL in the presence of 800 nmol/L 5′ and 3′ primers, 250 μmol/L dNTPs, 1 U Taq polymerase (TAKARA SHUZO, Otsu, Japan), and PCR buffer containing 10 mmol/L Tris-HCl (pH 8.3), 50 mmol/L KCl, and 2.5 mmol/L MgCl2 . The primers used for amplification of OX40 mRNA are forward primer (5′ TCTACAACGACGTGGTCA) and reverse primer (5′ CACAGTCAATCTCAGGCTTGTA). The primers (PRX3 and RPX4) were used to amplify HTLV-I pX(tax/rex) mRNA as previously described.20 PCR products were electrophoresed through 1.5% agarose gel, stained with ethidium bromide, and visualized on a UV transilluminator.
Cell adhesion assay.Cell adhesion assay was performed as described previously.7 In brief, HUVEC were plated onto gelatin-coated 96-well plates and cultured until confluence with or without prior exposure to 10 ng/mL of IL-1β (Otsuka Pharmaceutical Co) for 4 hours at 37°C. A total of 1 × 10551Cr-labeled ATL cells in the final volume of 60 μL were then added to each well. After being left for 15 minutes at 37°C, the wells were washed and the remaining adherent cells were lysed with 2% NP-40 in phosphate-buffered saline (PBS). One half of this volume was counted in a Top Count (Packard Instrument Company, Meriden, CT). To examine the effect of various MoAbs on the binding of ATL cells to IL-1–stimulated or unstimulated HUVEC, 51Cr-labeled ATL cells and HUVEC were incubated separately with MoAbs for 10 minutes at room temperature followed by preincubation with 5 mg/mL human IgG to block the nonspecific binding to Fc receptors. ATL cells were then added to the wells of HUVEC. All experiments were performed in triplicate.
Immunohistochemistry of skin biopsy specimens.Skin specimens were taken for biopsy from erythematous or papulous lesions of 4 patients who manifested skin involvement. They were embedded in Tissue-Tek OCT compound, and stored at −80°C until use. The serial cryostat sections (3 to 5 μm) were fixed in acetone. After air-drying, they were stained with control mouse IgG, OKT4, anti-Tac, and 315 MoAb, respectively using a DAKO PAP KIT (DAKOPATTS, Carpinteria, CA) according to the manufacturer's directions. All sections were counter-stained with Mayer's haematoxylin (Sigma, St Louis, MO) and mounted in glycerol gelatin.
RESULTS
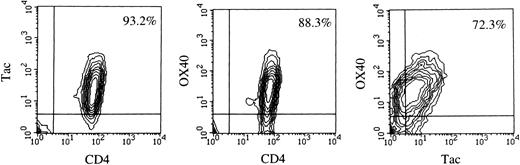
Flow cytometric analysis.Data of flow cytometry are summarized in Table 1. The majority of leukemic or LN cells in all of the cases examined were CD3+, CD4+, CD8−, Tac (IL-2 receptor α)+, which is the most common phenotype of ATL cells. Using 315 MoAb, we examined the expression of OX40 on these cells and found that fresh ATL cells from 15 of 17 cases expressed significant levels of OX40 without any stimulation, although its intensity varied among the cases. Dual color analysis clearly showed that most of the OX40+ cells were CD4+ and Tac+ (Fig 1), indicating that ATL cells expressed OX40. No apparent difference in OX40 expression was observed among the clinical subtypes of the disease or sources of the samples. In accordance with previous reports, we could not detect OX40 expression on fresh PBMC from normal individuals (data not shown). It is thus suggested that expression of OX40 is abnormally regulated in ATL, as is the case with Tac Ag/IL-2 receptor α.21 We could not detect expression of gp34, the ligand of OX40, on fresh ATL cells examined, although it is also known to be expressed preferentially on HTLV-I–infected T-cell lines.13-15
Two-color flow cytometric analysis of PBMC from case no. 13. Cells were first incubated with FITC-Leu3 (CD4) and biotin-anti–Tac (left), FITC-Leu3 and biotin-315 (OX40) (center), or FITC-anti–Tac and biotin-315 (OX40) (right), washed, and then incubated with PE-conjugated streptavidin. Samples were analyzed using a FACScan.
Two-color flow cytometric analysis of PBMC from case no. 13. Cells were first incubated with FITC-Leu3 (CD4) and biotin-anti–Tac (left), FITC-Leu3 and biotin-315 (OX40) (center), or FITC-anti–Tac and biotin-315 (OX40) (right), washed, and then incubated with PE-conjugated streptavidin. Samples were analyzed using a FACScan.
Cell lysates of fresh PBMC and PHA-stimulated PBMC (PHA-blasts, on day 3) from two donors, PBMC from case no. 13, LN cells from case no. 14, Jurkat, and Hut102 were mixed with SDS sample buffer, boiled for 5 minutes, and then applied to electrophoresis through a 7.5% polyacrylamide gel. Proteins were transferred to a sheet of PVDF membrane and the membrane was incubated with 10 μg/mL 315 MoAb. The binding of the MoAb was detected as described in Materials and Methods.
Cell lysates of fresh PBMC and PHA-stimulated PBMC (PHA-blasts, on day 3) from two donors, PBMC from case no. 13, LN cells from case no. 14, Jurkat, and Hut102 were mixed with SDS sample buffer, boiled for 5 minutes, and then applied to electrophoresis through a 7.5% polyacrylamide gel. Proteins were transferred to a sheet of PVDF membrane and the membrane was incubated with 10 μg/mL 315 MoAb. The binding of the MoAb was detected as described in Materials and Methods.
Western blot analysis.To examine if the Ag recognized by 315 MoAb on fresh ATL cells by flow cytometry is actually the OX40 protein, Western blot analysis was performed in two representative cases. As shown in Fig 2, a specific band with an apparent molecular weight (MW) of 50 kD was, present in PHA-stimulated normal PBMC (PHA-blasts), Hut102, but not in resting normal PBMC or Jurkat. A weak band with the same MW was clearly detected in both cases of ATL, indicating that these ATL cells express OX40 protein of the same MW, as has been described earlier. Since the same protein amount of the sample was loaded on each lane, it is assumed that the quantity of OX40 expressed on ATL cells is relatively low.
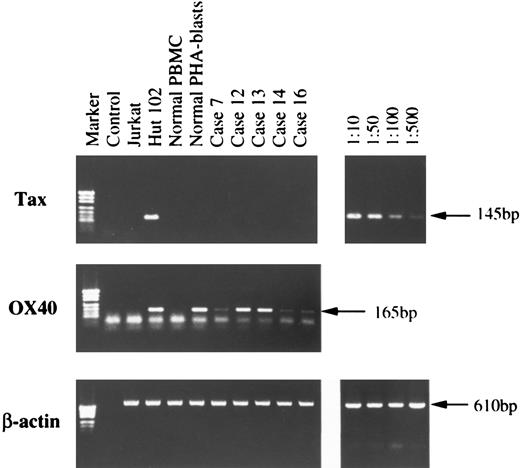
RT-PCR.Total RNA was isolated from fresh ATL cells and subjected to RT-PCR analysis for OX40, as well as HTLV-I viral mRNA expression. Corresponding to the results of flow cytometry and Western blot analysis, a unique PCR product band of OX40 was present in all five cases examined, Hut102, and PHA-blasts, but not in Jurkat or resting normal PBMC (Fig 3). Absence of OX40 mRNA in the latter two samples is not due to loss or degradation of mRNA, as RT-PCR of these using β-actin primers showed a specific band with similar intensity to the other samples. Thus, expression of OX40 is tightly regulated at the mRNA level and induced upon stimulation in normal T cells. Since HTLV-I infection, especially one of the virus products, p40tax, has been reported to induce OX40, as well as gp34 expression,15 we checked expression of HTLV-I mRNA by using primers designed to amplify processed tax/rex mRNA. We could not detect viral mRNA expression in any of the ATL cases examined under the conditions where viral mRNA could be amplified to a faint visible band from the mixed sample of 1 to 500 dilution of Hut102 cells in Jurkat cells (Fig 3, top).
Total cellular RNA was isolated by the acid-guanidium thiocyanate-phenol-chloroform method from Jurkat, Hut102, normal PBMC, normal PHA-blasts, and from PBMC or LN cells of five ATL cases. Single-strand cDNA synthesis and subsequent PCR reaction was done as described in Materials and Methods. PCR products were electrophoresed through 1.5% agarose gel, stained with ethidium bromide, and visualized on a UV-transilluminator. On the right, dilutions indicate the ratios of Hut102 cells to Jurkat cells.
Total cellular RNA was isolated by the acid-guanidium thiocyanate-phenol-chloroform method from Jurkat, Hut102, normal PBMC, normal PHA-blasts, and from PBMC or LN cells of five ATL cases. Single-strand cDNA synthesis and subsequent PCR reaction was done as described in Materials and Methods. PCR products were electrophoresed through 1.5% agarose gel, stained with ethidium bromide, and visualized on a UV-transilluminator. On the right, dilutions indicate the ratios of Hut102 cells to Jurkat cells.
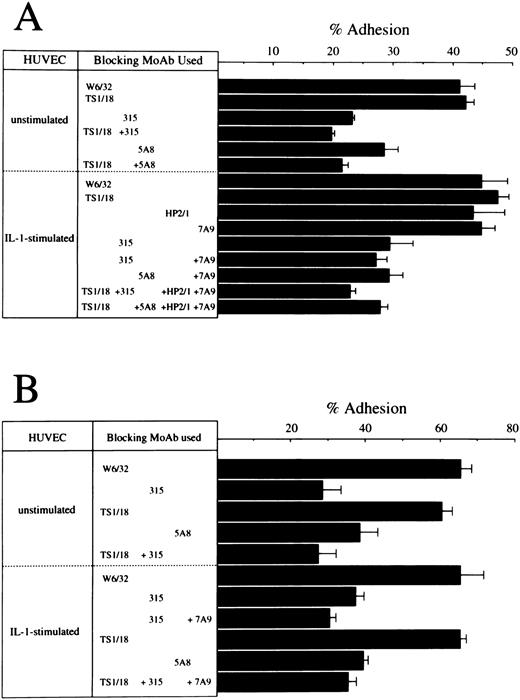
Cell adhesion assay.In a previous report, we demonstrated that the OX40/gp34 system directly mediates cell adhesion between activated or HTLV-I transformed T cells and HUVEC. So the next question was whether OX40 expressed on fresh ATL cells could play a role in adhesion of these cells to vascular endothelial cells. We performed cell adhesion assay with ATL cells from seven cases using HUVEC. Since the expression levels of OX40 on ATL leukemic cells were not so high as the relevant HTLV-I–infected T-cell lines, it was not possible to demonstrate specific binding through the OX40/gp34 system in all seven cases examined. However, in at least three cases, OX40-mediated adhesion was detected and representative experiments in two cases are shown in Fig 4. In both cases, ATL cells exhibit significant binding to resting HUVEC and this binding was inhibited by 315 (anti-OX40 MoAb) or 5A8 (anti-gp34 MoAb), but scarcely affected by TS1/18 (anti-LFA–1 MoAb). In case no. 9, binding of ATL cells to IL-1–activated HUVEC was also inhibited considerably by 315 or 5A8, but not TS1/18, HP2/1 (anti–VLA-4 MoAb) or 7A9 (anti-E–selectin MoAb). Combination of one or two of these MoAbs showed little additive effect to inhibition by 315 or 5A8 MoAb alone in this case. Similar results were obtained in case no. 14 in which additive effect of only 7A9 was examined. These data indicate that OX40 is involved in binding of ATL cells to HUVEC in at least some ATL cases, whether or not HUVEC are stimulated.
Effects of anti-LFA–1 MoAb, anti-VLA–4 MoAb, anti-E–selectin MoAb, anti-OX40 MoAb, and anti-gp34 MoAb on the binding of ATL cells to HUVEC. PBMC from case no. 6 (A) were preincubated with 10 μg/mL of W6/32 (anti-MHC class I MoAb), TS1/18 (anti-CD18 MoAb), 315 (anti-OX40 MoAb), or HP2/1 (anti-VLA–4 MoAb). HUVEC were also preincubated with 10 μg/mL of 7A9 (anti-E–selectin MoAb), or 5A8 (anti-gp34 MoAb). The binding to unstimulated or IL-1–stimulated HUVEC was then determined as described in Materials and Methods. A similar assay was done with PBMC from case no. 13 (B) except that HP2/1 MoAb was omitted.
Effects of anti-LFA–1 MoAb, anti-VLA–4 MoAb, anti-E–selectin MoAb, anti-OX40 MoAb, and anti-gp34 MoAb on the binding of ATL cells to HUVEC. PBMC from case no. 6 (A) were preincubated with 10 μg/mL of W6/32 (anti-MHC class I MoAb), TS1/18 (anti-CD18 MoAb), 315 (anti-OX40 MoAb), or HP2/1 (anti-VLA–4 MoAb). HUVEC were also preincubated with 10 μg/mL of 7A9 (anti-E–selectin MoAb), or 5A8 (anti-gp34 MoAb). The binding to unstimulated or IL-1–stimulated HUVEC was then determined as described in Materials and Methods. A similar assay was done with PBMC from case no. 13 (B) except that HP2/1 MoAb was omitted.
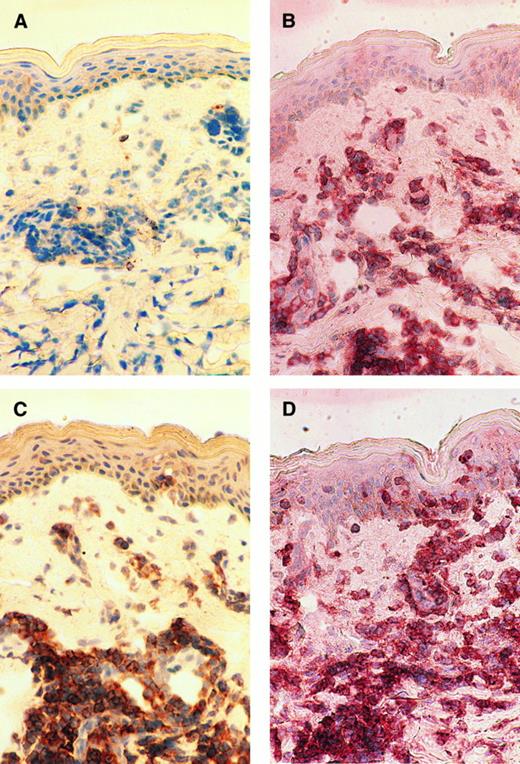
Immunohistochemical studies.Immunohistochemical examination was performed to see if OX40 was expressed on infiltrating ATL cells in vivo. Skin biopsy samples of the patients who manifested skin involvement were frozen, sliced onto the slide glasses, and examined by enzyme-linked immunostaining. We have so far examined four cases and found that infiltrating ATL cells express OX40 in all cases examined. A representative study of case no. 9 is shown in Fig 5. Massive mononuclear cell infiltration was observed in the upper dermis. The majority of the infiltrating cells were stained with both OKT4 and anti-Tac MoAb, indicating that these cells had the phenotype typical for ATL cells (Fig 5B and C). Staining of the serial section with 315 MoAb clearly showed that most of the infiltrating cells also expressed OX40 (Fig 5D).
Immunohistochemical findings of the skin biopsy from case no. 9. The serial cryostat sections of skin biopsy specimens fixed in acetone were stained with control mouse IgG (A), OKT4 (B), anti-Tac (C), and 315 MoAb (D). Mononuclear cells infiltrating into upper dermis are clearly positive for CD4, Tac (IL-2 receptor α), and OX40.
Immunohistochemical findings of the skin biopsy from case no. 9. The serial cryostat sections of skin biopsy specimens fixed in acetone were stained with control mouse IgG (A), OKT4 (B), anti-Tac (C), and 315 MoAb (D). Mononuclear cells infiltrating into upper dermis are clearly positive for CD4, Tac (IL-2 receptor α), and OX40.
DISCUSSION
In the present study, we have demonstrated that OX40 is expressed on fresh PBMC or LN cells from most, but not all ATL patients. Flow cytometric analysis showed that OX40 was predominantly expressed on CD4+ Tac+ cells. Considering that all the samples we examined contained more than 60% ATL cells based on morphology and surface phenotype, it is likely that most of the OX40+ cells we observed were ATL cells. It is possible that some normal activated T cells expressed OX40, but at most, constituted a minor population of OX40+ cells. There seems to be no apparent difference in OX40 expression among the clinical subtypes of the disease or sources of the samples. In Western blot analysis, a 50-kD protein band was detected by 315, an anti-OX40 MoAb, in two ATL cases examined, as well as PHA-blasts and Hut102, an HTLV-I–infected T-cell line, but not in resting PBMC or Jurkat. Furthermore, expression of OX40 mRNA was clearly visualized by RT-PCR in all ATL cases tested, PHA-blasts, and Hut102, but not in resting PBMC or Jurkat. Thus, fresh ATL cells make a contrast with normal PBMC that do not express detectable levels of OX40 without stimulation. Since expression of OX40 is thought to be strictly dependent on T-cell activation, it is suggested that the regulatory mechanism of OX40 expression may be deteriorated in ATL cells so that OX40 is constitutively expressed. A recent report described that OX40+ cells were occasionally detected in LN of some other types of T-cell lymphomas.22 In comparison to this, our data indicates that the frequency of OX40 positive cases in ATL is extraordinarily high.
During preparation of this report, a paper has been published indicating that HTLV-I tax protein can induce OX40 gene expression by the transacting mechanism.23 Although little is known about the regulatory mechanism of OX40 gene expression, it is implied that p40tax-mediated activation of transcription factors such as NF-κB and CREB may, as well, be involved in this abnormal expression of OX40. HTLV-I p40tax has also been reported to induce expression of gp34, the ligand of OX40, that is known to be preferentially expressed on HTLV-I–infected T-cell lines.15 Unlike OX40, however, expression of gp34 was not detectable on the fresh ATL cells we examined. RT-PCR analysis showed that ATL cells did not express detectable amounts of viral mRNA including tax. We confirmed the absence of p40tax expression in these cells also by Western blotting and immunofluorescence staining using Lt-4, an anti-p40tax MoAb24 (data not shown). Accordingly, it may be that expression of gp34 is strictly dependent on p40tax whereas the p40tax-mediated mechanism might not be essential for the constitutive expression of OX40 and Tac/IL-2 receptor α in fully transformed ATL cells. In this context, OX40 negative ATL cases seem to be interesting because they might represent a distinct regulatory mechanism of OX40 expression that is more dependent on viral expression. The difference between OX40 positive and exceptional OX40 negative ATL is to be analyzed in more detail in terms of gene regulation, as well as clinical features.
ATL patients often manifest infiltration of leukemic cells into various organs such as LN, liver, spleen, lung, skin, and intestinal tract, which occasionally causes poor prognosis of the disease. To elucidate the mechanism of leukemic cell infiltration, we attempted to define the molecules that were involved in cell adhesion between ATL cells, as well as HTLV-I–infected T-cell lines and endothelial cells. During the process of this investigation, we encountered OX40 and found that the OX40/gp34 system directly mediates cell adhesion between activated or HTLV-I–transformed T cells and HUVEC.7 It was important, therefore, for us to address the question if OX40 constitutively expressed on ATL cells can contribute to cell adhesion and eventually to organ infiltration of these cells. We performed adhesion assays using HUVEC and demonstrated that OX40 was involved in binding of ATL cells to HUVEC in at least three cases. It is noted that these ATL cells adhered to unstimulated, as well as IL-1–stimulated, HUVEC predominantly through the OX40/gp34 system, in which the involvement of the other adhesion molecules such as LFA-1, VLA-4, and E-selection seemed to be limited. As shown in the Western blot analysis, the amount of OX40 expressed on ATL cells was rather small compared with PHA-blasts or certain HTLV-I–infected T-cell lines, which presumably made it difficult to detect contribution of the OX40/gp34 system in all the cases examined. However, data with the three cases clearly indicate that OX40 expressed on ATL cells is functionally competent to mediate cell adhesion.
The immunohistochemical studies showed that infiltrating ATL cells in the skin expressed OX40 in vivo. It is known that adherence of lymphocytes to vascular endothelial cells is the first and limiting step for their extravasation leading to organ infiltration. Taken together with the data of cell adhesion assay, it is strongly suggested that OX40 is involved in cell adhesion and eventually in organ infiltration of ATL cells at least in some cases. Further studies are needed to determine whether or not any relationship exists between the level of OX40 expression and leukemic cell infiltration. As to the meaning of OX40 expression in ATL, cell adhesion and organ infiltration are not the only implication. Since it is known that OX40 transmits costimulatory signals into T cells upon binding of its ligand, gp34,16 17 OX40-mediated intracellular signals in ATL cells should also be deciphered in regard to cell activation and proliferation. With its functional multiplicity combining cytokine receptor, costimulatory molecule, and adhesion molecule in itself, OX40 may facilitate survival of ATL cells in microenvironments in vivo.
ACKNOWLEDGMENT
The authors thank Drs H. Sawada (Kokura Memorial Hospital, Kitakyushu), T. Ishikawa (Kobe City General Hospital, Kobe), N. Arima (Osaka Red Cross Hospital, Osaka), T. Kodaka (Shinko Hospital, Kobe), R. Onishi (Kitano Hospital, Osaka), and T. Nagai (Tsukaguchi Hospital, Amagasaki) for providing materials from the patients; K. Fukunaga, C. Fujiwara, and H. Komoda for technical assistance.
Supported in part by grants-in-aid from the Ministry of Education, Science and Culture of Japan.
Address reprint requests to Takashi Uchiyama, MD, Institute for Virus Research, Kyoto University, 53 Shogoin-Kawaracho, Sakyo, Kyoto 606, Japan.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal