To the Editor:
A 32-year-old woman of Greek origin was referred to the hospital for evaluation of fatigue and symptomatical anemia. The anemia was documented 10 years ago during her first pregnancy. During the last 10 years the patient received an unknown number of red blood cell transfusions. Otherwise, the medical history was unremarkable. There was no family history of anemia. Physical examination showed moderate hepato-splenomegaly. No jaundice was found.
The initial hemoglobin (Hb) concentration was 7 g/dL. Serum ferritin was 1,600 μg/L. The bone marrow cytology demonstrated hypercellularity and erythroid hyperplasia (E/G ratio was 1/0.75). Binuclear and polynuclear erythroblasts were the predominant morphologic features. A normal number of mature megakaryocytes and orderly myeloid maturation was present. Marrow iron was increased and pathological sideroblasts could not be observed. The incorporation of 59Fe(II) into erythrocytes was low, at a level of 44% (normal range, 70% to 90%). Intestinal iron absorption [measured after administration of a test dose of 10 μmol 59Fe(II) by whole body counting] was increased to 77% after 14 days (normal range, 10% to 50%). The liver-iron content amounted to 3.3 mg Fe per gram of liver tissue (wet weight; normal range, 0.1 to 0.5 mg/g).
Liver iron concentration was measured using a SQUID (Super Conducting Quantum Interference Device) biomagnetometer (Ferritometer; Bti, San Diego, CA) as described elsewhere.1,2 For measurement, the patient is lowered in a known, constant magnetic field (Bmax = 20mT, gradiometer first order) and the magnetic flux change versus a water reference medium is detected by two second order gradiometer pickup coils. The individual liver volume is assessed by sonography and the actual total liver iron is calculated from the respective liver iron concentration and liver volume.2
Increased agglutination with anti-i exceeding that on cord red blood cells was not observed. Based on these findings, a congenital dyserythropoietic anemia (CDA) type II was diagnosed.
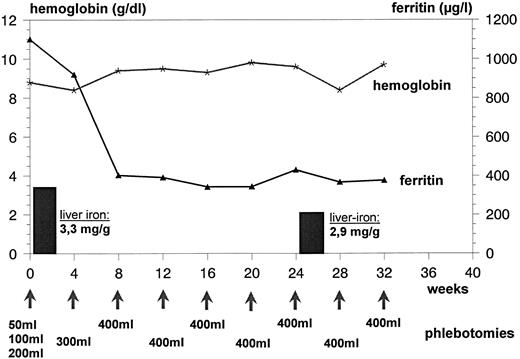
For treatment of the iron overload, phlebotomy was started despite the severe anemia with an amount of 50 mL of removed blood in week 1, followed by a stepwise increase to 100 mL, 200 mL, and 300 mL of blood in weeks 2, 3, and 4. This procedure was followed by phlebotomies of 400 mL blood every 4 weeks for 6 months. After 3 months, a considerable improvement of erythropoiesis could be observed. Hb levels increased to between 9 and 10 g/dL. Serum ferritin levels decreased to 380 μg/L. After 6 months of phlebotomy therapy (total amount, 2,900 mL of blood), the liver iron concentration was reduced to 2.9 mg Fe/g liver tissue (Fig 1).
Hb and serum ferritin concentrations during phlebotomy in the patient suffering from CDA II. Liver iron concentration (per gram wet weight) as measured by biomagnetometry is given in bars. Stepwise increase of removed blood in weeks 1 through 4 (see text).
Hb and serum ferritin concentrations during phlebotomy in the patient suffering from CDA II. Liver iron concentration (per gram wet weight) as measured by biomagnetometry is given in bars. Stepwise increase of removed blood in weeks 1 through 4 (see text).
CDAs are rare abnormalities of the red blood cell characterized by ineffective erythropoiesis and a variable anemia. On the basis of morphologic features, Heimpel and Wendt3 classified these disorders into three types. In 1971, an additional subtype was defined by McBride4 using serological findings. Type II, which is the most frequent type, with more than 100 reported cases, often is called hereditary erythroblastic multinuclearity associated with a positive acidified serum lysis test (HEMPAS), as described by Crookston et al.5 In vitro cultures of bone marrow cells from patients with CDA II showed a defective growth of the earliest commited erythroid progenitors, the erythroid burst-forming cells (BFU-E).6 Abnormalities in glycosylation of erythrocyte membrane proteins and lipids are also described.7 Furthermore, a deficiency of erythrocyte membrane CD44 was detected.8 The diagnosis of CDA II should be considered on bone marrow examination when erythroid hyperplasia and multiple binuclear and multinuclear erythroid cells are found.6 9 The iron incorperation into erythrocytes is significantly decreased, indicating a high grade of ineffective erythropoiesis.
CDA usually has a relatively benign course. Iron overload due to transfusion requirements may become a problem in patients with CDA, mostly in CDA type II.10 11 Additionally increased intestinal iron absorption due to the ineffectiveness of the erythropoiesis in CDA II in transfusion independent patients may contribute to iron overload [reported case: distinct increase to 77% of 59Fe(II) test dose absorption].
Irrespective of the cause of iron overload, patients who are not anemic can usually be treated effectively by phlebotomy,12 whereas patients with iron-loading anemias such as CDA usually require an iron chelation therapy. The clinical use of desferrioxamine with daily subcutaneous injections required is cumbersome.12 Orally active iron chelating compounds (such as 1,2-dimethyl-3-hydroxypyridin-4-one, also known as L1 or CP20) with, in animals, documented side effects such as bone marrow toxicity and teratogenicity13 are not yet available for clinical use.
To mobilize the surplus tissue iron, a careful phlebotomy treatment was started in our patient despite the severe anemia. The procedure was well tolerated, and the Hb concentration was relatively constant between 7 and 8 g/dL during the first 3 months of treatment. No increase of anemia-related symptoms was noted by the patient. After 3 months, an improvement of effective erythropoiesis was noticed, as indicated by an Hb increase up to 10 g/dL (Fig 1). There was a good correlation between the quantity of blood removed (2,900 mL corresponding to 1,200 mg Fe) and the measured decrease of liver iron concentration (loss of approximately 1,100 mg Fe).
We conclude that regular phlebotomy may be well tolerated in patients with iron-loading anemias such as CDA. It seems to be possible to correct serious iron overload in both transfused and nontransfused CDA patients and to prevent these patients from late complication of secondary hemochromatosis. In addition, there was also a considerable improvement of the erythropoiesis by a yet unknown mechanism.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal