Abstract
Ectopic expression of the erythropoietin receptor (EpoR) in the interleukin-3 (IL-3)–dependent cell line Ba/F3 results in growth and partial erythroid differentiation in Epo. In contrast, introduction and activation of the interleukin-5 receptor (IL-5R) or of the granulocyte-macrophage colony-stimulating factor receptor (GM-CSFR) results in proliferation only. As this effect is specific to the EpoR, the role of its extracellular or cytoplasmic domain in differentiation was tested after construction of two chimeric receptors. One receptor contained the extracellular domain of EpoR fused to the endodomain of IL-3R β-chain (E/β), while the other contained the EpoR cytoplasmic region fused to the extracellular domain of GM-CSFR α-chain (GMER). Surprisingly, both receptors induced differentiation ruling out a strict specificity of the extracellular or cytoplasmic region of EpoR in this process. Instead the ability to signal differentiation correlated with structural features shared by the EpoR, GMER, and E/β receptors. Dimerization of all three receptors results in the pairing of two signal transducing chains in the cytoplasm, in contrast to the mitogenic receptors IL-3R, IL-5R, GM-CSFR, which assemble as αβ heterodimers. Two new chimeric receptors that fulfilled the structural requirement exemplified by EpoR, but lacked any part of EpoR, were designed to consolidate this model. They consisted of the ectodomains of the GMR-α and IL-5Rα, respectively, fused to the endodomain of IL-3R β-chain. Both receptors were as effective as EpoR in signaling differentiation in response to their cognate ligand. Another property of receptors fulfilling these structural requirements is that they cause a marked delay in signal transducers and activators of transcription 5 (STAT5) activation on ligand stimulation. Taken together our studies show that structural assembly of receptors dictates their potential to signal erythroid differentiation in Ba/F3 cells, that differentiation can take place in the absence of Epo and that a delay in STAT5 activation is highly predictive of this process.
THE ERYTHROPOIETIN receptor (EpoR) is a member of the hematopoietic growth factor receptor family expressed on hematopoietic progenitor cells and committed erythroid precursor cells. Under the influence of Epo, progenitor cells first undergo a phase of pronounced expansion followed by a period of differentiation. Although multiple experiments show the pivotal role of the EpoR for growth,1-5 questions remain about the role of the Epo/EpoR interaction in differentiation. One line of evidence consistent with a stochastic model for erythroid differentiation, suggests that the EpoR has a marginal role in promoting differentiation, serving merely as a survival factor,6,7 whereas another, supporting an instructive model, claims that expression and activation of the EpoR is required for differentiation of late progenitor cells.8-11
To help define the role of the EpoR in differentiation, we took advantage of a model system provided by Ba/F3 cells.8,9 This strictly interleukin-3 (IL-3) dependent, murine progenitor cell line proliferates and undergoes limited erythroid differentiation in Epo on ectopic expression of the EpoR.10 11 Although this observation was consistent with the instructive model, it did not address two critical points. Is EpoR-mediated differentiation of Ba/F3 cells specific to this receptor, or is it merely a trivial consequence of the ectopic expression in Ba/F3 cells of a hematopoietic receptor other than the endogenous IL-3 receptor IL-3R? Second, if the EpoR differs from other receptors in its ability to support both proliferation and differentiation of Ba/F3 cells, what accounts for its unique specificity?
Initial studies with truncation mutants of the EpoR had failed to dissociate proliferation from differentiation.12 Furthermore, studies of chimeric receptors containing either the ectodomain or endodomain of the EpoR gave conflicting results: both the extracellular and the endodomain of the EpoR contributed independently to differentiation of Ba/F3 cells.12-14 As attempts to identify a differentiation-specific domain of the EpoR had remained uninformative, we decided to approach this question differently and investigate whether structural features in receptor assembly, characteristic of the EpoR and of the differentiation-competent chimeric receptors, might not provide an insight to their specificity. Indeed, structural differences in the assembled chains in the cytoplasm might lead to qualitative or quantitative differences in signal transduction, and thus explain why certain receptors primarily mediate proliferation while others support both proliferation and differentiation.
Although hematopoietic growth factor receptor subunits lack a cytoplasmic kinase activity, rapid tyrosine phosphorylation events follow activation of the receptor.15,16 In the case of the EpoR, the JAK2 kinase phosphorylates the EpoR itself,17,18 as well as signal transducers and activators of transcription (STAT) transcription factors.19,20 The integrity of the JAK/STAT pathway seems to be crucial for the proliferative response induced by hematopoietic growth factor receptors.21 Among the various STAT proteins identified, STAT5 (which exists as two isoforms, STAT5A and STAT5B22,23 ) is thought to be important in the proliferation of hematopoietic cell lines in response to IL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF ), IL-5, and Epo.24 25
In this report, we show that differentiation induced by the EpoR is a specific event, which cannot be achieved by signaling through other physiologic receptors such as the GM-CSF receptor (GMR) or the IL-5R. Furthermore, we present evidence that the cytoplasmic pairing of two signal transducing receptor chains is sufficient to promote differentiation of Ba/F3 cells. This observation reconciles all the previous findings and suggests that erythroid differentiation can be signaled by receptors containing no part of the EpoR. We also found an inverse correlation between early STAT5 activation and β-globin induction. Stimulation of differentiation-competent receptors led to a marked reduction in STAT5 binding, particularly in the early time points. This difference was not observed with other members of the STAT family. Our experiments offer biochemical evidence that EPO signaling in Ba/F3 cells is different from that of IL-3, GM-CSF, and IL-5. Furthermore they show that the status of early STAT5 activation can be used as a marker of erythroid differentiation in Ba/F3 cells.
MATERIALS AND METHODS
Cells
Ba/F3 cells8 were grown in a humidified incubator at 37°C with 5% CO2 in growth medium (RPMI 1640 medium supplemented with 10% fetal calf serum [FCS], penicillin [50 U/mL], streptomycin [50 μg/mL], glutamine [2 mmol/L], and murine rIL-3 [R&D, Minneapolis, MN] at 0.5 ng/mL).
Transfections
DNA constructs (25 μg) were stably introduced into 107 Ba/F3 cells by electroporation using a BioRAD apparatus set (Hercules, CA) at 300 V and 960 μF. Each construct was electroporated at least twice. Forty-eight hours after electroporation, cells were replated in growth medium supplemented with G418 (1 mg/mL). G418 resistant cells were kept in medium with IL-3 and G418 or switched to a given cytokine at the indicated concentration: murine rGM-CSF (R&D): 1 ng/mL; murine rIL-5 (R&D): 1 ng/mL; human rEpo: 0.5 U/mL.
Constructs
EpoR.The EpoR cDNA has been described.26
E/β.A BglII site was introduced over the codons encoding the 10th and 11th amino acid (a.a.) of the cytoplasmic region of murine βIL3 cDNA,27 and used to join in frame EpoR DNA (encoding a.a. 1 to 282) with βIL3 DNA (encoding a.a. 476 to 882) (see Fig 1).
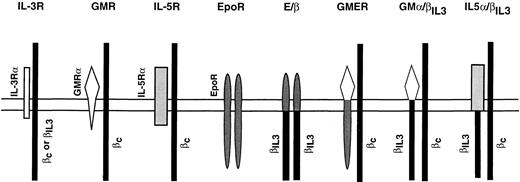
Schematic of the wild-type and chimeric receptors introduced into Ba/F3 cells. All receptor cDNAs were subcloned into the expression vector pLXSN, which contains a neomycin resistance gene as a selectable marker.30 Receptor complexes are shown as dimers. The endogenous βc chain is represented by a thin, black rectangle.
Schematic of the wild-type and chimeric receptors introduced into Ba/F3 cells. All receptor cDNAs were subcloned into the expression vector pLXSN, which contains a neomycin resistance gene as a selectable marker.30 Receptor complexes are shown as dimers. The endogenous βc chain is represented by a thin, black rectangle.
GMR-α.A cDNA for the GM-CSF receptor α-chain (GMR-α) was obtained by reverse transcriptse-polymerase chain reaction (PCR) of polyA+ RNA isolated from MKVS cells. MKVS is a murine myeloid cell line isolated after infection of Balb/c bone marrow cells with a retrovirus containing the v-myc and v-src oncogenes (B.M.-P., unpublished results, September 1988).
GM-CSFR α-chain (GMER).The chimeric receptor has a.a. 1 to 325 of the GMR-α chain fused to a.a. 250 to 571 of the EpoR (see Fig 1). The in-frame fusion was obtained using the gene splicing by overlap extension method.28
GMR-α/βIL3 . The chimeric receptor consists of a.a 1 to 325 of the GMR-α chain fused to a.a. 420 to 882 of βIL3 (see Fig 1). Construction of the bipartite cDNA was aided by PCR technology.
IL-5Rα.The cDNA was a kind gift of Dr Tavernier (Hoffmann LaRoche, Gent, Belgium).29
IL-5α/βIL3 . The chimeric receptor (see Fig 1) consists of a.a. 1 to 361 of the IL-5Rα chain fused to a.a. 466 to 882 of βIL3 using a strategy similar to that described above. All PCR-derived fragments in the above constructs were sequenced to confirm the integrity of the fusion and to rule out any additional unwanted mutations. cDNAs (wild-type or chimeric) were subcloned into the retroviral expression vector pLXSN, which contains the selectable marker neoR gene under the control of the SV40 promoter.30
RNA, Northern Blots, and RNase Protection Assays
RNA was isolated from cells grown in IL-3 or in the indicated cytokine, using TRIzol Reagent (GIBCO-BRL, Gaithersburg, MD) according to the manufacturer's recommendations.
Northern blot assay.RNA samples (15 μg) were run on a 1% agarose formaldehyde gel, transferred onto a nylon membrane (Duralon; Stratagene, La Jolla, CA), and hybridized in QuickHyb Solution (Stratagene) for 2 hours at 68°C. Blots were washed twice in 2 × saline sodium citrate /0.1% sodium dodecyle sulfate at room temperature for 15 minutes. Probes were 32P-labeled using the random primer method. Murine β-globin and (GAPDH) glyceraldehyde-3-phosphate dehydrogenase probes have been described.12
RNase protection.RNA samples (25 μg) were analyzed as previously described.31 The following probes were used: IL-5Rα riboprobe: the 169-base pair (bp) fragment (HincII-BglII) spanning the transmembrane region of the IL-5Rα chain cDNA was cloned into the Bluescript KS vector. Antisense riboprobe derived from that fragment was generated using T7 RNA polymerase. GMR-α riboprobe: Genomic DNA was used to amplify a GMR-α specific probe, directed against the 3′ coding region of the cDNA. A 110-bp fragment, containing 50 bp of the penultimate coding exon of GMR-α and extending into the following intron up to a Sty I site, was subcloned into Bluescript KS (EcoRI/Sma I). Antisense probe was generated with T7 polymerase. EpoR riboprobe: A 90-bp fragment from the BglII site to the 3′ Pst I site of the EpoR cDNA was subcloned into SP72 (BglII/Pst I). Antisense probe was generated with SP6 polymerase.
Dose Response Assays
The XTT reduction assay (Sigma, St Louis, MO) was performed as described.12 Each dose response curve was done three times in triplicate.
Nuclear Extracts and Electrophoretic Mobility Shift Assays
Cells were washed three times in phosphate-buffered saline (PBS) and starved in the absence of cytokines for 6 hours in RPMI 1640. Cells were then stimulated for 10 minutes with the indicated cytokine at a concentration used in growth medium (see above). Nuclear extracts were prepared as previously described.32 Oligonucleotides used were (top strand is indicated): β-casein promoter element: AGATTTCTAGGAATTCAAATC33; hSIE probe: GCATTTCCCGTAAATCC34; unrelated oligonucleotide: GATCCTCTCACCTTCTGCTAG. Nuclear extracts were incubated in binding buffer (13 mmol/L HEPES [pH 7.9], 65 mmol/L NaCl, 1 mmol/L DTT, 0.15 mmol/L EDTA, 8% Glycerol, and 1 μg of poydIdC)35 in the presence or absence of excess unlabeled competitor (40 ng/μL) before addition of the 32P-labeled oligonucleotide probe. Amounts of nuclear extracts were normalized on a per microgram of protein basis. Samples were electrophoresed on nondenaturing polyacrylamide gels, in 0.5 × (TBE) Tris/Borate/EDTA buffer.
Stat5 antiserum.Stat5 antiserum was raised in rabbits against a.a. 687 to 794 of ovine Stat5.
STAT6 antiserum.Polyclonal rabbit antiserum against Stat6 was raised in rabbits against a.a. 633 to 837 of murine Stat6. For supershift experiments, 1 μL of a 1:10 dilution of the crude antiserum was added to the reaction and incubated for 20 minutes on ice, before adding the radiolabeled oligonucleotide.
Flow Cytometry
Two-hundred thousand cells grown in IL-3 were used for each condition. Cells were washed in PBS containing 0.2% FCS and 0.02% NaAzide, and incubated with the first antibody for 30 minutes at 4°C. As isotype control, a polyclonal rabbit antimouse (Southern Biotech, Birmingham, AL) was used. Following two washes with PBS, cells were then incubated with the secondary antibody (biotinylated goat antirat or antimouse) for 30 minutes at 4°C. After two more washes, fluorescein isothiocyanate-labeled Streptavidin was added (30 minutes, 4°C), and cells were analyzed on a FACScan (Becton Dickinson & Co, San Jose, CA). Each experiment was performed three times.
RESULTS
The EpoR But Not the GMR Nor the IL-5R Signals Erythroid Differentiation in Ba/F3 Cells
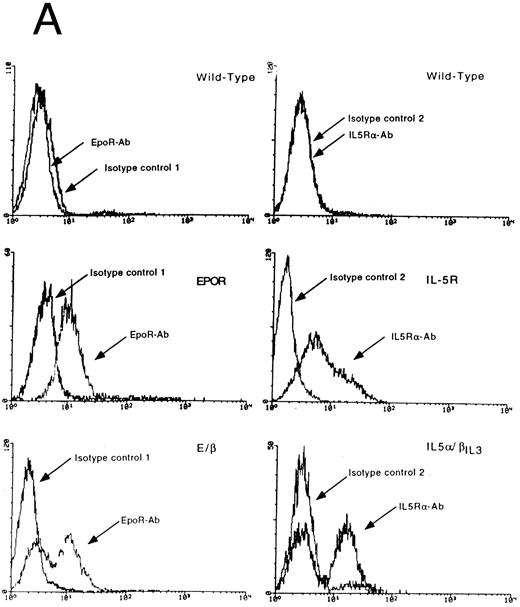
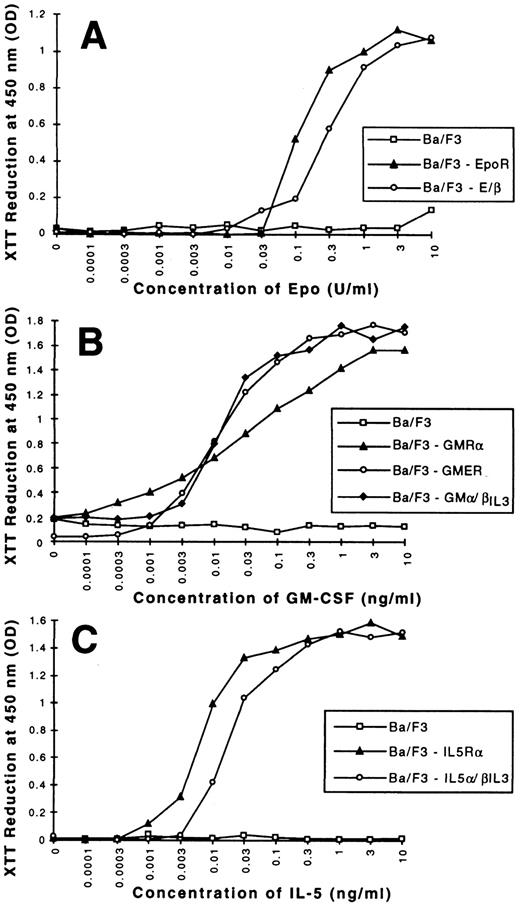
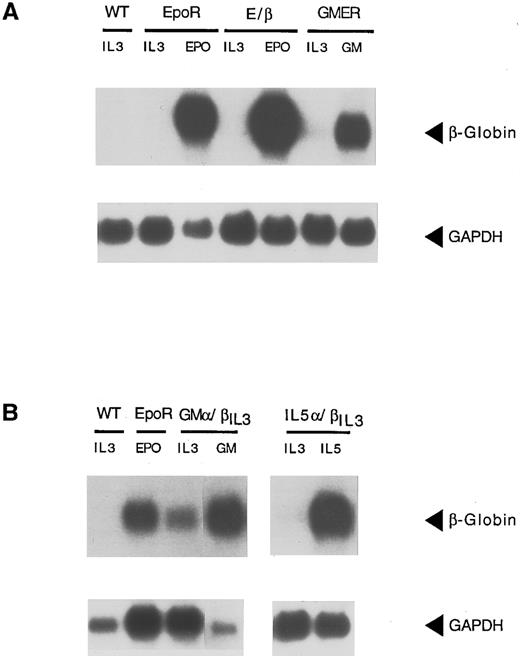
We first wanted to clarify whether β-globin production in Ba/F3 cells is a specific differentiation event in response to signaling through the EpoR or whether it is a general response of Ba/F3 cells grown in a factor other than IL-3. To this end, we subcloned the murine GM-CSFRα (GMR-α), IL-5Rα, and EpoR (shown schematically in Fig 1) into the pLXSN expression vector and introduced them independently into Ba/F3 cells. After neomycin selection, cells were maintained in IL-3 or switched to medium containing GM-CSF (1 ng/mL), IL-5 (1 ng/mL), and Epo (0.5 U/mL), respectively. In all cases, cells proliferated in the new cytokine. Ten days later, total RNA was isolated and probed for β-globin (Fig 2). As previously reported,10 Ba/F3 cells, as a population, are negative for β-globin mRNA (lane 1). On occasion, a low background level of β-globin can be detected in isolated clones, as reflected by some of the transfectants grown in IL-3 (Fig 2) The same low level can be seen in cells transfected with the pLXSN vector alone (data not shown). As expected, growth of Ba/F3-EpoR cells in Epo leads to a high level of β-globin expression. In contrast, activation of the GM-CSF or IL-5 receptors by their respective ligands results in little or no induction of β-globin above background. Levels were consistently 10- to 100-fold lower than those observed in cells expressing the EpoR. Identical findings were observed in three independent transfections (data not shown). Northern blot analysis confirmed equivalent amount of the three ectopically expressed receptor chain mRNAs (data not shown) and fluorescence-activated cell sorting (FACS) analysis confirmed surface expression for EpoR and IL-5Rα (Fig 3A). Lastly, all three receptors signaled proliferation in a dose dependent fashion and at comparable rates (Fig 4A through C). Thus, the difference in β-globin expression observed between Ba/F3-EpoR cells and parental or GM-CSF and IL-5 receptor bearing Ba/F3 cells is a specific response to the activation of the EpoR and is not due to gross differences in receptor expression or rates of proliferation, nor can it be attributed to the removal of IL-3 from the growth condition.
Unlike the EpoR, GMR or IL-5R do not signal differentiation in Ba/F3 cells. Ba/F3 cells WT and Ba/F3 cells transfected with the indicated receptor chains EpoR, GMR-α, and IL-5Rα were grown in IL-3 (0.5 ng/mL) and G418, or in Epo (0.5 U/mL), GM-CSF (1 ng/mL), and IL-5 (1 ng/mL), as shown above each lane. Total RNA was isolated after 10 days, and probed for β-globin expression. Fifteen micrograms was analyzed in each lane. Loading efficiency was controlled by hybridization of the same blot with a GAPDH probe (shown below).
Unlike the EpoR, GMR or IL-5R do not signal differentiation in Ba/F3 cells. Ba/F3 cells WT and Ba/F3 cells transfected with the indicated receptor chains EpoR, GMR-α, and IL-5Rα were grown in IL-3 (0.5 ng/mL) and G418, or in Epo (0.5 U/mL), GM-CSF (1 ng/mL), and IL-5 (1 ng/mL), as shown above each lane. Total RNA was isolated after 10 days, and probed for β-globin expression. Fifteen micrograms was analyzed in each lane. Loading efficiency was controlled by hybridization of the same blot with a GAPDH probe (shown below).
Expression of transfected wild-type and chimeric receptors but not of endogenous EpoR, GMR-α and IL-5Rα in Ba/F3 clones. (A) Surface expression of EpoR and E/β receptors (left-hand side) or IL-5Rα and IL-5α/βIL3 receptors (right-hand side). 2 × 105 cells were treated as described in Materials and Methods and processed for FACS analysis. The type of cells and the antibodies used are indicated in each panel. Anti-EpoR antibody: antibody 8866; isotype control 1: polyclonal rabbit antimouse. Anti-IL-5Rα antibody: antibody 20H9; isotype control 2: polyclonal rat antimouse IgG2b. A representative sample of 3 independent experiments is shown. (B) Detection of 3′ specific EpoR, GMR-α, and IL-5Rα sequences, respectively. Twenty-five micrograms of total RNA was analyzed in each lane. Probes are described in Materials and Methods. Migration of each protected fragment agrees with the expected size and is indicated by an arrow. Top panel: RNase protection assay for EpoR RNA sequences. M: DNA markers (pBR322 Msp I digest); t: tRNA. WT: Ba/F3 cells; EpoR: Ba/F3-EpoR cells; E/β: Ba/F3-E/β cells. Middle panel: RNase protection assay for GMR-α RNA sequences. t: tRNA; WT: Ba/F3 cells; GMR-α: Ba/F3-GMR-α cells; GMER: Ba/F3-GMER cells; GMα/βIL3 : Ba/F3-GMα/βIL3 . Bottom panel: RNase protection assay for IL-5Rα RNA sequences. M; DNA markers; t: tRNA; WT: Ba/F3 cells; IL-5Rα: Ba/F3-IL-5Rα; IL-5α/βIL3 : Ba/F3-IL-5α/βIL3 .
Expression of transfected wild-type and chimeric receptors but not of endogenous EpoR, GMR-α and IL-5Rα in Ba/F3 clones. (A) Surface expression of EpoR and E/β receptors (left-hand side) or IL-5Rα and IL-5α/βIL3 receptors (right-hand side). 2 × 105 cells were treated as described in Materials and Methods and processed for FACS analysis. The type of cells and the antibodies used are indicated in each panel. Anti-EpoR antibody: antibody 8866; isotype control 1: polyclonal rabbit antimouse. Anti-IL-5Rα antibody: antibody 20H9; isotype control 2: polyclonal rat antimouse IgG2b. A representative sample of 3 independent experiments is shown. (B) Detection of 3′ specific EpoR, GMR-α, and IL-5Rα sequences, respectively. Twenty-five micrograms of total RNA was analyzed in each lane. Probes are described in Materials and Methods. Migration of each protected fragment agrees with the expected size and is indicated by an arrow. Top panel: RNase protection assay for EpoR RNA sequences. M: DNA markers (pBR322 Msp I digest); t: tRNA. WT: Ba/F3 cells; EpoR: Ba/F3-EpoR cells; E/β: Ba/F3-E/β cells. Middle panel: RNase protection assay for GMR-α RNA sequences. t: tRNA; WT: Ba/F3 cells; GMR-α: Ba/F3-GMR-α cells; GMER: Ba/F3-GMER cells; GMα/βIL3 : Ba/F3-GMα/βIL3 . Bottom panel: RNase protection assay for IL-5Rα RNA sequences. M; DNA markers; t: tRNA; WT: Ba/F3 cells; IL-5Rα: Ba/F3-IL-5Rα; IL-5α/βIL3 : Ba/F3-IL-5α/βIL3 .
Dose-response curves of Ba/F3 cell clones. 5 × 104 cells were washed three times in PBS and starved for 6 hours in RPMI 1640 medium containing 10% FCS, and then stimulated with the indicated concentrations of Epo (A), GM-CSF (B), IL-5 (C). After 60 hours, XTT was added and the absorbance at 450 nm determined. RPMI medium was used as a blank. Each experiment was done in triplicates; values shown are means. Identical curves were observed in two other independent experiments.
Dose-response curves of Ba/F3 cell clones. 5 × 104 cells were washed three times in PBS and starved for 6 hours in RPMI 1640 medium containing 10% FCS, and then stimulated with the indicated concentrations of Epo (A), GM-CSF (B), IL-5 (C). After 60 hours, XTT was added and the absorbance at 450 nm determined. RPMI medium was used as a blank. Each experiment was done in triplicates; values shown are means. Identical curves were observed in two other independent experiments.
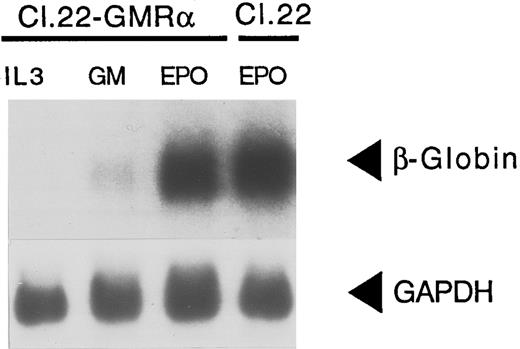
The difference in β-globin expression detected between Ba/F3-EpoR, Ba/F3-GMR, and Ba/F3-IL-5R cells was a consistent finding, as multiple clones isolated from independent electroporations were analyzed with identical results. In addition, varying GM-CSF and IL-5 concentrations over a 50-fold range (0.1 to 5 ng/mL) did not affect β-globin expression by Ba/F3-GMR and Ba/F3-IL-5R cells. This was in contrast to what was observed in Ba/F3-EpoR cells, where lowering Epo concentration enhanced the expression of β-globin mRNA (data not shown).36 To examine whether introduction of the GMR-α chain in Ba/F3 cells followed by growth in GM-CSF might select for a population of cells that have lost the capacity to differentiate, we transfected a cDNA for the GMR-α into Ba/F3-EpoR (clone 22) cells,36 which express the EpoR ectopically. Cells were maintained in IL-3 before electroporation and then directly selected in GM-CSF (1 ng/mL). Cells were either maintained in this cytokine, switched back to IL-3 (0.5 ng/mL) or switched to Epo (0.5 U/mL). Cells proliferated well in all three cytokines, with comparable kinetics (data not shown). There was no β-globin expression when the cells were grown in IL-3 (Fig 5). GM-CSF induced a low level of β-globin in clone 22-GMR-α (Fig 5), which is similar to that observed in Ba/F3 cells transfected with GMR-α (Fig 2). However, when clone 22-GMR-α cells were grown in Epo, there was a marked increase in β-globin expression (Fig 5). Therefore, the low level of β-globin observed in GM-CSF is not because of an inability of these cells to express a high level of β-globin mRNA, but reflects a minimal degree of differentiation caused by GMR activation.
Inability of GMR-α to signal differentiation is not caused by clonal restriction. Clone 22 (Cl 22), a Ba/F3 clone that expresses the EpoR ectopically was transfected with GMR-α cDNA (Cl 22-GMR-α). After selection in GM-CSF, cells were replated in IL-3, GM-CSF, and Epo, respectively. RNA was obtained and hybridized with a β-globin probe as described. Cytokines used to grow cells are indicated above each lane.
Inability of GMR-α to signal differentiation is not caused by clonal restriction. Clone 22 (Cl 22), a Ba/F3 clone that expresses the EpoR ectopically was transfected with GMR-α cDNA (Cl 22-GMR-α). After selection in GM-CSF, cells were replated in IL-3, GM-CSF, and Epo, respectively. RNA was obtained and hybridized with a β-globin probe as described. Cytokines used to grow cells are indicated above each lane.
Both the Endodomain and Ectodomain of the EpoR Can Signal Proliferation and Differentiation
To identify the region of the EpoR that is critically involved in differentiation, we constructed a series of chimeric receptors diagrammed in Fig 1. The ectodomain of the EpoR was initially fused to the cytoplasmic region of the IL-3RβIL3 chain. cDNA encoding the resulting chimeric receptor (E/β) was transfected into Ba/F3 cells, and cells were successfully switched to growth in Epo after a first selection in IL-3 and G418. Ba/F3-E/β cells proliferated in Epo in a dose-dependent manner with half-maximal growth at three times the concentration required for Ba/F3-EpoR cells (Fig 4A). This concentration is still in the range characteristic of high-affinity binding. Interestingly, E/β cells expressed a high level of β-globin mRNA after 10 days in Epo (Fig 6A). Expression of endogenous EpoR mRNA was ruled out by a highly sensitive RNase protection assay. Using a probe encoding a cytoplasmic portion of the EpoR, which is absent in E/β, no specific protected band arising from expression of endogenous EpoR mRNA was detected. As expected, the same probe protected a specific band of the predicted size in Ba/F3-EpoR cells (Fig 3B). E/β-receptor expression was confirmed at the RNA level by Northern blot analysis (data not shown) and FACS analysis showed surface expression of the chimeric receptor (Fig 3A). The fact that we can detect two populations that express E/β at different receptor densities reflects the polyclonal nature of E/β cells, and was observed in other transfection experiments. Taken together these results indicate that proliferation and differentiation of Ba/F3-E/β cells are a direct consequence of the activation of the E/β receptor.
Differentiation of Ba/F3 cells requires the cytoplasmic pairing of two signal transducing receptor chains. (A) RNA (15 μg) from Ba/F3 cells bearing the indicated receptors was analyzed as described in Fig 2. Cell lines and cytokines used for growth are indicated above each lane. β-Globin and GAPDH signals are shown with closed arrowheads. Abbreviations are as in Figure 3B. (B) β-Globin expression in IL-3 or GM-CSF (Ba/F3-GMa/βIL3 ) or in IL-3 and IL-5 (Ba/F3-IL-5a/βIL3 ). Ba/F3 cells and Ba/F3-EpoR cells are used as a negative and positive control, respectively.
Differentiation of Ba/F3 cells requires the cytoplasmic pairing of two signal transducing receptor chains. (A) RNA (15 μg) from Ba/F3 cells bearing the indicated receptors was analyzed as described in Fig 2. Cell lines and cytokines used for growth are indicated above each lane. β-Globin and GAPDH signals are shown with closed arrowheads. Abbreviations are as in Figure 3B. (B) β-Globin expression in IL-3 or GM-CSF (Ba/F3-GMa/βIL3 ) or in IL-3 and IL-5 (Ba/F3-IL-5a/βIL3 ). Ba/F3 cells and Ba/F3-EpoR cells are used as a negative and positive control, respectively.
We next examined the role of the EpoR endodomain by constructing a chimeric receptor (GMER) containing the extracellular domain of the GMR-α chain fused to the endodomain of the EpoR (Fig 1). As the GMR-α chain forms a high-affinity receptor with the βc chain (which, like βIL3 , is endogenously expressed in Ba/F3 cells31 37 ) it is likely that GMER associates with βc on ligand binding. After selection in IL-3 and G418, Ba/F3-GMER cells were switched to GM-CSF and proliferated in a dose dependent fashion identical to Ba/F3-GMR cells (Fig 4B). Importantly, high β-globin expression was observed in Ba/F3-GMER cells after 10 days in GM-CSF (Fig 6A). While GMER mRNA was highly expressed in these cells (data not shown), no GMR-α mRNA could be detected by RNase protection, ruling out expression and activation of a potential endogenous GMR-α chain (Fig 3B). These results implied that the EpoR endodomain encodes the critical information necessary for differentiation, even though the E/β chimera had previously implicated the EpoR ectodomain as being crucial for β-globin expression. These conflicting results raised an interesting paradox.
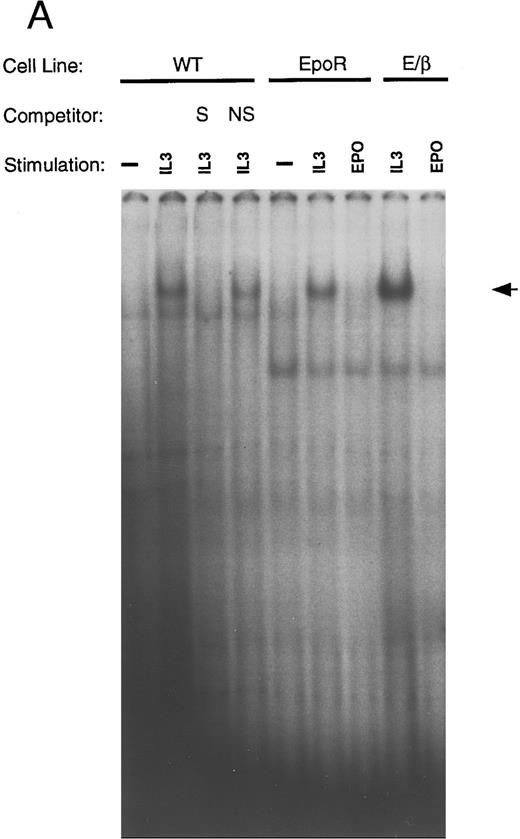
Inverse correlation between STAT5 activation and differentiation. Ba/F3 cells bearing the indicated receptors were washed 3 times in PBS, starved for 6 hours in RPMI, and then stimulated with the indicated growth factor for 10 minutes. Nuclear extracts were obtained as described. Electrophoretic mobility shift assays (EMSA) were performed using a β-casein probe.33 Competition with unlabeled oligonucleotides were done with either the β-casein probe S or an unrelated nonspecific oligonucleotide (NS). Migration of the specific complex is shown with an arrow. (A) EMSA with nuclear extracts from wild-type Ba/F3 cells, Ba/F3-EpoR, and Ba/F3 E/β treated as indicated above each lane. (B) EMSA with nuclear extracts from Ba/F3-GMR-α and Ba/F3-GMα/βIL3 cells.
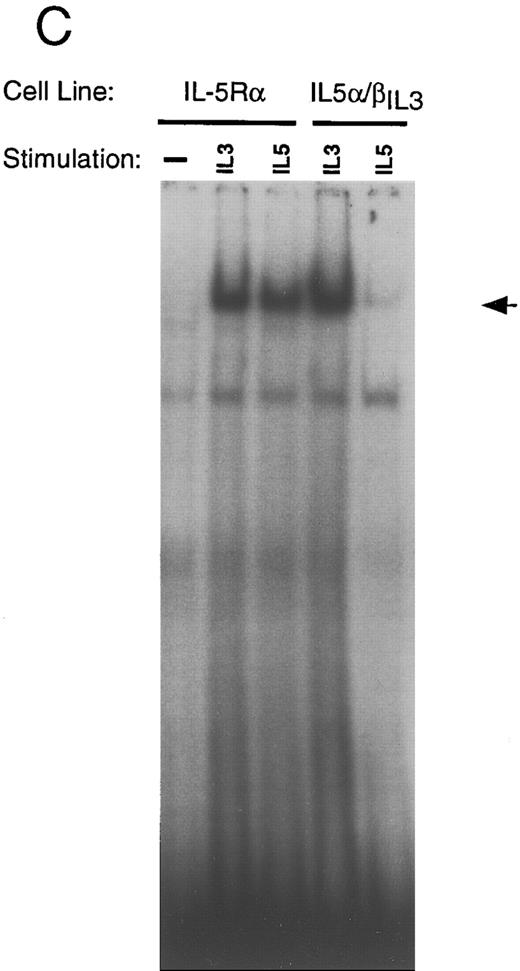
(C) EMSA with nuclear extracts from Ba/F3-IL-5Rα and Ba/F3-IL-5α/βIL3 cells. (D) EMSA were performed as in (A), in the absence or presence (+) of specific anti-STAT5 (αSTAT5) or anti-STAT6 (αSTAT6) antisera.
Inverse correlation between STAT5 activation and differentiation. Ba/F3 cells bearing the indicated receptors were washed 3 times in PBS, starved for 6 hours in RPMI, and then stimulated with the indicated growth factor for 10 minutes. Nuclear extracts were obtained as described. Electrophoretic mobility shift assays (EMSA) were performed using a β-casein probe.33 Competition with unlabeled oligonucleotides were done with either the β-casein probe S or an unrelated nonspecific oligonucleotide (NS). Migration of the specific complex is shown with an arrow. (A) EMSA with nuclear extracts from wild-type Ba/F3 cells, Ba/F3-EpoR, and Ba/F3 E/β treated as indicated above each lane. (B) EMSA with nuclear extracts from Ba/F3-GMR-α and Ba/F3-GMα/βIL3 cells.
(C) EMSA with nuclear extracts from Ba/F3-IL-5Rα and Ba/F3-IL-5α/βIL3 cells. (D) EMSA were performed as in (A), in the absence or presence (+) of specific anti-STAT5 (αSTAT5) or anti-STAT6 (αSTAT6) antisera.
Erythroid Differentiation in the Absence of Epo and of Its Receptor
To reconcile these experimental data we hypothesized that receptor complexes, which failed to induce differentiation had an intracellular composition of a short α-chain paired to a longer, signal transducing β-chain (eg, IL-3, GM-CSF, and the IL-5 receptors). In contrast, ligand-induced dimerization of two signal transducing receptor chains, supported differentiation (Fig 1). In such cases, intracellular pairing of two EpoR chains (EpoR), two βIL3 (E/β), or of an EpoR and βc chains (GMER) was equally effective. To test our model, we constructed two additional chimeric receptors. GMα/βIL3 and IL-5α/βIL3 are composed of the ectodomains of the GMR-α and IL-5Rα chains, respectively, fused to the endodomain of the βIL3 chain (Fig 1). cDNAs encoding these constructs were transfected into Ba/F3 cells. After selection in IL-3 and G418, cells were successfully switched to GM-CSF or IL-5, respectively. Similar chimerae had been shown previously to form high-affinity receptors in hematopoietic cells by dimerizing with βc .38,39 Ba/F3 cells expressing GMα/βIL3 and IL-5α/βIL3 were strictly factor-dependent and proliferated either in IL-3, or in the presence of their cognate ligand (GM-CSF or IL-5, respectively). As predicted by our model, growth of Ba/F3-GMα/βIL3 and Ba/F3-IL-5α/βIL3 in GM-CSF or IL-5, respectively, resulted in high β-globin expression (Fig 6B). Although a higher background of β-globin expression is detected in Ba/F3-GMα/βIL3 grown in IL-3, expression in GM-CSF is induced in excess of 10-fold when differences in loading are taken into account (as controlled by GAPDH expression). Expression of the chimeric chains was confirmed at the RNA level (data not shown), and surface expression of IL-5α/βIL3 protein was shown by FACS analysis (Fig 3A). As in the case of E/β cells, two populations of cells bearing different receptor densities were detected. Since we lacked an antibody directed against the ectodomain of the murine GMR-α chain, a similar experiment could not be performed with Ba/F3-GMR-α, Ba/F3-GMER, and Ba/F3-GMα/βIL3 cells. However, Northern blot analysis confirmed that all three ectopic chains were expressed at similar levels (data not shown). There was no evidence of endogenous GMR-α or IL-5Rα mRNA in Ba/F3-GMα/βIL3 or IL-5α/βIL3 cells by RNase protection, using probes directed against a specific 3′ fragment of the GMR-α and IL-5Rα chains, respectively. In contrast, these probes protected fragments of the appropriate size in Ba/F3-GMR-α and Ba/F3-IL-5Rα cells, respectively (Fig 3B). Dose dependent proliferation curves indicate that GMα/βIL3 supports half-maximal growth at a concentration similar to that of wild-type GMR, whereas IL-5α/βIL3 requires a threefold higher concentration of IL-5 than does the IL-5R. A slightly lower affinity for a similar chimera was also observed in another report.39 These results indicate for the first time that differentiation of Ba/F3 cells can be achieved in the absence of Epo or an EpoR, as long as signaling is mediated by a dimer of two signal transducing chains and not by an α/β heterodimer.
Differentiation Correlates With Delayed STAT5 Binding
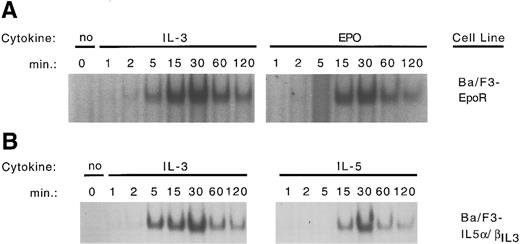
In an attempt to link downstream signaling events to the differentiation process, we investigated activation of STAT proteins in Ba/F3 cells expressing the various wild-type and chimeric receptors. Cells were grown in IL-3, washed, and starved for 6 hours. At that time, cells were stimulated for 10 minutes with physiological concentrations of either IL-3 or the ligand recognized by the ectopically expressed receptor. Nuclear extracts were prepared and were used in a binding reaction with a β-casein GAS consensus sequence.33 A distinct complex is detected by the β-casein probe on IL-3 stimulation of all of the above cell lines. This complex is specific, since it is competed with excess unlabeled β-casein oligonucleotides but not by unrelated oligonucleotides (Fig 7A through C). Supershift experiments show that the complex is comprised of STAT5 proteins, as it reacts with anti-STAT5 antibody (Fig 7D), but fails to be recognized by antibodies directed against STAT1-4 (data not shown) or against STAT6 (Fig 7D). Stimulation of cells expressing the wild-type GMR or IL-5R with their respective cytokine leads to the same high level binding of STAT5 complex. In contrast, a 10-minute stimulation of any receptor capable of mediating differentiation (EpoR, E/β, GMα/βIL3 , IL-5α/βIL3 ) fails to fully activate STAT5 binding (Fig 7A through C). This difference can not be accounted by differences in nuclear protein concentration as an Oct-1 specific probe bound the same amount of Oct-1 complex in our nuclear extracts (data not shown). A time course experiment was performed with Ba/F3-EpoR cells and Ba/F3-IL-5α/βIL3 cells. Cells were stimulated either with IL-3 (0.5 ng/mL) and Epo (0.5 U/mL) (Fig 8A), or with IL-3 and IL-5 (1 ng/mL) (Fig 8B). Electrophoretic mobility shift assay performed with the β-casein probe revealed that STAT5 activation in Ba/F3-EpoR is significantly delayed on Epo stimulation (Fig 8A) compared to IL-3 stimulation. A similar delay was observed in Ba/F3-IL-5α/βIL3 cells on IL-5 stimulation (Fig 8B). This delay was observed whether the cells were maintained in Epo (Ba/F3-EpoR), IL-5 (Ba/F3-IL-5α/βIL3 ), or IL-3 containing medium before starvation. Interestingly, when supraphysiological concentrations of Epo (10 U/mL) or IL-5 (10 ng/mL) were used, β-globin expression was dramatically decreased and the delay in STAT5 activation was abrogated (data not shown).
Time course of STAT5 binding in Ba/F3-EpoR and Ba/F3 -IL-5α/βIL3 cells. (A) Ba/F3-EpoR cells were maintained in Epo (0.5 U/mL) washed 3× in PBS and starved for 6 hours in RPMI medium containing 10% FCS. Cells were then stimulated with either IL-3 (0.5 ng/mL) or Epo (0.5 U/mL) for the indicated period of time (1 × 107 cells per time point). Nuclear extracts were obtained and used for an EMSA with a β-casein probe as described in Materials and Methods. (B) Ba/F3-IL-5α/βIL3 were maintained in IL-5 (1 ng/mL) and treated as above. Stimulation was performed with either IL-3 (0.5 ng/mL) or IL-5 (1 ng/mL).
Time course of STAT5 binding in Ba/F3-EpoR and Ba/F3 -IL-5α/βIL3 cells. (A) Ba/F3-EpoR cells were maintained in Epo (0.5 U/mL) washed 3× in PBS and starved for 6 hours in RPMI medium containing 10% FCS. Cells were then stimulated with either IL-3 (0.5 ng/mL) or Epo (0.5 U/mL) for the indicated period of time (1 × 107 cells per time point). Nuclear extracts were obtained and used for an EMSA with a β-casein probe as described in Materials and Methods. (B) Ba/F3-IL-5α/βIL3 were maintained in IL-5 (1 ng/mL) and treated as above. Stimulation was performed with either IL-3 (0.5 ng/mL) or IL-5 (1 ng/mL).
DISCUSSION
Proliferation and differentiation of hematopoietic progenitor cells are promoted by activation of hematopoietic receptors by their respective ligands. To preserve specificity in these responses, receptors are likely to encode unique features that distinguish them functionally from one another. Here, we show that the ability of the EpoR to signal limited differentiation in Ba/F3 cells is not shared by other wild-type hematopoietic receptors like the IL-3, GM-CSF, and IL-5 receptors, even though these receptors are fully mitogenic in these cells. To determine what was unique about the EpoR, chimeric receptors were designed to test the role of the extracellular and cytoplasmic regions of the EpoR in supporting both growth and differentiation. The E/β chimera, in which the endodomain of the EpoR was replaced by the corresponding βIL3 receptor cytoplasmic chain behaved identically to the wild-type EpoR and retained the ability to induce β-globin. This finding confirmed our previously published report describing similar chimeric EpoR mutants.12 Taken together, these results suggested that the EpoR ectodomain was a critical region for differentiation. Although important, the extracellular domain of the EpoR is not sufficient on its own unless it is coupled to a signal transducing cytoplasmic receptor unit. Indeed, truncation mutants of EpoR without its cytoplasmic region, or a chimera containing the EpoR ectodomain fused to the cytoplasmic region of GMR-α lack mitogenic and differentiating activities2 (data not shown).
If differentiation mapped to the ectodomain of the EpoR, what was then the function of the cytoplasmic portion of the EpoR? In our earlier study,12 this question had not been addressed. However, Maruyama et al14 had shown that a chimeric receptor carrying the extracellular domain of the epidermal growth factor (EGF ) receptor linked to the cytoplasmic domain of EpoR signaled both proliferation and erythroid differentiation of the leukemic cell line TSA8 in response to EGF. Likewise, when we replaced the EpoR ectodomain by the GMR-α chain (GMER), this receptor was equivalent to wild-type EpoR or E/β in inducing differentiation. This raised the following conundrum: one set of experiments identified the extracellular domain of the EpoR as a region necessary, but not sufficient for differentiation; another found the cytoplasmic region to be responsible for differentiation. This apparent paradox had been raised previously.11,14 Although it had created considerable interest, it had not been resolved, in part because of a controversy over low level expression of endogenous EpoR in the studies of Chiba et al.11
A closer look at the predicted composition of the various receptor complexes suggested a unifying model. Receptors consisting of an αβ heterodimer only allowed proliferation of Ba/F3 cells. On the other hand, receptor complexes characterized by an association of two signal transducing chains in the cytoplasm, or alternatively, by the lack of an α-chain in the receptor complex, supported both cell growth and differentiation. This model made no obligate reference to having any part of the EpoR in the differentiation of Ba/F3 cells as long as the necessary structural requirements were met. Our model was confirmed with the chimeric receptors GMα/βIL3 and IL-5α/βIL3 . Both receptors fulfill the postulated requirements and lack any part of the EpoR (Fig 1). Importantly, they induce high β-globin expression indicating that they are differentiation competent. When expressed in Ba/F3 cells, GMα/βIL3 , and IL-5α/βIL3 subunits associate with the endogenous βc chain in the presence of GM-CSF and IL-5, consistent with the dose responsiveness of these cells to the two cytokines (Fig 4). This association was independently confirmed by others. A similar IL-5Rα/β chimera was shown to coprecipitate with βc in the presence of IL-5,39 while a human GMR-α/β chimeric receptor required coexpression of human βc in Ba/F3 cells for high-affinity binding of human GM-CSF.38
Removal of IL-3 from the medium has been described to trigger differentiation of Ba/F3-EpoR cells by impairing proliferation.40 This argument does not apply to our chimeric receptors since all experiments for differentiation were performed at saturating doses of cytokines. Thus, differentiation in Ba/F3 cells is not the result of a defect in proliferative potential, nor can it be attributed to dimerization and activation of endogenous EpoR induced by the Epo in the serum. Rather it is a specific outcome dictated by the growth factor receptor complex.
Although our data support a direct role for receptors in signaling differentiation, the cellular milieu in which they are acting is also critical. We have previously observed that the IL-2–dependent T-cell line CTLL can proliferate in Epo after introduction of the EpoR, but shows no sign of β-globin production even after prolonged growth in Epo.10 It is likely that Ba/F3 cells are precommitted, or at least predisposed, to undergo erythroid differentiation. Therefore, cytokine receptors, as long as they meet certain structural requirements, may have the function of enabling progenitor cells to complete genetic programs already in place. It is tempting to speculate that receptors may have different effects depending on the array of transcription factors expressed at the time of receptor activation. Therefore, specificity and cellular fate in vivo may be achieved by carefully regulating the expression of a receptor both in a temporal and lineage-specific manner.
Our experiments indicate that functional distinctions exist between receptors that form homodimers (eg, EpoR) and those that form αβ heterodimers (eg, IL-3R, GMR, and IL-5R). To understand how two signal transducing chains might act differently from an α/β heterodimer, we attempted to link downstream signaling events to the process of differentiation. A newly described class of signaling proteins, STATs, has gained much attention. Receptor-mediated activation leads to phosphorylation and translocation of STAT proteins to the nucleus where they serve as transcription activators. Recently, activation of STAT5 has been reported in hematopoietic cells after binding of IL-2, IL-3, GM-CSF, IL-5, and Epo to their receptors,19,20,22,23,41,42 and correlated with proliferation in response to the various cytokines.5 Our data confirm that STAT5 can be activated by these cytokines in Ba/F3 cells; however, with a notable difference. Among the wild-type receptors, the EpoR was unique in that it induced no or much less of the activated STAT5 complex after a 10 minute stimulation. This dramatic decrease in early STAT5 binding was also observed after stimulation of the E/β, the GMR-α/βIL3 and IL-5α/βIL3 receptors with their respective ligands. Thus, diminished STAT5 binding at 10 minutes correlates with differentiation. Additionally, early STAT5 activation provides another assay for the ability of receptors to support differentiation of Ba/F3 cells. Using high-affinity probes for STAT1 and 3,34,43 and for STAT6,44 no discernible pattern of activation emerged for these transcription factors that could be linked to either proliferation or differentiation (data not shown). Thus, STAT 1, 3, and 6 are less likely to be involved in differentiation of Ba/F3 cells. The striking differences in STAT5 activation were most pronounced after a 10-minute stimulation. However, at later time points in Epo and IL-5, respectively, (20 and 30 minutes) the EpoR and IL-5α/βIL3 receptors were also capable of inducing STAT5 binding, albeit to a lesser extent than after IL-3 stimulation (Fig 8). This delay can not be explained by impaired signaling by chimeric receptors, since activation of wild-type EpoR shows the same kinetics of STAT5 binding. Whether the delay in STAT5 activation influences the fate of a cell to choose between proliferation and differentiation can not be answered at this point and will be an area of future investigation. However, recent evidence suggests that erythroid differentiation of an erythroleukemic cell line correlates with impaired function of STAT5 in response to Epo.45 Therefore, it is possible that the strength or the timing of the STAT5 signal may be important determinants in choosing between proliferation and differentiation. Neuronal differentiation offers an interesting parallel. Nerve growth factor (NGF ), which causes neuronal differentiation of PC12 cells, leads to a sustained activation of the mitogen-activated protein kinase cascade. In contrast to NGF, EGF induces proliferation of PC12 cells only, and leads to the transient activation of the same cascade.46
In conclusion, our data support an enabling role for the EpoR in erythroid differentiation in an appropriate cellular environment. They further define the necessary structural requirements for differentiation at the receptor level and show that by complying with these requirements, the EpoR can be replaced by other receptors without a loss of differentiation capacity. An intriguing interpretation of our results is to propose an important role for the α-chain in preventing differentiation and promoting full STAT5 activation, rather than attribute differentiation and the delay of STAT5 activation to the presence of a second signal transducing receptor chain. Indeed, Krosl et al47 have reported that Epo-induced differentiation of Ba/F3 cells can be blocked by an interfering IL-3Ra chain.47 Finally, our results support a direct link between differentiation signals in hematopoiesis and the STAT pathway.
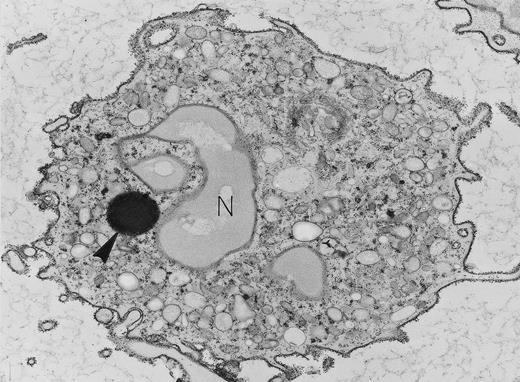
Neutrophil with lipid body. Specialized ultrastructural procedures were used to image this peripheral blood neutrophil. A reduced osmium postfixation step renders nuclear lobes (N) and secretory granules poorly electron-dense but each individual particle of cytoplasmic glycogen is electron-dense, and the single, large, round, membrane-free lipid body (arrowhead) is highly electron-dense. Exposure to cationized ferritin after fixation has provided a uniform layer of electron-dense ferritin particles bound to the plasma membrane. Original magnification × 8,000. (Courtesy of Ann M. Dvorak, MD, Department of Pathology, Beth Israel Hospital, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215.)
Neutrophil with lipid body. Specialized ultrastructural procedures were used to image this peripheral blood neutrophil. A reduced osmium postfixation step renders nuclear lobes (N) and secretory granules poorly electron-dense but each individual particle of cytoplasmic glycogen is electron-dense, and the single, large, round, membrane-free lipid body (arrowhead) is highly electron-dense. Exposure to cationized ferritin after fixation has provided a uniform layer of electron-dense ferritin particles bound to the plasma membrane. Original magnification × 8,000. (Courtesy of Ann M. Dvorak, MD, Department of Pathology, Beth Israel Hospital, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215.)
ACKNOWLEDGMENT
We thank E.C. TePas for her contribution in cloning the murine GMR-α cDNA. We are also indebted to Dr Tavernier and his group for their generosity in providing us with both murine IL-5R cDNA and monoclonal antibody against the IL-5Rα chain. We thank Dr David Nathan for his support, his thoughtful insights, and for his critical reading of our manuscript.
Supported in part by National Institutes of Health Grant No. 2P01 HL32262 (Bethesda, MD). M.P. is a recipient of fellowships by the Swiss National Research Foundation, by the Cantonal Cancer Leagues of Basel and Solothurn, and by the Margarete and Walther Lichtenstein Foundation. K.N. is a recipient of a Fulbright Scholarship and of a D. Collen Research Foundation fellowship. M.C. is a recipient of a fellowship by the American Cancer Society. B.M.-P. receives partial support from Genetics Institute.
Address reprint requests to Miklos Pless, MD, Dana-Farber Cancer Institute, Division of Pediatric Oncology, 44 Binney St, Boston, MA 02115.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal