Abstract
Expression of the apoptosis inhibitory protein Bcl-x was studied in CD34+ hematopoietic precursor cells and in the promyelocytic leukemia cell line HL-60. The enriched population of CD34+ cells (more than 95%) was cultured in the presence of stem cell factor, interleukin-3 (IL-3), IL-6, and either granulocyte colony-stimulating factor or macrophage colony-stimulating factor to achieve granulocyte or monocyte/macrophage differentiation, respectively. The expression of Bcl-x increased in the early stages of both differentiation pathways. However, by day 21 of culture mature granulocytes were Bcl-x–negative, whereas monocytes/macrophages either maintained or increased the expression of Bcl-x. The pattern of Bcl-x expression in the differentiated CD34+ cells was similar to that observed in HL-60 cells differentiated along the granulocyte lineage (induced by incubation with retinoic acid), or along the monocyte/macrophage lineage (induced by incubation with phorbol diester). The bcl-x transcript predominant in HL-60 and CD34+ cells differentiated into monocytes/macrophages was bcl-xL . Although little is yet known regarding the functional significance of Bcl-x within the granulomonocytic compartment, marked changes in the pattern of its expression, as observed during granulomonocytic differentiation of HL-60 and CD34+ cells, are likely to alter the life span of mature granulocytes and monocytes/macrophages.
ALTHOUGH GRANULOCYTES and monocytes/macrophages derive from common progenitor cells, neutrophilic granulocytes have limited life span in vitro and in vivo whereas monocytes/macrophages are long-living cells. The difference in life span between these two lineages might reflect a difference in the expression of survival genes. Promyelocytes and myelocytes have been shown to express high levels of the apoptosis inhibitor Bcl-2, whereas metamyelocytes display decreased expression and mature polymorphonuclear cells are negative.1 Similarly, Delia et al2 showed that the number of Bcl-2–positive myeloid cells in normal bone marrow as detected by immunostaining decrease from myeloblasts and monoblasts to neutrophils and monocytes, which are mostly negative. These results are consistent with those obtained with the promyelocytic cell line HL-60, which can be induced to differentiate toward the granulocyte or monocyte/macrophage lineages when treated with chemical inducers. It has been demonstrated that both retinoic acid (RA)-induced granulocyte and phorbol-12-myristate-13-acetate (PMA)-induced monocyte/macrophage maturation of HL-60 cells is accompanied by a progressive decrease in the expression of Bcl-2.2 3
Recently, it has been shown that another member of the Bcl-2 family, Bcl-xL , which functions as a cell death effector protein,4 may be essential for the long-term survival of the hematopoietic stem cell population, since the most primitive, small to medium lymphocyte-sized subset of CD34+lin− precursor cells express Bcl-x but not Bcl-2.5 Bcl-x immunostaining has been detected in myeloid precursors, including metamyelocytes. However, the majority of bone marrow polymorphonuclear cells do not contain Bcl-x and spleen and peripheral blood granulocytes are mostly Bcl-x–negative.6 A major role for Bcl-xL in maintaining the survival of hematopoietic cells is supported by recent studies of mutant mice in which Bcl-x has been disrupted by homologous recombination.7 Mice deficient in Bcl-x develop massive apoptosis of hematopoietic progenitors and die around 13 days of gestation. In contrast, mice deficient in Bcl-2 exhibit increased spontaneous apoptosis of lymphoid cells after birth but no apparent abnormalities of the myeloid compartment.8 These observations suggest that Bcl-xL may be an important factor to regulate the survival of myeloid cells.
Studies of gene expression during myelopoiesis have been largely dependent on the use of cell lines, which recapitulate many of the developmental steps of normal myeloid cells.9 However, these cell lines have been established from patients with myeloid leukemias and the majority of them carry genetic abnormalities. Cells expressing the CD34 surface antigen constitute a heterogeneous population of hematopoietic cells that includes primitive, uncommitted stem/progenitor cells capable of initiating long-term hematopoiesis in vitro, as well as more mature progenitors committed to different lineages of differentiation.10-13 In the present study we have examined the granulomonocytic-specific expression of Bcl-x in a cell model allowing inducible differentiation of normal CD34+ progenitor cells in cytokine-supported liquid culture. We have shown that the expression of Bcl-x during the monocyte/macrophage differentiation is either maintained or increased, whereas it is downregulated as myeloid progenitors mature toward polymorphonuclear cells. Similar results were obtained in HL-60 cells induced to undergo granulomonocytic differentiation. This differential expression of Bcl-x suggests that this anti-apoptotic protein may account for, or at least contribute to, the difference in cell life span of mature granulocytes and monocytes/macrophages.
MATERIALS AND METHODS
Cell culture.The HL-60 promyelocytic leukemia cell line was maintained at 5 to 9 × 105 cell/mL in RPMI 1640 medium (Seromed Biochrom KG, Berlin, Germany) supplemented with 10% heat-inactivated fetal calf serum (FCS; Flow Laboratories, Irvine, CA), nonessential aminoacids, 2 mmol/L glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycine. To induce differentiation, cells were cultured in the presence of 1 μmol/L RA or 20 ng/mL PMA (both from Sigma, St Louis, MO) for the indicated times. RA was dissolved and stored in DMSO at 5 mmol/L and diluted 1:5,000 in culture medium to induce differentiation. Fresh medium containing the differentiation inducers was added to the cultures every 48 to 72 hours.
Isolation and flow cytometry analysis of CD34+ cells.CD34+ cells were purified using the MiniMACS CD34 progenitor cell isolation kit (Miltenyi Biotech, Auburn, CA), following the manufacturer's recommendations. Briefly, peripheral blood progenitor cells from patients with solid tumors were recruited by standard-dose chemotherapy and subsequent administration of granulocyte colony-stimulating factor (G-CSF ). Informed consent was obtained in all cases. Collected cells were washed and resuspended in phosphate buffered saline (PBS) containing 0.5% bovine serum albumin (BSA) (Fraction V; Sigma) and 5 mmol/L EDTA. Cells were incubated first with QBEND-10 antibody (mouse antihuman CD34) (Miltenyi Biotech) in the presence of human IgG as a blocking reagent and then with 100 μL MACS microbeads per 108 cells. Labeled cells were filtered through a 30 μm nylon mesh before loading onto a column installed in the magnetic field. Trapped cells were eluted after the column was removed from the magnet and further depleted of contaminant CD34− cells by a second MACS passage.
The purity of the CD34+ population ranged from 95% to 98% as evaluated by flow cytometry using an EPICS Elite ESP analyzer (Coulter, Hialeah, FL). Newly isolated CD34+ cells as well as cells that had been cultured for various time intervals, were phenotyped by flow cytometry as previously described.3 Antibodies used for the cytometric analysis included anti-CD34 labeled with phycoerythrin (PE) (Becton Dickinson, San Jose, CA) and anti-CD33 labeled with fluorescein isothiocyanate (FITC) (Coulter).
Expression of Bcl-x and Bcl-2 was determined by flow cytometric analysis using mouse antihuman Bcl-x (kindly provided by Dr Craig Thompson, University of Chicago), or 6C8, a hamster antihuman Bcl-2 monoclonal antibody, respectively, followed by biotin-conjugated goat antimouse or antihamster IgG (Sigma) and PE-labeled streptavidin (Cappel, Durham, NC) as described.3 Mouse Leu-4 (Becton Dickinson) and hamster 3F11 monoclonal antibodies were used as control.
Ex vivo differentiation of CD34+ cells.Purified CD34+ cells were seeded into 24-well culture plates (Becton Dickinson) at 5 × 104 cells/mL in Iscove's modified Dulbecco's medium (IMDM; GIBCO-BRL, Grand Island, NY) containing 20% FCS and recombinant human stem cell factor (SCF ), interleukin-3 (IL-3), and IL-6 (kindly provided by Immunex, Seattle, WA) at a final concentration of 100 ng/mL. To induce granulocyte or monocyte/macrophage differentiation of the CD34+ cells, 100 ng/mL G-CSF (Amgen, Thousand Oaks, CA) or 500 U/mL macrophage-CSF (M-CSF; kindly provided by Dr C. Bello-Fernandez, University of Vienna) were also added to the culture. At the indicated time points cells were collected for morphological, immunocytochemical, and flow cytometric analysis.
Immunocytochemical staining.Cells cytocentrifuged onto slides were fixed in 100% ethanol at 4°C for 10 minutes and subsequently blocked with 5% normal goat serum diluted in Tris-buffered saline (TBS) pH 8.0 for 30 minutes at room temperature. Rabbit antihuman Bcl-x antibody (Transduction Laboratories, Lexington, KY) at a 1:200 dilution was incubated with the fixed cells, and this was followed by an incubation with biotinilated goat antirabbit IgG (Sigma) at a dilution of 1:4,000 and ExtrAvidin alkaline phosphatase (Sigma) diluted 1:500. All incubations were performed at room temperature for 1 hour. The primary and secondary reagents were diluted in TBS containing 5% normal goat serum. Staining was developed using bromochlorindolyl phosphate and nitroblue tetrazolium (BCIP/NBT) substrate, and then counterstained using 0.1% acridine orange and 0.1% safranin O. Cells incubated with normal rabbit serum instead of primary antibodies were used as a negative control.
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis.Total RNA was prepared using TRIZOL reagent (GIBCO-BRL). To assess mRNA expression, a RT-PCR method was used as previously described.14 Briefly, for the RT reaction, RNA was primed with random hexamer and reverse transcribed with Superscript MMLV reverse transcriptase (GIBCO-BRL) in a 20-μL volume. A fragment from position 441 to position 780 of the human bcl-xL cDNA sequence4 was amplified by using specific primers (5′CGGGCATTCAGTGACCTGAC and 5′TCAGGAACCAGCGGTTGAAG). A 50 μL PCR mixture contained 1 μL of the RT reaction, 20 pmol of each primer, each dNTP (0.2 mmol/L), 10 mmol/L Tris-HCL (pH 8.3), 50 mmol/L KCl, 1.5 mmol/L MgCl2 , and 2.5 U of Taq DNA polymerase (Bioline, London, UK). The PCR reaction profile was as follows: 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. After 30 cycles of amplification, the expected PCR products (340 bp for bcl-xL , and 151 bp for bcl-xs ), were size fractionated onto a 2% agarose gel and stained with ethidium bromide.
RESULTS
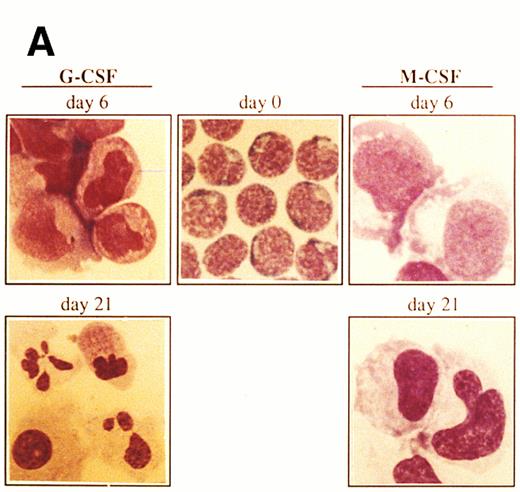
Ex vivo culturing of isolated CD34+ cells.Mobilized progenitor cells were enriched for the CD34+ cell fraction by using an immunomagnetic system as described above. The CD34+-selected population was cultured with a combination of three cytokines previously shown to induce ex vivo expansion of committed clonogenic progenitor cells (ie, SCF, IL-3, IL-6) and with either G-CSF or M-CSF to induce granulocyte or monocyte/macrophage maturation, respectively.15-17 The CD34+ cells (more than 95%) showed a blast-like morphology with very little cytoplasm (Fig 1A). Within the CD34+ cell population, less than 3% expressed CD33, a marker for myeloid cells18 (Fig 1B). Contaminating cells with a mature phenotype (CD34− cells), including granulocytes, lymphocytes, and monocytes was always less than 5% as determined by FACS analysis and morphology. After 6 days of culture in the presence of SCF, IL-3, IL-6, and G-CSF, the number of CD34+ cells decreased to 71%, with the early myeloid cell population (CD34+CD33+) moving increased from 1.6% at day 0 to 56% at day 6 (Fig 1B). Furthermore, by day 6 of culture the morphology of cells changed drastically with a concomitant decrease in the number of blast-like cells and with the development of a heterogeneous cell population of myeloblasts, promyelocytes, and myelocytes (Fig 1A). At day 21, 98.4% of cells had lost the progenitor cell marker CD34 and were positive for CD33, indicating a differentiation into mature myeloid cells (Fig 1B). In addition to the immunophenotypic features, at day 21 most of the cells (87%) had the morphology of mature granulocytes (metamyelocytes and band and segmented neutrophilic granulocytes) (Fig 1A).
Morphology and phenotypic characterization of CD34+ cells undergoing granulocyte or monocyte/macrophage differentiation. Isolated CD34+ cells were cultured in the presence of SCF, IL-3, IL-6, and either G-CSF or M-CSF. (A) At the indicated times, cells were cytocentrifuged and stained with May-Grunwald-Giemsa solution (original magnification × 1,000). (B) Bidimensional dot plots of cells labeled with anti-CD34 and anti-CD33 monoclonal antibodies. Numbers within quadrants indicate the percentage of cells in the different populations. Quadrants were set according to isotype-matched negative control stainings. All stainings and dot plots are from a representative experiment (n = 3).
Morphology and phenotypic characterization of CD34+ cells undergoing granulocyte or monocyte/macrophage differentiation. Isolated CD34+ cells were cultured in the presence of SCF, IL-3, IL-6, and either G-CSF or M-CSF. (A) At the indicated times, cells were cytocentrifuged and stained with May-Grunwald-Giemsa solution (original magnification × 1,000). (B) Bidimensional dot plots of cells labeled with anti-CD34 and anti-CD33 monoclonal antibodies. Numbers within quadrants indicate the percentage of cells in the different populations. Quadrants were set according to isotype-matched negative control stainings. All stainings and dot plots are from a representative experiment (n = 3).
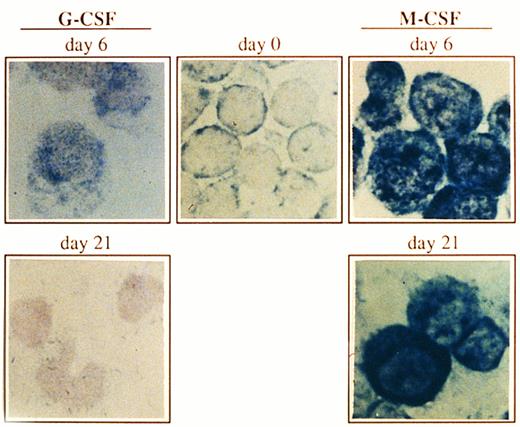
Expression of Bcl-x in CD34+ cells undergoing granulocyte or monocyte/macrophage differentiation. Cells were cultured as described in Fig 1, and at the indicated time points, Bcl-x protein was analyzed by an immunocytochemical procedure using a rabbit antibody against human Bcl-x. After incubation with biotinilated goat antirabbit IgG and ExtrAvidin alkaline phosphatase, staining was developed using BCIP/NBT substrate (original magnification × 1,000). No signal was detectable in the negative controls. All stainings are from a representative experiment (n = 3).
Expression of Bcl-x in CD34+ cells undergoing granulocyte or monocyte/macrophage differentiation. Cells were cultured as described in Fig 1, and at the indicated time points, Bcl-x protein was analyzed by an immunocytochemical procedure using a rabbit antibody against human Bcl-x. After incubation with biotinilated goat antirabbit IgG and ExtrAvidin alkaline phosphatase, staining was developed using BCIP/NBT substrate (original magnification × 1,000). No signal was detectable in the negative controls. All stainings are from a representative experiment (n = 3).
When culturing of cells in the presence of SCF, IL-3, IL-6, and M-CSF, a clear pattern of monocyte/macrophage maturation was observed (Fig 1A). By day 6 of culture the cells showed a monoblast morphology with wide cytoplasm and vacuolization. The percentage of myeloid progenitor cells (CD34+CD33+) was 92.4% and this number decreased to 1.3% after 21 days of culturing (Fig 1B) with a concomitant increase in the number of CD34−CD33+ cells (96%), which were majoritary mature monocytes/macrophages as determined by morphology (Fig 1A). Although the results shown in Fig 1 were obtained from a single experiment, similar conclusions were reached on the proportions of the different populations from two other experiments using the same labeling and culture conditions (data not shown).
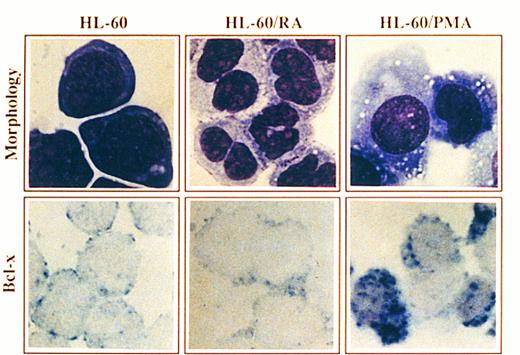
Morphology and Bcl-x expression in HL-60 cells treated with PMA or RA. HL-60 cells untreated or after culturing in the presence of PMA for 4 days or RA for 6 days were cytocentrifuged and either stained with May-Grunwald-Giemsa solution or analyzed for the expression of Bcl-x using a rabbit anti–-Bcl-x antibody. After incubation with biotinilated goat antirabbit IgG and ExtrAvidin alkaline phosphatase, staining was developed using BCIP/NBT substrate (original magnification × 1,000). No signal was detectable in the negative controls. All stainings are from a representative experiment (n = 3).
Morphology and Bcl-x expression in HL-60 cells treated with PMA or RA. HL-60 cells untreated or after culturing in the presence of PMA for 4 days or RA for 6 days were cytocentrifuged and either stained with May-Grunwald-Giemsa solution or analyzed for the expression of Bcl-x using a rabbit anti–-Bcl-x antibody. After incubation with biotinilated goat antirabbit IgG and ExtrAvidin alkaline phosphatase, staining was developed using BCIP/NBT substrate (original magnification × 1,000). No signal was detectable in the negative controls. All stainings are from a representative experiment (n = 3).
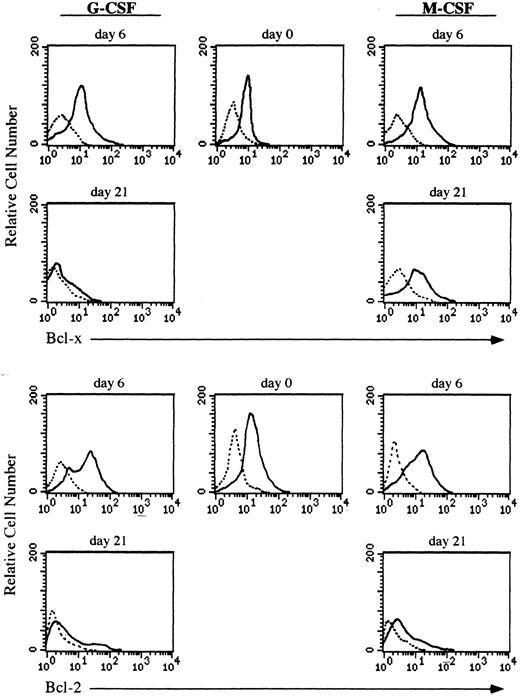
Expression of the Bcl-x protein during culturing of CD34+ cells.Both granulocytes and monocytes/macrophages arise from common committed progenitor cells, which may undergo two different pathways of maturation after induction with different CSF, (ie, G-CSF and M-CSF ). Since the life span of granulocytes is much shorter than that of monocytes/macrophages in the periphery, we argued that this may reflect a differential expression of survival genes in these two lineages. Given that Bcl-2 is absent in both granulocytes and monocytes/macrophages,2,3 we focused our studies on another member of the Bcl-2 family, Bcl-x. We have analyzed the expression of Bcl-x along the granulomonocytic differentiation of CD34+ cells incubated with SCF, IL-3, IL-6, and either G-CSF or M-CSF. A representative experiment is shown in Fig 2. Bcl-x was uniformly expressed in the majority of CD34+ cells (>96%) before culturing; however, it showed a different pattern of expression when CD34+ cells were cultured in the presence of G-CSF or M-CSF (Fig 2). After 6 days of culture in the presence of G-CSF a moderate increase in the expression of Bcl-x in more than 45% of cells (myeloid precursors) was detected, and at day 21, more than 80% of cells (mature granulocytes) were Bcl-x–negative. Interestingly, when the CD34+ population was cultured for 6 days in the presence of M-CSF, the expression of Bcl-x was increased in those cells undergoing morphologic differentiation (>50%) and by day 21 of culture, more than 85% of cells (mature monocytes/macrophages) either maintained or increased the expression of Bcl-x. These results were confirmed by flow cytometric analysis (Fig 3). The expression of Bcl-x was selectively downregulated when the CD34+ population was cultured with G-CSF. By day 21 of culture, only those cells induced to undergo monocytic differentiation expressed Bcl-x (Fig 3). As shown in Fig 3, flow cytometric analysis also detected that Bcl-2 was expressed in the majority of CD34+ cells lacking CD33, which is in agreement with previous results.2 After 6 days of culture in the presence of G-CSF or M-CSF more than 70% of cells still expressed Bcl-2, and at day 21 most of the cells were Bcl-2–negative (Fig 3).
Flow cytometric analysis of Bcl-x and Bcl-2 in CD34+ cells undergoing granulocyte or monocyte/macrophage differentiation. Cells were cultured in the presence of G-CSF or M-CSF and at the indicated time points differentiating cells were stained with mouse antihuman Bcl-x, hamster antihuman Bcl-2 (6C8) or irrelevant antibody as a background control (dotted lines) followed by biotin-conjugated goat antimouse or antihamster IgG and PE-conjugated streptavidin. All histograms are from a representative experiment (n = 3).
Flow cytometric analysis of Bcl-x and Bcl-2 in CD34+ cells undergoing granulocyte or monocyte/macrophage differentiation. Cells were cultured in the presence of G-CSF or M-CSF and at the indicated time points differentiating cells were stained with mouse antihuman Bcl-x, hamster antihuman Bcl-2 (6C8) or irrelevant antibody as a background control (dotted lines) followed by biotin-conjugated goat antimouse or antihamster IgG and PE-conjugated streptavidin. All histograms are from a representative experiment (n = 3).
Expression of Bcl-x during granulomonocytic differentiation of HL-60 cells.It has been previously shown that treatment of HL-60 promyelocytic cells with RA or PMA, which induced granulocyte or macrophage-like cell differentiation, respectively, was coupled to downregulation of the Bcl-2 protein as assessed by Western blot, immunocytochemistry and FACS analysis.2,3 To assess whether the endogenous levels of Bcl-x are regulated during granulomonocytic differentiation of HL-60 cells, the expression of Bcl-x was examined by immunocytochemistry before and after treatment with RA and PMA. A representative experiment is shown in Fig 4. HL-60 cells showed a low expression of Bcl-x (Fig 4, see page 3201), which is in agreement with previous data assessed by flow cytometry.14 Incubation of HL-60 cells with RA for 6 days induced granulocyte differentiation as detected by morphology (Fig 4), and also by the NBT reduction capacity and the decrease in the expression of transferrin receptors (data not shown). This pattern of differentiation was accompanied by a decrease in the expression of the Bcl-x protein (Fig 4). In contrast, HL-60 cells treated with PMA for 4 days, acquired a macrophage-like morphology (Fig 4) and most of the cells exhibited intense adherence to plastic with prominent pseudopodia formation and also marked adherence to each other. In addition, PMA-treated cells exhibited nonspecific esterase activity and the expression of surface transferrin receptors declined (data not shown). Interestingly, Bcl-x expression was upregulated in those cells that acquired a mature, macrophage-like phenotype (>50% of cells) (Fig 4).
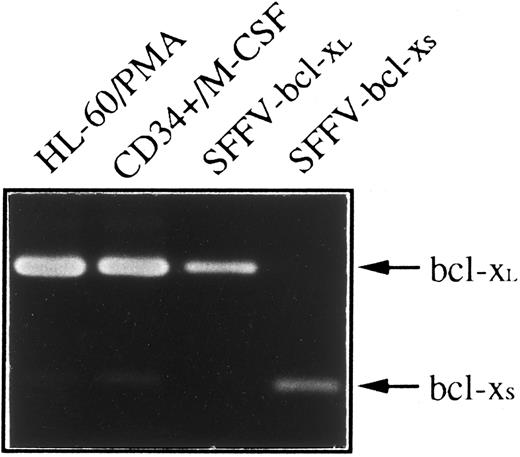
Since two species of human bcl-x mRNA, bcl-xL and bcl-xS that exhibited distinct biological function have been described,4 we have used an RT-PCR method to determine whether bcl-xL or bcl-xS was responsible for anti–Bcl-x staining shown in HL-60 and CD34+ cells differentiated toward monocytes/macrophages. Figure 5 shows that a weak expression of bcl-xS could be detected in both cell types; however, bcl-xL was the bcl-x form predominant in CD34+ cells after 21 days of culture in the presence of M-CSF and in HL-60 cells treated with PMA for 4 days.
Expression of bcl-x mRNA in HL-60 and CD34+ cells differentiated into monocytes/macrophages. Total RNA was purified from HL-60 cells treated with PMA for 4 days and CD34+ cells after 21 days of culture in the presence of M-CSF, and subjected to RT-PCR analysis with oligonucleotide primers that amplify both bcl-xL (340 bp) and bcl-xS (151 bp). Plasmids containing bcl-xL (SFFV-bcl-xL ) or bcl-xS (SFFV-bcl-xS ) cDNAs were also amplified as positive controls. After 30 cycles, PCR products were electrophoresed in a 2% agarose gel and stained with ethidium bromide.
Expression of bcl-x mRNA in HL-60 and CD34+ cells differentiated into monocytes/macrophages. Total RNA was purified from HL-60 cells treated with PMA for 4 days and CD34+ cells after 21 days of culture in the presence of M-CSF, and subjected to RT-PCR analysis with oligonucleotide primers that amplify both bcl-xL (340 bp) and bcl-xS (151 bp). Plasmids containing bcl-xL (SFFV-bcl-xL ) or bcl-xS (SFFV-bcl-xS ) cDNAs were also amplified as positive controls. After 30 cycles, PCR products were electrophoresed in a 2% agarose gel and stained with ethidium bromide.
DISCUSSION
Clonal analysis has demonstrated that the CD34+ population contains multipotential hematopoietic precursors, and that the type of cytokine used plays a major role in lineage commitment and differentiation.19-21 Stimulation of bone marrow progenitors with IL-3 gives rise to colonies consisting of granulocytes, macrophages, megakaryocytes, or mast cells, whereas the developmental potential of G-CSF and M-CSF is more restricted, resulting mainly in the formation of granulocyte and monocyte/macrophage colonies, respectively.22 23
Although granulocytes and monocytes/macrophages derive from common progenitor cells, neutrophilic granulocytes have limited life span in vitro and in vivo, whereas monocytes/macrophages are long-living cells. The difference in life span between these two lineages might reflect a difference in the expression of survival genes. In this report we have provided novel data on the expression of the apoptosis inhibitory protein Bcl-x during the granulocyte and monocyte/macrophage differentiation of CD34+ cells. Bcl-x is expressed in all subsets of pluripotent hematopoietic cells, suggesting an important role for this protein in the maintenance of survival in early stages of hematopoiesis.5 Whether Bcl-x could be relevant to maintain survival at particular stages of hematopoietic differentiation is uncertain. However, our results show that Bcl-x is lost in mature granulocytes derived from CD34+ cells cultured in the presence of G-CSF, whereas it is upregulated when CD34+ cells are differentiated toward monocytes/macrophages. In line with this, we have also analyzed the expression of Bcl-x by immunocytochemistry in peripheral blood monocytes and alveolar macrophages and shown that both cell types are clearly positive for Bcl-x, whereas peripheral blood neutrophils are negative (data not shown). As it has been previously reported in bone marrow cells and differentiating HL-60 cells2,3 we find that Bcl-2, which presents a widespread tissue distribution in hematopoietic cells and promotes cell survival1,2,24 is downregulated during granulocyte and monocyte/macrophage differentiation of CD34+ cells. The pattern of expression of Bcl-x in CD34+ cells cultured with M-CSF or G-CSF appears concordant with the in vitro findings on HL-60 cells. Of the human leukemic cell lines that have a restricted capacity for differentiation, the best known are HL-60 promyelocytic cells.25 HL-60 cells proliferate in suspension and can differentiate into four types of myeloid cell, depending on the inducing agent. Thus, RA can induce granulocyte differentiation and PMA causes macrophage-like cells to develop. Since HL-60 cells represent a progenitor population of cells that are already committed to develop along the granulomonocytic lineages, several investigators have used this cell line as a model to study the expression of a number of genes during differentiation of HL-60 cells into granulocytes or macrophage-like cells.26 27
We have shown that bcl-xL is the predominant bcl-x form expressed in HL-60 and CD34+ cells differentiated toward monocytes/macrophages. Interestingly, in hematopoietic precursors, the predominant bcl-x form is bcl-xS ,5 suggesting that the expression of both bcl-x forms are inversely regulated along the monocyte/macrophage pathway of differentiation. It has been recently shown that Bcl-xS can effectively inhibit the protective effects of Bcl-xL following growth factor withdrawal and chemotherapeutic drug treatment.28 Thus, the relative expression levels of the two Bcl-x protein isoforms may be critical to achieve an optimum balance between life and death during differentiation of some hematopoietic lineages. A major role for Bcl-xL in maintaining the survival of hematopoietic cells is supported by recent studies of mutant mice in which Bcl-x has been disrupted by homologous recombination.7 Mice deficient in Bcl-x develop massive cell death of hematopoietic progenitor cells. In contrast, mice deficient in Bcl-2 exhibited no apparent abnormalities of the nonlymphoid compartments.8 These data suggest that the expression of Bcl-xL may be critical to control the survival of proliferating hematopoietic precursor cells, which is consistent with the results presented here, since the expression of Bcl-x has been detected in both granulocyte and monocyte/macrophage precursors. It would be of interest to know whether the expression of Bcl-x along the differentiation of CD34+ cells is regulated by the growth factors added to the culture (ie, G-CSF, M-CSF ). In line with this, it has been demonstrated that erythropoietin (Epo) regulates the expression of both Bcl-xL and Bcl-2 in the Epo-dependent cell line HCD-57, and this seems to be a major mechanism by which Epo maintains the viability of erythroid progenitor cells.29 Whether G-CSF and M-CSF are involved in a similar regulation pathway is uncertain yet; however, it has been shown that in addition to promoting the proliferation and the differentiation of immature myeloid cells, G-CSF and M-CSF are also able to increase survival of mature granulocytes and monocytes, respectively.30
In conclusion, we envision a model in which the expression of Bcl-xL during the monocyte/macrophage differentiation pathway, favors the long-living capacity of monocytes/macrophages. By contrast, the loss of Bcl-xL in granulocytes restricts the life span of these mature cells.
ACKNOWLEDGMENT
We thank I. Orman for excellent technical assistance.
Supported by Fondo de Investigaciones Sanitarias Grant No. 94/1415 and an AMGEN grant to J.L.F.-L. and by Comision Interministerial de Ciencia y Tecnologia Grants No. SAF-96/0274 to J.L.F.-L. and SAF-95/1548 CO2-01 to J.A.B.
Address reprint requests to Jose Luis Fernández-Luna, PhD, Servicio de Inmunologia, Hospital Universitario Marques de Valdecilla, Insalud, 39008 Santander, Spain.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal