Abstract
B-chronic lymphocytic leukemia (B-CLL) is characterized by the accumulation of long-lived B lymphocytes that express high levels of Bcl-2. We examined the involvement of CED-3/ICE-like proteases in the apoptosis of B-CLL cells. One of the substrates of these proteases is poly(ADP [adenosine 5′-diphosphate]-ribose) polymerase (PARP). The effect of different factors that induce the apoptosis of B-CLL cells on the proteolytic cleavage of PARP has been studied. Treatment of B-CLL cells with different concentrations of dexamethasone (1 to 1,000 μmol/L) induced in a dose-dependent manner the cleavage of PARP. Dexamethasone induced PARP cleavage after 12 hours of incubation, which was almost complete at 48 hours. PARP cleavage during apoptosis of B-CLL cells was studied in cells from eight patients and a correlation was found between cell viability and the degree of PARP cleavage. Incubation in vitro of B-CLL cells with fludarabine for 48 hours induced PARP cleavage in all the cases studied. Protein kinase C (PKC) activation with 100 nmol/L TPA (12-O-tetradecanoylphorbol 13-acetate) or incubation with interleukin-4 (10 ng/mL) prevented either dexamethasone- or fludarabine-induced proteolysis of PARP. Incubation of B-CLL cells with the CED-3/ICE–like protease inhibitor Z-VAD.fmk inhibited spontaneous and dexamethasone-induced PARP cleavage and DNA fragmentation in a dose-dependent manner. Furthermore, Z-VAD.fmk prevented the cytotoxic effect of dexamethasone. These results indicate that CED-3/ICE–like proteases play an important role in the apoptosis of B-CLL cells.
B-CHRONIC LYMPHOCYTIC leukemia (B-CLL) is characterized by the accumulation of monoclonal CD5+ B lymphocytes.1 The majority of circulating cells appear to be nondividing and it has been suggested that the clonal excess of B cells results from decreased cell death rather than increased proliferation.2
Apoptosis is the physiological process whereby most cells, including B lymphocytes, are eliminated, which leads to homeostasis.3 Apoptosis is characterized by morphological and biochemical changes that include cell shrinkage, membrane blebbing, chromatin condensation, and endogenous endonuclease activation.4 Apoptosis of B-CLL lymphocytes can be regulated by different cytokines.5 When B-CLL cells are placed in culture medium, they undergo apoptosis.6 This spontaneous apoptosis in cultured B-CLL cells is probably triggered by the absence of survival factors present in vivo. Candidate survival factors that prevent apoptosis of B-CLL cells in vitro are: interleukin-4 (IL-4), interferon-γ (IFN-γ), IFN-α, IL-2, IL-6, IL-8, and IL-13.7-14 On the other hand, IL-10 and IL-5 induce apoptosis in these cells.15,16 Glucocorticoids and other chemotherapeutic agents used clinically, like chlorambucil, 2-chloro-2′-deoxyadenosine and fludarabine, induce apoptosis in CLL lymphocytes,17-21 suggesting that apoptosis is one of the mechanisms of their therapeutic action.
The proto-oncogene product Bcl-2 has been shown to inhibit cell death induced by a variety of apoptotic stimuli and in numerous cell types.22 The Bcl-2 protein is overexpressed in B-CLL lymphocytes,23-25 which may inhibit the apoptosis of these cells. The mechanism of action of Bcl-2 is still unknown.26,27 It has been proposed that Bcl-2 has either an antioxidant activity28,29 or the capacity to interfere with intracellular calcium signaling.30 Protein kinase C (PKC) activation23 and also incubation with different cytokines, including IL-4,7 IL-8,13 and IFN-α,10 increase Bcl-2 levels in B-CLL cells and consequently block apoptosis. These findings suggest that a common step, inhibitable by Bcl-2, is activated during B-CLL apoptosis.
It is becoming evident that cysteine-proteases of the CED-3/ICE family play an important role in apoptosis.31-33 Inhibition of these proteases can block apoptosis triggered by different stimuli. They are synthesized as proenzymes, which are proteolytically processed to form active heterodimeric enzymes, but the mechanisms of control are largely unknown. Several substrates of these proteases have been described.32 Some of these proteases can cleave and inactivate poly(ADP-ribose) polymerase (PARP), an enzyme that participates in DNA repair and genome maintenance.34 Although the significance of PARP cleavage during apoptosis is not clear, it can be used as a marker of activation of CED-3/ICE–like proteases and apoptosis.35-38
CED-3/ICE–like proteases are involved in the apoptosis of different cell lines. Recently, involvement of CED-3/ICE–like proteases in the apoptosis of Ramos-Burkitt lymphoma cells has been reported.39 However, the implication of these proteases in the apoptosis of primary tumor cells has not been studied. Here we attempt to determine whether CED-3/ICE–like proteases are involved in the apoptosis of B-CLL cells. The knowledge of the mechanisms underlying B-CLL apoptosis could contribute to the design of new treatments for B-CLL.
MATERIALS AND METHODS
Patients.Eleven patients (six men and five women) with B-CLL, who had not received treatment, median age 64 years (range, 47 to 75 years) were studied. B-CLL was diagnosed according to standard clinical and laboratory criteria. The median peripheral blood leukocytosis was 104 × 109 leukocytes per liter (range, 19 to 425 × 109). Leukemic cells were phenotyped for cell surface markers by flow cytometry and were positive in all cases for CD5 and CD19. According to Binet's classification,40 at the time of inclusion four patients were at stage A, three patients were at stage B, and four patients were at stage C.
Reagents.12-O-tetradecanoylphorbol 13-acetate (TPA) and 3,(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma Chemical Co (St Louis, MO). Dexamethasone was obtained from Merck KGaA (Darmstadt, Germany). Fludarabine was obtained from Schering AG (Berlin, Germany). Recombinant human IL-4 was obtained from Genzyme (Cambridge, MA). N-benzyloxycarbonyl-Val-Ala-Asp-fluromethyl ketone (Z-VAD.fmk) and N-benzyloxycarbonyl-Phe-Ala-fluromethyl ketone (Z-FA.fmk) were obtained from Enzyme Systems Products (Dublin, CA). Anti-PARP polyclonal antibody (Vi.5) raised against the recombinant human PARP overproduced in Sf9/baculovirus was kindly provided by Dr Gilbert de Murcia (Strasbourg, France).
Isolation of B-CLL cells.Peripheral blood lymphocytes from B-CLL patients were obtained from the Hematology Laboratory at Hospital Clinic, Barcelona, Spain. Mononuclear cells from peripheral blood samples were isolated by centrifugation on a Ficoll/Hypaque (Seromed, Berlin, Germany) gradient and cryopreserved in liquid nitrogen in the presence of 10% dimethyl sulfoxide (DMSO).
Cell culture.B-CLL lymphocytes were cultured immediately after the thawing of the cells at a concentration of 2 to 5 × 106 cells/mL in RPMI 1640 culture medium (GIBCO-BRL, Paisley, Scotland) supplemented with 10% heat inactivated fetal calf serum (FCS), 2 mmol/L glutamine and gentamicin 0.04 mg/mL at 37°C in a humidified atmosphere containing 5% carbon dioxide.41 Factors were added at the beginning of the culture.
Cell viability assay.Cell viability was determined by the MTT assay.42 B lymphocytes (5 × 105 cells/well) were incubated in 96-well plates in the absence or presence of factors in a final volume of 100 μL. After 48 hours, 10 μL of MTT (5 mg/mL in phosphate-buffered saline) was added to each well for an additional 6 hours. The blue MTT formazan precipitated was dissolved in 100 μL of isopropanol: 1 mol/L HCl (24:1). After 1 hour of incubation at 37°C, the absorbance values at 540 nm were determined on a multiwell plate reader. Cell viability is expressed in percentage with respect to cells incubated with 100 nmol/L TPA, which maintains cell viability.17 43
Western blot analysis of PARP cleavage.Cells were lysed with Laemmli sample buffer44 and equal amounts of protein estimated by the BCA Protein Assay (Pierce, Rockford, IL) were separated by electrophoresis on 10% or 12% polyacrylamide gel and transferred to Immobilon-P (Millipore, Bedford, MA) membranes. After blocking for 1 hour with 5% dried skimmed milk in TBST (50 mmol/L Tris HCl pH 8.0, 150 mmol/L NaCl, 0.5% Tween-20), the filters were incubated with Vi.5 PARP antibody diluted 1:1,000 in 5% dried skimmed milk in TBST. Antibody binding was detected using a secondary antibody (swine antirabbit immunoglobulin, DAKO, Glostrup, Denmark) conjugated to horseradish peroxidase diluted 1:500 in 5% dried skimmed milk in TBST and an enhanced chemiluminescence (ECL) detection kit (Amersham, Buckinghamshire, UK).
Analysis of DNA fragmentation.Analysis of DNA fragmentation by agarose gel electrophoresis was performed essentially as previously described.45 Five million cells were pelleted and lysed in 10 mmol/L Tris-HCl pH 7.4, 0.2% Triton X-100, 1 mmol/L EDTA. After incubating for 20 minutes at 4°C, cell lysates were centrifuged at 14,000g for 15 minutes to separate low molecular weight DNA from intact chromatin. The supernatant was treated with 0.2 mg/mL of proteinase K in a buffer containing 150 mmol/L NaCl, 10 mmol/L Tris HCl pH 8.0, 40 mmol/L EDTA and 1% sodium dodecyl sulfate (SDS) for 6 hours at 37°C. The DNA preparations were extracted twice with buffered phenol to remove proteins. DNA was precipitated with 140 mmol/L NaCl and two volumes of ethanol at −20°C overnight. DNA precipitates were recovered by centrifugation at 14,000g for 15 minutes, washed twice in 70% ethanol, and air dried. DNA pellets were dissolved in 15 μL of double-distilled water and treated for 1 hour at 37°C with RNase (Boehringer Mannheim, Mannheim, Germany). A total of 5 μL of loading buffer was added to each tube and the DNA preparations were electrophoresed in 1% agarose gels. Gels were stained with ethidium bromide and visualized under ultraviolet (UV) light.
RESULTS
PARP cleavage during glucocorticoid-induced apoptosis of B-CLL cells.First, the effect of the incubation in the absence of any factor or in the presence of glucocorticoids on the viability of B-CLL lymphocytes from different patients was analyzed (Table 1). Different degrees of spontaneous and dexamethasone-induced loss of viability were observed after 48 hours of incubation. In agreement with previous reports,6 17 both spontaneous and dexamethasone-induced loss of viability were confirmed to be apoptosis by analysis of fragmented DNA (results not shown).
Cytotoxic Effect of Dexamethasone and Fludarabine on B-CLL Lymphocytes
| Patient . | % Viability . | ||
|---|---|---|---|
| No. . | Control . | Dexamethasone . | Fludarabine . |
| 1 | 99 ± 9 | ND | 54 ± 9 |
| 2 | 102 ± 18 | 31 ± 2 | 28 ± 2 |
| 3 | 65 ± 1.5 | 52 ± 1 | 27 ± 3 |
| 4 | 29 ± 1 | 13 ± 0.2 | 7 ± 0.1 |
| 5 | 55 ± 3 | 25 ± 2 | 21 ± 2 |
| 6 | 25 ± 1 | 18 ± 1 | 15 ± 1 |
| 7 | 83 ± 13 | 52 ± 12 | 37 ± 19 |
| 8 | 69 ± 0.3 | 22 ± 0.3 | 26 ± 2 |
| 9 | 65 ± 4 | 27 ± 0.4 | 32 ± 2 |
| 10 | 100 ± 2.5 | ND | 32 ± 4 |
| 11 | 68 ± 2 | 50 ± 0.3 | 25 ± 5 |
| Patient . | % Viability . | ||
|---|---|---|---|
| No. . | Control . | Dexamethasone . | Fludarabine . |
| 1 | 99 ± 9 | ND | 54 ± 9 |
| 2 | 102 ± 18 | 31 ± 2 | 28 ± 2 |
| 3 | 65 ± 1.5 | 52 ± 1 | 27 ± 3 |
| 4 | 29 ± 1 | 13 ± 0.2 | 7 ± 0.1 |
| 5 | 55 ± 3 | 25 ± 2 | 21 ± 2 |
| 6 | 25 ± 1 | 18 ± 1 | 15 ± 1 |
| 7 | 83 ± 13 | 52 ± 12 | 37 ± 19 |
| 8 | 69 ± 0.3 | 22 ± 0.3 | 26 ± 2 |
| 9 | 65 ± 4 | 27 ± 0.4 | 32 ± 2 |
| 10 | 100 ± 2.5 | ND | 32 ± 4 |
| 11 | 68 ± 2 | 50 ± 0.3 | 25 ± 5 |
B-CLL lymphocytes were incubated at 37°C for 48 hours in the absence of any factor (control) or with 10 μmol/L dexamethasone or fludarabine (5 μg/mL). Cell viability was determined by the MTT assay as described in Materials and Methods. Data are shown as the mean value ± SD.
Abbreviation: ND, not determined.
To study the involvement of CED-3/ICE–like proteases during the apoptosis of B-CLL cells, we determined whether PARP, the best-known substrate of these proteases, was cleaved when apoptosis was induced. Time-course and dose-response studies of PARP cleavage during glucocorticoid-induced apoptosis were performed with cells from one of the patients (patient no. 1). PARP cleavage was analyzed by immunoblotting using Vi.5 antibody against the enzyme, which recognizes both the native enzyme (116 kD) and the cleavage product (≈85 kD). Figure 1A shows that the 85-kD PARP proteolytic fragment appeared after 12 hours of incubation with 10 μmol/L dexamethasone and that this cleavage was more pronounced at 24 hours. At 48 hours, the native 116-kD PARP had almost disappeared and the total amount of PARP protein (116 kD plus 85 kD) had clearly decreased, indicating that the 85-kD protein was also being degraded. The finding that the proteolytic fragment of PARP was also observed in cells incubated without glucocorticoids for 48 hours is due to the spontaneous apoptosis that takes place in these cells when placed in culture medium.
PARP cleavage in B-CLL cells. (A) Time course of dexamethasone-induced PARP cleavage. B-CLL lymphocytes were incubated with or without 10 μmol/L dexamethasone for the indicated times. (B) Dose response of PARP cleavage. Cells were incubated for 48 hours with various concentrations of dexamethasone as indicated. PARP cleavage was analyzed by Western blot as described in Materials and Methods. The position of the native PARP (116 kD) and the proteolytic fragment (85 kD) is indicated.
PARP cleavage in B-CLL cells. (A) Time course of dexamethasone-induced PARP cleavage. B-CLL lymphocytes were incubated with or without 10 μmol/L dexamethasone for the indicated times. (B) Dose response of PARP cleavage. Cells were incubated for 48 hours with various concentrations of dexamethasone as indicated. PARP cleavage was analyzed by Western blot as described in Materials and Methods. The position of the native PARP (116 kD) and the proteolytic fragment (85 kD) is indicated.
The dose-response study after 48 hours of incubation with dexamethasone (Fig 1B) showed that PARP cleavage was dose-dependent and detectable in the presence of 1 μmol/L dexamethasone. The proteolysis of PARP was complete when a high dose of dexamethasone (1 mmol/L) was used.
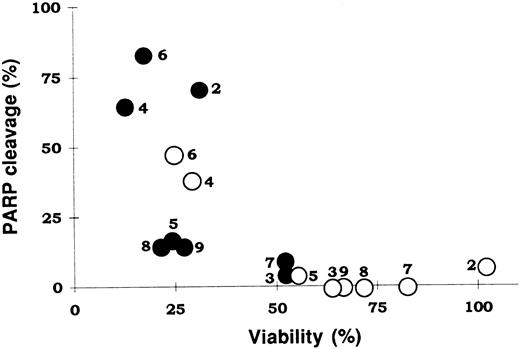
PARP cleavage was further studied in lymphocytes from eight additional B-CLL patients. Cells were incubated with two doses of dexamethasone, for 24 hours. DNA and protein extracts were obtained following incubation of cells for 24 hours in 1 or 10 μmol/L dexamethasone. Agarose gel electrophoresis of DNA had the characteristic ladder-like pattern of apoptosis (results not shown). Analysis of the protein extracts by Western blot showed that in all cases dexamethasone treatment increased the proteolysis of PARP, which was shown by an increase in the cleavage product and/or a decrease in the quantity of native enzyme. A correlation was found between viability and the degree of PARP cleavage (Fig 2). Thus, the cells most sensitive to dexamethasone (patients no. 2 and 8) showed a high dexamethasone-induced proteolysis of PARP, and the least sensitive cells (patients no. 3 and 7) had a very low degree of PARP cleavage. The spontaneous cleavage of PARP also depended on the patient studied, so cells from patients 4 and 6 had a very high degree of spontaneous cleavage of PARP, which correlated with a high spontaneous loss of viability.
Correlation between cell viability and PARP degradation. The values of cell viability for cells incubated without (○) or with 10 μmol/L dexamethasone (•) are shown in Table 1. PARP degradation was quantified by densitometric scanning of the immunoblots. Numbers beside the symbols indicate patient numbers.
Correlation between cell viability and PARP degradation. The values of cell viability for cells incubated without (○) or with 10 μmol/L dexamethasone (•) are shown in Table 1. PARP degradation was quantified by densitometric scanning of the immunoblots. Numbers beside the symbols indicate patient numbers.
Effect of fludarabine on the cleavage of PARP.The effect of another drug that also induces apoptosis but has a different mechanism of action was next studied. Fludarabine, a purine analogue commonly used in B-CLL treatment,46 induces apoptosis in these cells.21 The effect of 5 μg/mL fludarabine on the viability of lymphocytes from B-CLL patients after 48 hours of incubation is shown in Table 1. All patients were sensitive to fludarabine and this compound induced apoptosis as shown by DNA fragmentation analysis (results not shown). We next analyzed whether fludarabine induced PARP cleavage. Incubation of B-CLL cells from six patients with fludarabine for 48 hours in vitro induced PARP cleavage in all cases (results not shown). The degree of proteolysis depended on the patient studied and ranged from 57% to 95% (mean value, 83.4 ± 14).
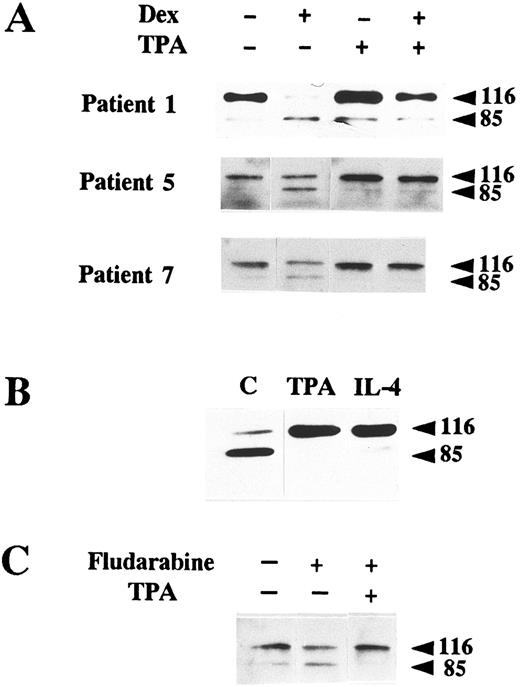
PKC activation and IL-4 inhibit PARP proteolysis.Factors such as the phorbol ester TPA, an activator of PKC, and IL-4 have been described as inhibitors of apoptosis in B-CLL lymphocytes.7,17 19 We studied whether these factors produced any effect on PARP cleavage. As seen in Fig 3A, incubation of B-CLL cells from three patients with 100 nmol/L TPA inhibited glucocorticoid-induced PARP cleavage. Incubation of cells from patient 6 (which had a high degree of spontaneous apoptosis) with 100 nmol/L TPA blocked PARP cleavage (Fig 3B). This effect was also observed when cells were incubated with 10 ng/mL IL-4. Finally, we tested if TPA blocked fludarabine-induced apoptosis. TPA inhibited both DNA degradation (results not shown) and PARP cleavage (Fig 3C).
Inhibition of PARP proteolysis by TPA and IL-4. (A) Inhibition of dexamethasone-induced PARP proteolysis by TPA. B-CLL lymphocytes from three patients were incubated with 100 nmol/L TPA in the presence or absence of 10 μmol/L dexamethasone for 48 hours. (B) Inhibition of spontaneous PARP proteolysis. Cells from patient 6 were incubated for 48 hours either in the absence of any factor (C) or with 100 nmol/L TPA, or IL-4 (10 ng/mL). (C) Inhibition of fludarabine-induced PARP cleavage. Cells from patient 5 were incubated with the indicated factors for 24 hours. The concentrations used were 100 nmol/L TPA and 5 μg/mL fludarabine. Western blot of PARP was performed with protein extracts from these cells as previously described.
Inhibition of PARP proteolysis by TPA and IL-4. (A) Inhibition of dexamethasone-induced PARP proteolysis by TPA. B-CLL lymphocytes from three patients were incubated with 100 nmol/L TPA in the presence or absence of 10 μmol/L dexamethasone for 48 hours. (B) Inhibition of spontaneous PARP proteolysis. Cells from patient 6 were incubated for 48 hours either in the absence of any factor (C) or with 100 nmol/L TPA, or IL-4 (10 ng/mL). (C) Inhibition of fludarabine-induced PARP cleavage. Cells from patient 5 were incubated with the indicated factors for 24 hours. The concentrations used were 100 nmol/L TPA and 5 μg/mL fludarabine. Western blot of PARP was performed with protein extracts from these cells as previously described.
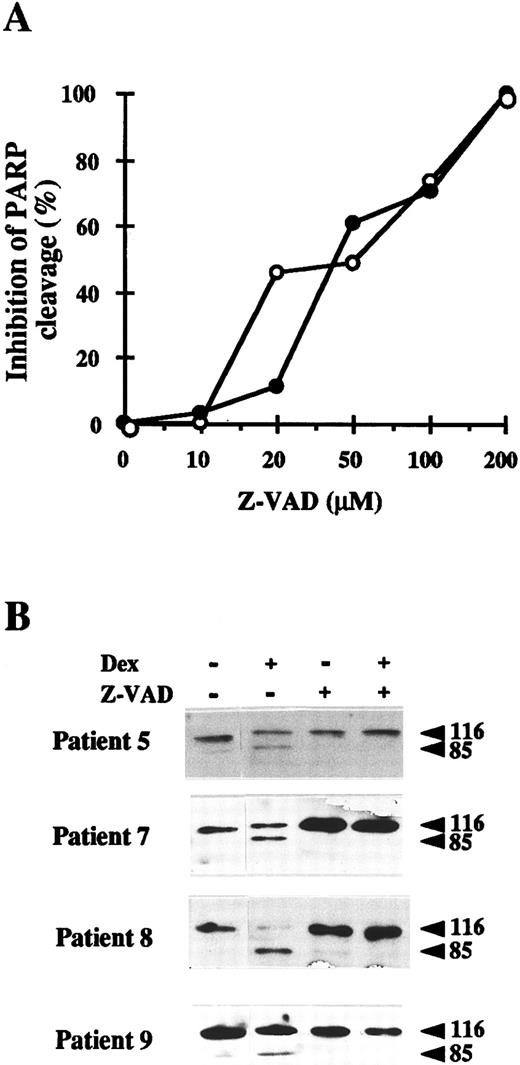
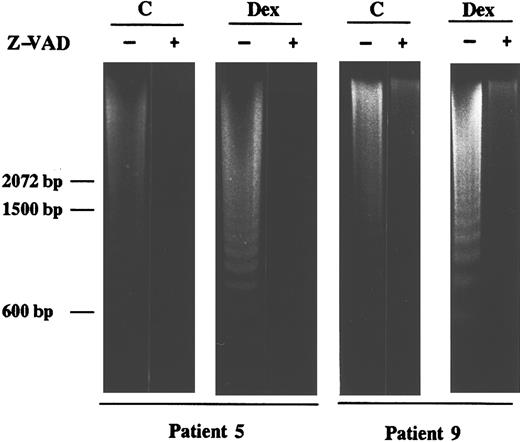
Inhibition of CED-3/ICE–like proteases blocks apoptosis of B-CLL cells.To confirm the involvement of CED-3/ICE–like proteases in the apoptosis of B-CLL cells, we used a specific inhibitor of these proteases, Z-VAD.fmk.47 The dose response study of Z-VAD.fmk on the inhibition of spontaneous and dexamethasone-induced apoptosis was performed with cells from patients 6 and 9, respectively. As seen in Fig 4A, 20 to 50 μmol/L Z-VAD.fmk decreased the proteolysis of PARP and 200 μmol/L completely blocked it. Incubation of B-CLL cells from four patients with 200 μmol/L Z-VAD.fmk for 48 hours in the presence or absence of dexamethasone showed that PARP proteolysis was inhibited in all cases studied (Fig 4B). Z-FA.fmk (200 μmol/L), a compound similar to Z-VAD.fmk, which lacks the aspartic acid residue that is necessary to inhibit CED-3/ICE–like proteases, did not block PARP degradation (data not shown). Furthermore, analysis by agarose gel electrophoresis showed that 200 μmol/L Z-VAD.fmk also inhibited both spontaneous and dexamethasone-induced DNA fragmentation almost completely (Fig 5).
Effect of the CED-3/ICE–like protease inhibitor Z-VAD.fmk on PARP proteolysis in B-CLL cells. (A) Dose response of the inhibitory effect of Z-VAD.fmk on PARP proteolysis. Cells from patient 6 were incubated without dexamethasone (○) and cells from patient 9 were incubated with 10 μmol/L dexamethasone (•), in the presence of various concentrations of Z-VAD.fmk for 48 hours. Western blots of PARP were performed as described in Materials and Methods and the inhibition of PARP cleavage was quantified by densitometric scanning of the immunoblots. (B) B lymphocytes from four B-CLL patients were incubated for 48 hours with 10 μmol/L dexamethasone in the presence or absence of 200 μmol/L Z-VAD.fmk. Z-VAD.fmk was added 1 hour before dexamethasone administration. Cells were lysed and analyzed by Western blot as described in Materials and Methods.
Effect of the CED-3/ICE–like protease inhibitor Z-VAD.fmk on PARP proteolysis in B-CLL cells. (A) Dose response of the inhibitory effect of Z-VAD.fmk on PARP proteolysis. Cells from patient 6 were incubated without dexamethasone (○) and cells from patient 9 were incubated with 10 μmol/L dexamethasone (•), in the presence of various concentrations of Z-VAD.fmk for 48 hours. Western blots of PARP were performed as described in Materials and Methods and the inhibition of PARP cleavage was quantified by densitometric scanning of the immunoblots. (B) B lymphocytes from four B-CLL patients were incubated for 48 hours with 10 μmol/L dexamethasone in the presence or absence of 200 μmol/L Z-VAD.fmk. Z-VAD.fmk was added 1 hour before dexamethasone administration. Cells were lysed and analyzed by Western blot as described in Materials and Methods.
Effect of the CED-3/ICE–like protease inhibitor Z-VAD.fmk on DNA fragmentation in B-CLL cells. Cells from patients 5 and 9 were incubated for 24 hours with 10 μmol/L dexamethasone in the presence or absence of 200 μmol/L Z-VAD.fmk. Z-VAD.fmk was added 1 hour before dexamethasone administration. DNA was extracted and subjected to agarose gel electrophoresis as described in Materials and Methods. Similar results were obtained with cells from patients 7 and 8.
Effect of the CED-3/ICE–like protease inhibitor Z-VAD.fmk on DNA fragmentation in B-CLL cells. Cells from patients 5 and 9 were incubated for 24 hours with 10 μmol/L dexamethasone in the presence or absence of 200 μmol/L Z-VAD.fmk. Z-VAD.fmk was added 1 hour before dexamethasone administration. DNA was extracted and subjected to agarose gel electrophoresis as described in Materials and Methods. Similar results were obtained with cells from patients 7 and 8.
Finally, we studied the effect of this inhibitor on the viability of B-CLL cells. Z-VAD.fmk (200 μmol/L) maintained B-CLL lymphocytes viability for 4 days, although incubation for longer periods of time decreased it (64% ± 8% after 7 days of incubation). The effect of Z-VAD.fmk on the viability of B-CLL lymphocytes incubated for 48 hours in the absence or presence of dexamethasone was studied. As shown in Fig 6, Z-VAD.fmk inhibited the cytotoxic effect of dexamethasone in all the patients studied. Although Z-VAD.fmk had no significant effects on B lymphocytes from patients 7, 8, and 9, in those patients with high spontaneous apoptosis (patients no. 5 and 6), Z-VAD.fmk reduced the spontaneous loss of viability.
Effect of Z-VAD.fmk on the viability of B-CLL cells. Cells from five patients were seeded in 96-microwell plates and incubated for 48 hours with the indicated factors. The concentrations used were 10 μmol/L dexamethasone and 200 μmol/L Z-VAD.fmk. Z-VAD.fmk was added 1 hour before dexamethasone administration. Cytotoxicity was analyzed by the MTT method as described in Materials and Methods. Data are shown as the mean value ± SD of two (patients 6, 8, and 9) or four (patients 5 and 7) independent experiments. Statistical significance of differences between treatments without or with Z-VAD.fmk was assayed by analysis of variance (ANOVA) (Fisher's Protected Least Significant Difference [PLSD]): * = P < .12, ** = P < .05, *** = P < .01.
Effect of Z-VAD.fmk on the viability of B-CLL cells. Cells from five patients were seeded in 96-microwell plates and incubated for 48 hours with the indicated factors. The concentrations used were 10 μmol/L dexamethasone and 200 μmol/L Z-VAD.fmk. Z-VAD.fmk was added 1 hour before dexamethasone administration. Cytotoxicity was analyzed by the MTT method as described in Materials and Methods. Data are shown as the mean value ± SD of two (patients 6, 8, and 9) or four (patients 5 and 7) independent experiments. Statistical significance of differences between treatments without or with Z-VAD.fmk was assayed by analysis of variance (ANOVA) (Fisher's Protected Least Significant Difference [PLSD]): * = P < .12, ** = P < .05, *** = P < .01.
DISCUSSION
This report describes the involvement of CED-3/ICE–like proteases in the apoptosis of B-CLL cells. To our knowledge, this is the first report of a role for CED-3/ICE–like proteases in the apoptosis of primary cancer cells. The results presented herein show that activation of CED-3/ICE–like proteases in B-CLL cells is a common step in spontaneous, glucocorticoid- and fludarabine-induced apoptosis. Furthermore, apoptosis of B-CLL cells is blocked by a specific inhibitor of CED-3/ICE proteases, suggesting that these proteases are essential for the apoptosis of these cells.
At present, the mechanisms that control CED-3/ICE–like proteases are largely unknown.31,32 There seems to be a cascade of proteases similar to the one involved in the activation of the complement, but the signal that starts this apoptotic cascade is not known. Recently, it has been reported that CD95/FasR uses the adaptor protein FADD/MORT1 physically to engage FLICE/MACH, the apical component of a proteolytic cascade of CED-3/ICE–like proteases.48 49
Our results also show that PKC activation inhibits spontaneous, glucocorticoid- and fludarabine-induced apoptosis and blocks PARP cleavage. Furthermore, PARP cleavage is blocked by IL-4. These findings suggest that both PKC and IL-4 signal transduction pathways cross-talk with the different apoptotic pathways in a common step upstream of the activation of the proteases that cleave PARP. This common step could be Bcl-2, as both PKC activation23 and IL-47 increase the levels of Bcl-2 protein. Recently, biochemical evidence that Bcl-2 functions upstream of the CED-3/ICE family proteases has been reported,50-52 as Bcl-2 prevents the processing of these proteases and the cleavage of poly(ADP-ribose) polymerase.
Although it has been used as a marker of apoptosis,35-38 the significance of PARP cleavage during this process is unknown. Mice with a disrupted PARP gene do not show altered apoptosis,53 however, a dominant negative of PARP induces apoptosis of transfected cells.54 PARP has been implicated in different processes including DNA repair, DNA replication, and transcription.34 Interestingly, in B-CLL cells PARP is the most abundant protein to bind to damaged DNA.55 It has been proposed that a central function of CED-3/ICE proteases in apoptosis is the cleavage of PARP and other nuclear repair proteins, thereby abolishing their critical homeostatic functions.56
The CED-3/ICE–like proteases present in B-CLL cells have not been identified, but some of these proteases are expressed in B lymphocytes.57-59 The identification of these proteases in B-CLL cells and the knowledge of their mechanisms of control could be very important to design new therapies for this disease. In this respect, direct activation of these proteases to induce apoptosis may be achieved.
ACKNOWLEDGMENT
We are indebted to Dr G. de Murcia for the kind gift of PARP antibodies. We thank Drs G. Pons, A. López-Rivas, J.L. Vives-Corrons, N. Villamor, and C. Pastor and A. Becerra for their help and suggestions, Drs J. Esteve and J. Briones for their help in providing peripheral blood from patients, and R. Rycroft for language assistance.
Supported by “Fondo de Investigaciones Sanitarias de la Seguridad Social” (FIS 95/0873) and by the “Generalitat de Catalunya” (1995 SGR 00427), Spain. B.B. is the recipient of a research fellowship from Comissió Interdepartamental de Recerca i Innovació Technològica (CIRIT, Spain).
Address reprint requests to Joan Gil, PhD, Unitat de Bioquı́mica, Facultat de Medicina, Campus de Bellvitge, Universitat de Barcelona, Pavelló de Govern, 1a planta, 08907 L'Hospitalet, Spain.


![Fig. 6. Effect of Z-VAD.fmk on the viability of B-CLL cells. Cells from five patients were seeded in 96-microwell plates and incubated for 48 hours with the indicated factors. The concentrations used were 10 μmol/L dexamethasone and 200 μmol/L Z-VAD.fmk. Z-VAD.fmk was added 1 hour before dexamethasone administration. Cytotoxicity was analyzed by the MTT method as described in Materials and Methods. Data are shown as the mean value ± SD of two (patients 6, 8, and 9) or four (patients 5 and 7) independent experiments. Statistical significance of differences between treatments without or with Z-VAD.fmk was assayed by analysis of variance (ANOVA) (Fisher's Protected Least Significant Difference [PLSD]): * = P < .12, ** = P < .05, *** = P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/9/10.1182_blood.v89.9.3378/3/m_bl_0038f6.jpeg?Expires=1769550003&Signature=5Pv6SRkB7HbUMFpRVE8MDnfPCIFwbe9fNK~igcDTDc92W92lxTBjDpTDbfX8OWXwuBa08dYbxRtNjybHc7Vwb47wlfgkSk6E4yMJEDHTJsD-K2V576RWK7A3UHkv6VBxiB5DzlLaT5RdKZA2MDBio4OLp6N1Ypk-pyQmLq0Ni63NP9jcqys-mol4QkmBQOns03u2xeRWsO~ZsoBkY4zyrYtTtLgcKfBtKjbUPMDqXuNcWJ5v~5PK8jio~udhh9TU~qXKJluE-NfmUNJAPXt6s0wunZtmbpq1fLr0OjHLaIu2C52Oc6JOjfzkcCfZRP795DAIbT44miO-zrs5pgs4pw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal