Abstract
By immunization with nuclear lysates of L428 cells, we raised a monoclonal mouse antibody, Ki-S2 (IgG1 ). In Western blots, this antibody recognizes a nuclear antigen with an apparent molecular mass of 100 kD, termed p100. Protein sequencing of p100 showed that this is a hitherto unknown protein. Immunohistochemical examination of cryostat and paraffin sections of nearly all human tissue types and neoplasms showed that p100 was exclusively expressed in the nuclei of a fraction of proliferating cells. Cell sorting and fluorescence-activated cell sorting analysis of stimulated peripheral blood mononuclear cells showed that p100 was exclusively expressed in proliferating cells from the transition G1/S until the end of cytokinesis. During mitosis, this protein is strictly associated with the spindle pole and with the mitotic spindle, whereas during S and G2 , p100 is diffusely distributed throughout the cell nucleus. Immediately after completion of cytokinesis, p100 was rapidly degraded. In L428 cells, p100 is phosphorylated at least during mitosis. It has a turnover time of about 1 hour. Studies on routinely processed paraffin sections of specimens of malignant lymphoma, benign and malignant nevocellular tumors, and breast cancer showed that in all cases less than 40% of the Ki-67–positive growth fraction expressed p100. Thus, p100 might prove to be a more reliable measure of cellular proliferation and one that is more closely correlated to cancer prognosis, beyond its general biologic relevance as a cell cycle protein.
RECENT PROGRESS IN analyzing the structure and function of cell cycle-associated proteins such as cyclins, cyclin-dependent kinases, and their inhibitors has considerably promoted our understanding of the mechanisms controlling cellular proliferation.1-3 Among the large number of proteins involved, only a few have proven useful for monitoring proliferative activity and growth fraction. The ability to identify antigens that are reliably associated with cell proliferation in normal and neoplastic tissue, such as Ki-67 and topoisomerase-IIα, by means of highly specific antibodies has proven invaluable in diagnostic histopathology and cell biology.4-10
Both antigens have proven their diagnostic and prognostic value in several retrospective and prospective studies.5,8 These proliferation markers have found widespread application, but some studies have failed to show a close correlation between antigen expression and either the growth fraction or the prognosis of tumors.11,12 The major shortcoming has been an overestimation of the size of the proliferation compartment.11-13 One of the major reasons for this failure is the fact that proteins such as Ki-67 and topoisomerase-IIα are expressed in the cell cycle phases S, G2 , M, and G1.4,7,14 However, the G1 phase can easily be influenced by several external and internal factors.15-17 Cells in G1 quiescence may pass the restriction point G1/S and contribute directly to the magnitude of the proliferation compartment, or they may senesce in G0 and succumb by apoptosis.15-17 In contrast to G1 , the S, G2 , and M phases of the cell cycle are relatively constant in duration.17 Moreover, cells in G1 make up the largest fraction of the cycling subpopulation. Thus, G1 is the major source of uncertainty in estimations of the growth fraction.
We describe a hitherto unknown proliferation-associated protein expressed exclusively from the transition G1/S until the end of cytokinesis.
MATERIALS AND METHODS
Generation of monoclonal antibodies (MoAbs).L428 cells (a generous gift from Prof V. Diehl, Köln, Germany) were lysed and the nuclei were washed and harvested by centrifugation. These nuclei were used in incomplete Freund's adjuvant to immunize female BALB/C mice by three intraperitoneal injections at 10-day intervals. After boostering, the splenocytes were fused with P3x63-Ag.8653 mouse myeloma cells. Hybridomas were grown in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and subcloned. Supernatants were screened for their reactivity with proliferating cells in normal human tissue.
Tissue samples.The specificity of the MoAb was tested on approximately 4-μm–thick cryostat sections of nearly all normal human tissue types. In addition, Ki-S2 was tested on routinely processed paraffin sections of the same samples. Peripheral blood mononuclear cells (PBMC) were prepared as cytospin slides. To show the positive correlation between the MoAb Ki-S2 and other established proliferation markers reported earlier from our laboratory,7,9 47 cases of non-Hodgkin's lymphoma classified according to the updated Kiel classification18 and the Working Formulation19 were studied using Ki-S2 and Ki-S5 on routinely processed paraffin sections. The latter antibody is directed against the Ki-67 antigen.9 Twenty-six of the cases had been classified as chronic B-lymphocytic leukemia (B-CLL) and 21 as Burkitt's type high-grade B-cell lymphoma. In addition, 47 cases of melanocytic lesions and 100 cases of stage I nodal negative ductal invasive carcinoma of the breast were included.
Immunocytochemistry.Tissue samples fixed in 5% to 10% formaldehyde for an unknown period of time were routinely processed for paraffin embedding. Approximately 4-μm–thick tissue sections were fixed onto sialinized slides. For antigen retrieval, sections were treated in a microwave oven as described earlier for other proliferation-specific nuclear antigens.7,9 Cryostat sections and cytospin slides were fixed in acetone at room temperature for 10 minutes and incubated with the primary antibody (Ki-S2 and Ki-S5 [specific for the Ki-67 antigen]) for 30 minutes. The immunoreaction was visualized by means of the alkaline phosphatase antialkaline phosphatase (APAAP) method.20 The slides were briefly counterstained with Meyer's hemalum (all reagents from Dianova, Hamburg, Germany). Immunofluorescence stainings of the L428 cells were performed with the MoAb Ki-S2 and a goat antimouse polyclonal antibody coupled with Cy3. A polyclonal rabbit anti Ki-67 serum (kindly provided by Prof Dr J. Gerdes, Borstel, Germany) was used for double immunofluorescence stainings.
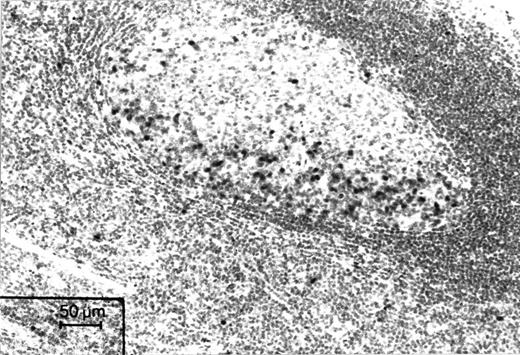
Immunohistochemical staining of a microwave-processed paraffin section of a formalin-fixed human tonsil with Ki-S2. Cell nuclei in the dark zone of a germinal center, which harbors proliferating cells, are strongly positive. APAAP staining.
Immunohistochemical staining of a microwave-processed paraffin section of a formalin-fixed human tonsil with Ki-S2. Cell nuclei in the dark zone of a germinal center, which harbors proliferating cells, are strongly positive. APAAP staining.
Flow cytometry.PBMC from healthy donors were obtained by density centrifugation of heparinized blood.21 PBMC (106/mL) were cultured with 1 μg/mL phytohemagglutinin A (PHA; Wellcome, Maidstone, UK) in RPMI 1640 supplemented with 10% human serum. Stimulated PBMC were harvested daily. Some of them were used to prepare cytospin slides, which were immunostained with Ki-S2 and Ki-S5. Differential counts based on 500 cells each in Ki-S5 and Ki-S2 immunostained cytospin slides were correlated. Further portions were fixed for 40 minutes with fresh paraformaldehyde solution (0.5 g/mL) in phosphate-buffered saline (PBS) at 4°C. The cells were washed and subsequently permeabilized with 0.1% Triton-X 100 in PBS. PBMC (5 × 105) were incubated with Ki-S2 at 4°C for 1 hour, washed, and labeled with fluorescein isothiocyanate goat antimouse serum for 30 minutes. An anti–IL-2 antibody of the same isotype (IgG1) was used as the negative control. Cytometry was performed on a FACScan flow cytometer (BectonDickinson, Mountain View, CA). DNA staining was recorded on a linear scale and antibody staining on a log scale. A total of 1,024 channels were analyzed.
Cell sorter analysis.On day 3, PHA-stimulated (10 μL/mL) PBMC were sorted twice with a cell sorter according to their DNA/RNA (Hoechst 33342, 2 mg/mL; pyronin Y, 5 mmol/L) content into G0/G1 and S/G2/M phase cells.22 Cytospin preparations of the two sorted fractions were immunostained with Ki-S2 and Ki-S5 using the APAAP technique.
Biolabeling and determination of the molecular mass of the antigen.L428 cells (3 × 106/mL) were labeled with [35S]methionine (50 mCi) overnight. All further steps were performed on ice or at 4°C. After intensive washing with ice-cold PBS, the cells were lysed with 2% Triton-X 100, 1 mmol EDTA, and 1 mmol phenylmethanesufonyl fluoride (PMSF ) in PBS, pH 7.4, for 5 minutes. After a short centrifugation step, the pelleted nuclei were lysed with 0.35 mol/L NaCl, 1 mmol PMSF for 30 minutes. The lysate of the nuclei was then centrifuged for 10 minutes at 15,000g, and the supernatant was used for immunoprecipitation with Ki-S2. The MoAb Ki-S2 was coupled to rabbit antimouse IgG-coupled protein A Sepharose CL-4B (Sigma, München, Germany). After 1 hour of incubation, the immunoprecipitates were washed several times with a low (PBS, 0.4% Triton-X 100) and high salt solution (PBS, 0.5 mol/L NaCl, 0.4% Triton-X 100). After washing, the immunoprecipitate was boiled in loading buffer under reducing conditions for 5 minutes. The immunoprecipitate was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) at a gradient of 5% to 10% or 7.5% to 15%. The gels were subjected to autoradiography as described previously.23 A molecular weight standard (SDS-6H; Sigma) served as reference.
To determine the turnover time of the Ki-S2 antigen, we labeled L428 cells for 1 hour. Subsequently, the cells were intensively washed and immunoprecipitation experiments were performed as described above at various time intervals after the end of labeling. In addition to immunoprecipitation, Western blotting was performed with Ki-S2. In these experiments lysates of L428 were separated by SDS-PAGE at a gel gradient of 5% to 10% or 7.5% to 15%) and then transferred to nitrocellulose membranes overnight. After blocking with 3% bovine serum albumin, the membranes were incubated with supernatant containing Ki-S2. Visualization was performed with 4-chloro 1-naphthol after the membranes had been incubated with peroxidase-conjugated rabbit antimouse IgG (Sigma). To see whether the Ki-S2 antigen is subject to phosphorylation, radiolabeling experiments were performed with [32P]orthophosphate (Amersham Buchler, Braunschweig, Germany). L428 cells (1 × 107) were washed with phosphate-free buffer and then incubated in phosphate-free medium (minimal essential medium [MEM]; Bioconcept, Umkirch, Germany) supplemented with 20 mmol/L glutamine and 10% FCS for 30 minutes. Then, 1 mCi [32P]orthophosphate (carrier-free) was added. After 1 hour, cells were harvested and prepared for immunoprecipitation as described above.
PHA Stimulation of Peripheral Blood Lymphocytes
| Days . | n . | Ki-S2 . | Ki-S3 . | % Ki-S2 . |
|---|---|---|---|---|
| 0 | 8 | >1% | >1% | |
| 1 | 8 | 2% | 5% ± 2% | 40% |
| 2 | 8 | 19% ± 5% | 37% ± 5% | 51% |
| 3 | 8 | 25% ± 4% | 64% ± 9% | 39% |
| 4 | 8 | 17% ± 3% | 43% ± 10% | 39% |
| 5 | 8 | 10% ± 4% | 34% ± 9% | 29% |
| 6 | 8 | 9% ± 2% | 26% ± 6% | 35% |
| Days . | n . | Ki-S2 . | Ki-S3 . | % Ki-S2 . |
|---|---|---|---|---|
| 0 | 8 | >1% | >1% | |
| 1 | 8 | 2% | 5% ± 2% | 40% |
| 2 | 8 | 19% ± 5% | 37% ± 5% | 51% |
| 3 | 8 | 25% ± 4% | 64% ± 9% | 39% |
| 4 | 8 | 17% ± 3% | 43% ± 10% | 39% |
| 5 | 8 | 10% ± 4% | 34% ± 9% | 29% |
| 6 | 8 | 9% ± 2% | 26% ± 6% | 35% |
Evaluation of immunohistochemical staining with Ki-S2 and Ki-S5. Numbers represent mean and standard deviation of percentages.
Abbreviation: n, number of blood donors.
Immunohistochemical Staining of Different Tumor Types: Comparison of Ki-S2 and the Anti–Ki-67 Antigen-Specific MoAb Ki-S5
| Diagnosis . | n . | Ki-S2 . | Ki-S5 . | % Ki-S2 . |
|---|---|---|---|---|
| B-CLL | 26 | 4% ± 3% | 14% ± 6% | 28% |
| Burkitt's lymphoma | 21 | 95% ± 18% | 36% ± 9% | 37% |
| Breast cancer | 100 | 17% ± 6% | 43% ± 18% | 39% |
| Malignant melanoma | 18 | 16% ± 6% | 48% ± 22% | 33% |
| Benign nevi | 29 | >1% | 4% ± 3% | 25% |
| Diagnosis . | n . | Ki-S2 . | Ki-S5 . | % Ki-S2 . |
|---|---|---|---|---|
| B-CLL | 26 | 4% ± 3% | 14% ± 6% | 28% |
| Burkitt's lymphoma | 21 | 95% ± 18% | 36% ± 9% | 37% |
| Breast cancer | 100 | 17% ± 6% | 43% ± 18% | 39% |
| Malignant melanoma | 18 | 16% ± 6% | 48% ± 22% | 33% |
| Benign nevi | 29 | >1% | 4% ± 3% | 25% |
Numbers represent the mean and standard deviation of percentages. All tissue samples were routinely processed paraffin sections.
Abbreviation: n, number of cases.
Cell culture and synchronization.All experiments were performed with the cell line L428. L428 cells were grown in RPMI 1640 supplemented with 10% FCS and 2 mmol/L L-glutamine. Exponentially growing cells were arrested with 0.15 μg/mL colcemid (Boehringer Mannheim, Mannheim, Germany) overnight.24
Protein sequencing.For protein sequencing experiments, a nuclear lysate preparation of 1 × 1010 HeLa cells was used. Immunoprecipitation was performed with Ki-S2 coupled to protein A Sepharose Cl-4B as described above. The immunoprecipitates were separated by SDS-PAGE and then transferred by blotting to polyvinylidene fluoride (PVDF) membranes (Immobilon P; Millipore, Eschborn, Germany). The membranes were stained with Coomassie brilliant blue R 250. After destaining with 50% methanol, the protein band was excised from the membrane and further processed for sequencing. Because attempts to sequence the intact proteins from the blot membrane were unsuccessful, the protein was digested with Lys C, as described by Bauw et al,25 and the proteolytic fragments were separated by narrowbore high-performance liquid chromatography (130 A; Applied Biosystems, Weiterstadt, Germany) on a reverse-phase column (Vydac C4; 300 A pore size; 5 mm particle size; 2.1 × 125 mm). Peptides were eluted with a linear mobile phase gradient (0% to 80% B in 50 minutes; solvent A, water/0.1% TFA; solvent B, 70% acetonitrile/0.09% trifluoroacetic acid [TFA]) at a flow rate of 200 mL/min. Peptide-containing fractions detected at 214 nm were collected manually into siliconized Eppendorf tubes and frozen immediately. Protein sequences of 10 peptides were determined by standard Edman degradation on an automatic sequencer (473 A; Applied Biosystems).
RESULTS
Immunoreactivity of Ki-S2 in normal tissue.Ki-S2 was shown to be a mouse monoclonal IgG1 antibody that specifically detects an antigen in the nuclei of proliferating cells in sections of frozen and paraffin-embedded tissues. There was no difference in the number of positive cells and in the intensity of the reaction between frozen sections and paraffin-embedded samples. The distribution of this nuclear antigen in normal human tissue was found to be restricted to sites harboring cycling cells, such as the germinal centers of lymphoid tissue (Fig 1). Nonspecific cross-reactivities in normal human tissue were not observed. Extensive immunohistochemical investigation of all normal tissue types showed that p100 and the Ki-S2 antibody are highly specific to the nuclei of cycling cells in characteristic tissue sites. In the epithelial coverings, a few basal epithelial cells and a moderate number of suprabasal epithelial cells showed a positive reaction. In the mucosa of the gastrointestinal tract, Ki-S2–positive cells were restricted to the lower part of the crypts. In lymphoid tissue, the dark zone of the germinal centers of lymphoid B follicles was highly reactive, whereas the number of positive cells did not exceed 5% in other areas of the lymph nodes. In testicular tissue, spermatogonia reacted strongly, whereas mature spermatozoa were invariably negative. In the bone marrow, promyelocytes, myelocytes, proerythroblasts, and erythroblasts (E1 and E2) were positive, whereas mature granulocytic cells and erythroblasts were consistently negative. Normal nonstimulated PBMC were negative for Ki-S2 and Ki-S5, and only occasional cells (<1%) showed faint nuclear reactivity. After stimulation of PBMC with PHA, the expression of the Ki-S2 antigen increased, paralleling that of the Ki-S5–reactive Ki-67 antigen. On day 3, 25% ± 4% of the stimulated cells expressed the Ki-S2 antigen. The Ki-S2 antigen expression correlated positively with the expression of the Ki-67 antigen determined by the MoAb Ki-S5 (r = .89, P < .01; Table 1).
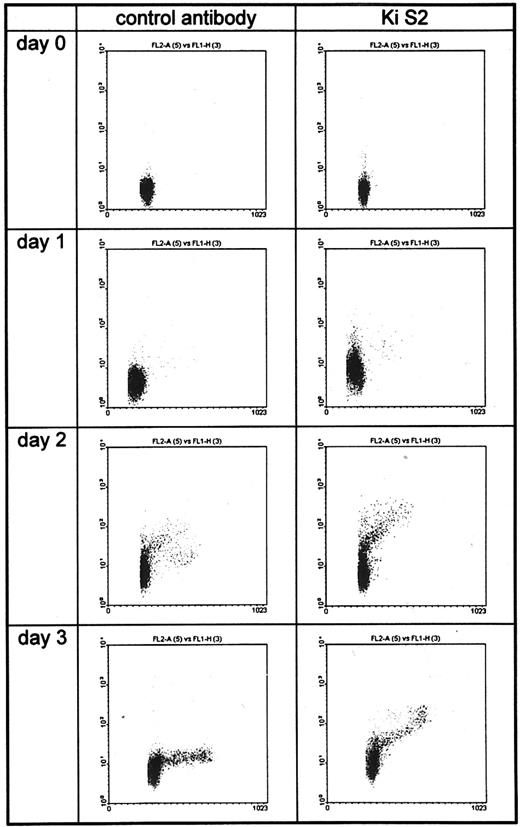
Flow cytometric analysis of unstimulated (day 0) and PHA-stimulated PBMC (days 1 through 3). DNA staining was registered on a linear scale (FL1-A) and Ki-S2 staining on a log scale (FL2-H). Less than 1% of the unstimulated PBMC were positive. Twenty-four hours after PHA stimulation, a small population of the G1 cells became positive. On days 2 and 3, all cells in S/G2 and M phases bound Ki-S2.
Flow cytometric analysis of unstimulated (day 0) and PHA-stimulated PBMC (days 1 through 3). DNA staining was registered on a linear scale (FL1-A) and Ki-S2 staining on a log scale (FL2-H). Less than 1% of the unstimulated PBMC were positive. Twenty-four hours after PHA stimulation, a small population of the G1 cells became positive. On days 2 and 3, all cells in S/G2 and M phases bound Ki-S2.
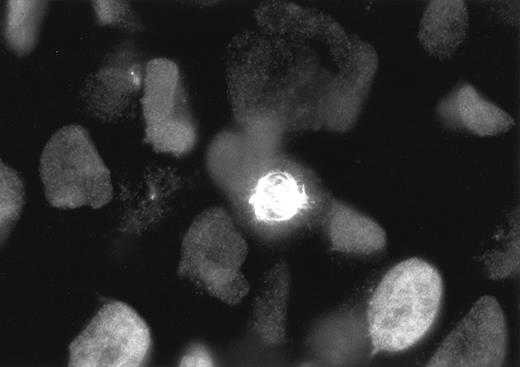
Staining of L428 cells with Ki-S2 and a Cy3-labeled goat antimouse polyclonal antibody. During the different phases of mitosis, the Ki-S2 antigen is strongly associated with the spindle poles and the mitotic spindle, whereas in the S and G2 phases, cells the antigen is diffusely distributed throughout the cell nucleus.
Staining of L428 cells with Ki-S2 and a Cy3-labeled goat antimouse polyclonal antibody. During the different phases of mitosis, the Ki-S2 antigen is strongly associated with the spindle poles and the mitotic spindle, whereas in the S and G2 phases, cells the antigen is diffusely distributed throughout the cell nucleus.
Expression of Ki-S2 in neoplasms.With Ki-S5, the Ki-67 proliferation marker included in this study, the cases of B-CLL showed a low percentage of positive cells. In contrast to this typical low-grade B-cell lymphoma, considerably higher values were detected in the Burkitt's lymphomas. In all lymphoma cases there was a significant (P < .01) positive correlation (r = .97) between the results achieved with the antibody recognizing the Ki-67 antigen (Ki-S5) and with Ki-S2 (Table 2).
Similar results were obtained when other neoplasms were studied with Ki-S2 in comparison to Ki-S5. Cases of stage I, nodal-negative ductal invasive adenocarcinoma of the breast showed 42% ± 11% Ki-67–positive tumor cells, as detected with the antibody Ki-S5. Only around 40% of the Ki-67–positive cells (17% ± 9%) expressed the Ki-S2–reactive nuclear antigen (Table 2).
Of the melanocytic tumors, in benign nevi (n = 29), less than 2% of the cells were positive for the Ki-67 antigen. Ki-S2 was also detectable in less than 1%. In cases of malignant melanoma (n = 18), 38% ± 12% of the cells showed Ki-67 expression, whereas only 15% ± 6% of the cells were positive for Ki-S2. The quantitative results with both antibodies showed a highly significant correlation (r = .97 and .98; P < .01) for all tumor types (Table 2). All immunohistochemical stainings to determine the expression of the Ki-S2 antigen in the different neoplasms were performed on routinely processed paraffin sections.
Cell sorting and flow cytometry.Cell sorting of stimulated PBMC into highly purified G0/G1 and S/G2/M cell populations showed a clear difference with respect to Ki-S2 expression. Whereas only occasional (>1%) positive cells were detectable in the G0/G1 fraction, slides prepared from the S/G2/M phase fraction showed considerably higher reactivity, exceeding 95% of the sorted cells. These results were well in line with those obtained by fluorescence-activated cell sorting (FACS) analysis (Fig 2). Twenty-four hours after stimulation, the percentage of positive cells was low. It increased on days 2 and 3, as the cells traversed the S, G2 , and M phases of the cell cycle.
Immunofluorescence staining showed that the antigen recognized by the antibody was diffusely distributed throughout the cell nuclei during S and G2 phases. In M phase, the antigen was strictly associated with the spindle poles and the mitotic spindles (Fig 3). Double immunofluorescence stainings with the MoAb Ki-S2 and a polyclonal anti–Ki-67 antigen serum showed that Ki-S2 antigen expression ceased immediately after the termination of cytokinesis (data not shown).
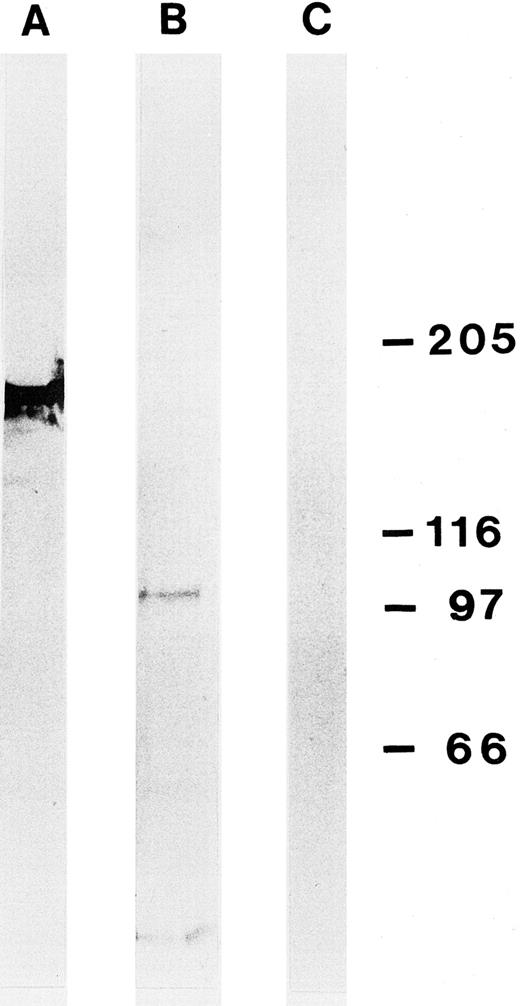
Determination of the molecular mass and first characterizations of the Ki-S2 antigen.The molecular mass of the Ki-S2 antigen in L428 cells was determined by immunoprecipitation and Western blotting. Under reducing conditions, one antigen with a molecular mass of about 100 kD, termed p100, could be detected with both methods (Fig 4). An antibody of the same isotype (IgG1) specific for topoisomerase-IIα (170 kD) served as positive control.26 After L428 cells were radiolabeled with [32P]orthophosphate, a signal could be detected that was strongly enhanced when L428 cells were arrested in mitosis with colcemid for 18 hours (Fig 5). After 18 hours, about 35% of the L428 cells were in mitosis. To determine the turnover time of the Ki-S2 antigen, L428 cells were labeled with [35S]methionine for 1 hour. The cells were washed and immunoprecipitation experiments were performed at different time intervals after the end of labeling. Two hours after the end of labeling only a weak signal could be detected, which indicates a short half-life of about 1 hour for p100 (Fig 6).
Western blot of lysed L428 cells using the MoAb Ki-S2 (B) after SDS-PAGE (gradient gel 5% to 10%). Ki-S2 detects a protein of about 100 kD. (A) Control experiment with an MoAb of the same isotype (IgG1) specific for topoisomerase-IIα (170 kD). (C) Control without primary antibody. Molecular weight standards are shown on the right (in kilodaltons).
Western blot of lysed L428 cells using the MoAb Ki-S2 (B) after SDS-PAGE (gradient gel 5% to 10%). Ki-S2 detects a protein of about 100 kD. (A) Control experiment with an MoAb of the same isotype (IgG1) specific for topoisomerase-IIα (170 kD). (C) Control without primary antibody. Molecular weight standards are shown on the right (in kilodaltons).
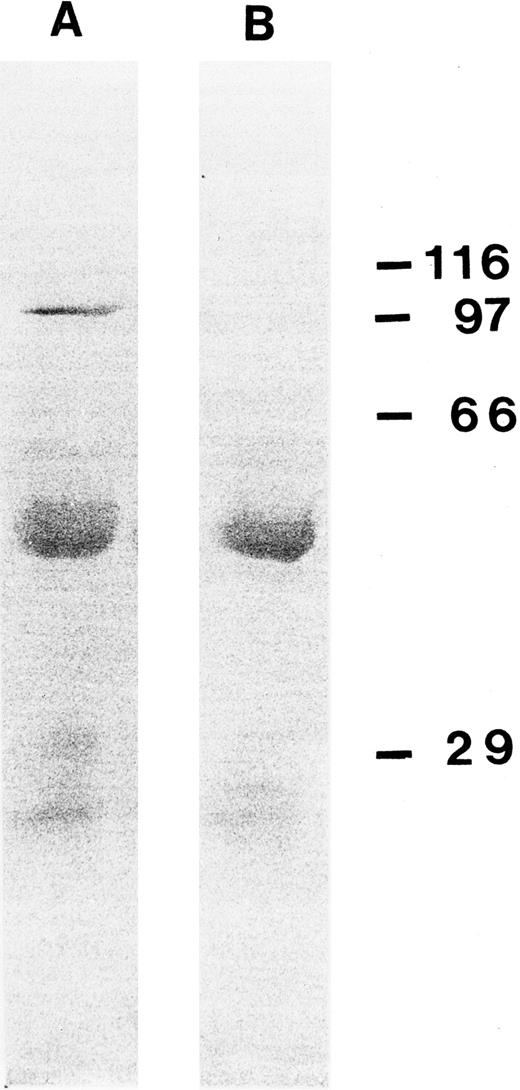
Immunoprecipitation experiment with Ki-S2 on L428 cells after the cells were labeled with [32P]orthophosphate (A). In lane B, L428 cells were arrested with colcemid (0.15 μg/mL) overnight before labeling with [32P]. (C) Isotype control experiment. Molecular weight standards are shown on the right (in kilodaltons).
Immunoprecipitation experiment with Ki-S2 on L428 cells after the cells were labeled with [32P]orthophosphate (A). In lane B, L428 cells were arrested with colcemid (0.15 μg/mL) overnight before labeling with [32P]. (C) Isotype control experiment. Molecular weight standards are shown on the right (in kilodaltons).
Determination of the turnover time of the Ki-S2 antigen. L428 cells were labeled for 1 hour with [35S]methionine and intensively washed. Immunoprecipitation experiments were performed immediately after the end of labeling (A) and 90 minutes (B), 120 minutes (C), and 180 minutes (D) after the end of labeling. (E) Isotype control experiment after the end of labeling. Molecular weight standards are shown on the right (in kilodaltons).
Determination of the turnover time of the Ki-S2 antigen. L428 cells were labeled for 1 hour with [35S]methionine and intensively washed. Immunoprecipitation experiments were performed immediately after the end of labeling (A) and 90 minutes (B), 120 minutes (C), and 180 minutes (D) after the end of labeling. (E) Isotype control experiment after the end of labeling. Molecular weight standards are shown on the right (in kilodaltons).
Protein sequence data.To look for protein sequence homologies with already characterized proteins, amino acid sequences were analyzed. For this purpose, nuclear lysates of 1 × 1010 HeLa cells were used for immunoprecipitation. The precipitated antigen was blotted onto an Immobilon P membrane and stained with Coomassie blue (Fig 7). This antigen preparation was monitored by Western blot experiments with the MoAb Ki-S2. Only antigen preparations with a molecular mass of about 100 kD that could be stained with the MoAb Ki-S2 were used for protein sequencing. Because attempts to sequence the N-terminus of the Ki-S2 antigen p100 failed, the protein was digested with Lys C. Ten peptides of two independent immunoprecipitations were sequenced. The sequences of these peptides were compared with protein sequences from various protein data banks (PIR, Swiss Prot, Geneva, Switzerland). No homology with known proteins could be detected.
A Coomassie blue staining of a PVDF membrane after an immunoprecipitation experiment using cell nuclei of 1 × 1010 HeLa cells with Ki-S2. The 100-kD protein band of this experiment was excised and used for digestion with Lys C and peptide sequencing. Molecular weight standards are shown on the right (in kilodaltons).
A Coomassie blue staining of a PVDF membrane after an immunoprecipitation experiment using cell nuclei of 1 × 1010 HeLa cells with Ki-S2. The 100-kD protein band of this experiment was excised and used for digestion with Lys C and peptide sequencing. Molecular weight standards are shown on the right (in kilodaltons).
DISCUSSION
We report a new formalin- and paraffin-resistant, proliferation-associated nuclear antigen with a molecular mass of about 100 kD. This antigen, p100, is specifically recognized by a mouse IgG1 MoAb designated Ki-S2. Extensive immunohistochemical investigations on frozen sections and paraffin-embedded samples of all normal tissue types showed that p100 and the Ki-S2 antibody are highly specific for the nuclei of cycling cells in characteristic tissue sites. In the epithelial coverings, a few basal epithelial cells and a moderate number of suprabasal epithelial cells showed a positive reaction. In the mucosa of the gastrointestinal tract, Ki-S2–positive cells were restricted to the lower part of the crypts. In lymphoid tissue, the dark zone of the germinal centers of lymphoid B follicles was highly reactive, whereas the number of positive cells did not exceed 5% in other areas of the lymph nodes. In testicular tissue, spermatogonia showed high reactivity, whereas mature spermatozoa were invariably negative. In the bone marrow, promyelocytes, myelocytes, proerythroblasts, and erythroblasts (E1 and E2) were positive, whereas mature granulocytic cells and erythroblasts were consistently negative. Although nearly all normal leukocytes of the peripheral blood were negative for this antigen, PHA stimulation induced increasing expression of Ki-S2 from less than 1% on day 0 to a maximum of 25% ± 4% on day 3.
Ki-S2 showed the same distribution pattern in normal tissue as the established proliferation markers Ki-67 and topoisomerase-IIα.4,7,9,26 However, in quantitative terms, Ki-S2 immunolabeled only 30% to 40% of the Ki-67– and topoisomerase-IIα–expressing cell population.27 All computations showed a significant positive correlation between the expression rates of the two antigens.
In neoplastic tissue, these antigens showed similar behavior. In cases of indolent low-grade B-cell lymphomas, such as B-CLL, and benign tumors, such as benign nevi, the expression of the p100 and Ki-67 antigens was extremely low. High activity levels were found for aggressive B-cell lymphomas of Burkitt's type and for other malignant tumors such as ductal invasive cases of breast carcinomas, whereby the p100 level did not exceed about 40% of the Ki-67 levels, on average. The highest Ki-67 antigen expression, exceeding 90% of the tumor cells, was found in cases of Burkitt's lymphoma. p100 expression was also high, although considerably lower than Ki-67 expression. These results imply that p100 is expressed in only a certain portion of the cell cycle phases.
Cell sorting studies, separating cells in G0/G1 from cells in the S, G2 , and M phases of the cell cycle, showed that, unlike Ki-67, the p100 antigen was selectively expressed during the cell cycle phases S, G2 , and M. Flow cytometric studies confirmed these results. In addition, these results lend support to conclusions deduced from immunoprecipitation studies on [35S]methionine-labeled cells at different time intervals, which implied that p100 is rapidly degraded after the end of cytokinesis and promptly expressed as the cells traverse the G1/S restriction point. The turnover time of p100, estimated from the degradation kinetics, is around 1 hour. Immunofluorescence studies with Ki-S2 or Ki-S2 in combination with a polyclonal anti–Ki-67 antibody in double immunofluorescence stainings show that, until mitosis, p100 is evenly distributed throughout the nucleus, whereas during mitosis, it is strictly associated with the spindle poles and the mitotic spindle.
Immunoprecipitation of radiolabeled p100 showed a molecular mass of about 100 kD. This result was also confirmed by Western blotting. Amino acid analysis of the purified protein after proteolytic degradation showed no significant homology with any protein sequence known so far. Thus, p100 is a new nuclear proliferation-associated protein selectively expressed in the S, G2 , and M phases of the cell cycle. p100 differs in the above-mentioned features from all other known cell cycle-associated proteins and from those associated with proliferating cells, such as p350/320 (Ki-67 antigen), p170 (topoisomerase-IIα), p36 PCNA (proliferating cell nuclear antigen), p210 (nuclear matrix antigen), p125 (mitotin).27-31 Nuclear proteins with a nearly identical molecular mass of 100 kD, such as nucleolin and inner centromere binding proteins (INCENPs), have no sequence homologies with the 10 peptides resolved from p100 by proteolytic digestion. In line with these considerations, the corresponding immunohistochemical studies show a completely different staining pattern.32 33
No information is available on the functional aspects of p100. Its restricted expression between the G1/S restriction point and termination of cytokinesis, as detected by cell sorter analysis and by double immunofluorescence stainings, and its close association with the spindle poles, plus the fact that p100 is phosphorylated during the M phase, might indicate a role in the mechanics of cytokinesis. Activation by phosphorylation is a phenomenon regularly found in cell cycle-associated proteins, such as PCNA and topoisomerase-IIα.34-36
To our knowledge, p100 is the first cell cycle-associated protein with such a restricted expression pattern. The highly specific MoAb Ki-S2 can probably replace S phase labeling with radiolabeled DNA precursors37 and thus yield proliferation data with a closer correlation to tumor prognosis.
Considering the importance of determining cell proliferation in cell biology and clinical oncology,11,38,39 several different methods for the assessment of cellular proliferation have been developed. One of the most effective ways to survey the proliferative activity of a cell population is the mean population doubling time.38 However, this is feasible only for tissue culture conditions. This also applies to radiomonitoring or immunohistochemical detection of DNA precursors, such as [3H]thymidine and bromodeoxyuridine (BrDU). Like Ki-S2, antibodies to BrDU are expressed in about 30% of the Ki-67–positive cells.37 The introduction of immunohistochemical methods for monitoring cellular proliferation in cell suspensions or fixed tissue sections using MoAbs to proliferation-specific cell proteins, such as Ki-67 antigen and topoisomerase-IIα, has been of considerable help in cell biology and in the evaluation of tumor growth.7,9 40 These antigens are also expressed during the G1 cell cycle phase. Because the G1 phase, which accounts for a major part of cell cycle, varies extremely in duration, such markers are unreliable for tumor entities with prolonged G1. The immunohistochemical assessment of p100 should become a powerful tool for diagnostic and prognostic purposes. Further studies on this hitherto unknown proliferation-associated protein will increase our knowledge of the cell cycle puzzle.
ACKNOWLEDGMENT
The authors thank M. Hauberg and G. Jopp for their excellent technical assistance, K. Herwartz for performing the flow cytometry experiments, and K. Dege for helping prepare the manuscript.
Address reprint requests to Dr H.J. Heidebrecht, Department of Hematopathology, University of Kiel, Michaelisstr. 11, D-24105 Kiel, Germany.

![Fig. 5. Immunoprecipitation experiment with Ki-S2 on L428 cells after the cells were labeled with [32P]orthophosphate (A). In lane B, L428 cells were arrested with colcemid (0.15 μg/mL) overnight before labeling with [32P]. (C) Isotype control experiment. Molecular weight standards are shown on the right (in kilodaltons).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/1/10.1182_blood.v90.1.226/5/m_bl_0002f5.jpeg?Expires=1766008304&Signature=0jTB7LJ0W9t227UUtqX5sC18e7ngJ1nC45vnLJ8rjBcxpjN9qSOvRwYtMfdzN-D0dS9MJBCJFQX6wQxM6b4NXuaO-0ZQYuxqPi8WLvMOsSTUyb9O9TZqpeIxIaa7POjmFY42rVRXTYU1gKaCDU6mZ3~cgTIx6ZRK1LRGwxTS~ks8I6N3lL0D~EWqd2mO1-I4x12T1aOEufpD4aD5iRf7iGZz44TpHODk1isrb6VLFBLwZLNY1ByLpkXwTY~WFFkQTqnwYC8G8RGMMWmCxGpMl-wgi8sDxSnOjCAbCu~5U8noOANoruOp1aAv6fA2T06-kdbb2RiS~xyHHv1w26sBrg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Determination of the turnover time of the Ki-S2 antigen. L428 cells were labeled for 1 hour with [35S]methionine and intensively washed. Immunoprecipitation experiments were performed immediately after the end of labeling (A) and 90 minutes (B), 120 minutes (C), and 180 minutes (D) after the end of labeling. (E) Isotype control experiment after the end of labeling. Molecular weight standards are shown on the right (in kilodaltons).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/1/10.1182_blood.v90.1.226/5/m_bl_0002f6.jpeg?Expires=1766008304&Signature=a3lxYsHBW4SfgMZKi4HbuKK3Kek7aeMs~GORCGzSU7hf2SBHf5ztcR0YdvMv5cy-SM0JxqY4IeoZGAk1y5XojBIKA3MGJImVhr1Kt0tzPPY5VnclgClN~r-mq356xUymKnSvoFlObmJjxm8bwCIQHxCalScoOKgmt3Z9VRjbn0ke86LrE8ZCLWOp9Pwfpg182IQIoDfm5G1SiaeLqlNfLl57He487QUzSv05K6COz7ZLgRJS~XD0OKSsWz5tHMwKAnLjdTYkFXoM9bf8gzs2qiIlODmlAiRUIjWsSjK7PuDoZBrxhWb9cSCpMgvTyYfFK1MpIgicwOsfSipimMayPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal