Abstract
Continuous indomethacin (INDO) administration in the drinking water (10 to 20 μg/mL) profoundly inhibited plasmacytoma (PCT) development initiated by three 0.2- or 0.5-mL intraperitoneal (i.p.) injections of pristane in hypersusceptible BALB/c.DBA/2-Idh1-Pep3 congenic mice. The most effective inhibitions were obtained with continuous INDO treatment. When treatment was delayed until 50 to 60 days after the first pristane injection, there was approximately a 50% reduction in PCT incidence. The primary action of pristane is the induction of a chronic inflammation in the peritoneal connective tissues and the formation of a microenvironment where PCTs develop. INDO, a powerful inhibitor of prostaglandin synthases (cyclooxygenases 1 and 2), did not inhibit the formation of mesenteric oil granuloma nor the appearance of cells in this chronic inflammatory tissue carrying c-myc illegitimately joined to an Ig heavy chain switch region, ie, the t(12; 15) chromosomal translocation. INDO inhibited PCT induction by the i.p. implantation of 21 × 2 mm polycarbonate discs. These solid objects predominantly induce the formation of a patchy fibroplastic tissue on contacting peritoneal surfaces. These and previous data indicate that indomethacin inhibits an intermediate stage in PCT development after the arrival of cells bearing the T(12; 15) translocation in the oil granuloma and before these cells acquire transplantability to a pristane-conditioned host. The biological mechanism that explains how INDO inhibits PCT development is not yet established but appears to result from decreased production of prostaglandins in chronic inflammatory tissues (oil granuloma, fibroplasia), suggesting that prostaglandins play an active role in oil and solid plastic induced PCT formation.
PLASMACYTOMAS (PCTs) are induced in genetically susceptible strains of mice such as BALB/cAn and BALB/cAn.DBA/2-Idh1-Pep3 mice (C.D2 I/P)1 by the intraperitoneal (i.p.) injections of paraffin oils, eg, pristane (2,6,10,14, tetramethylpentadecane),2 silicone gels3 and various plastic objects.4 These materials are poorly if at all metabolized and act by inducing the formation of various kinds of chronic inflammatory reactive tissues such as oil granulomas (OG) or fibroplasias on peritoneal surfaces. Histological studies of pristane induced PCTs have shown that these tumors proliferate in this inflammatory tissue, but other evidence indicates that the process of PCT development can begin before the OG is induced.5
More than 95% of PCTs possess consistent chromosomal translocations involving regions near or in the c-myc protooncogene on chromosome (chr) 15 and an Ig locus on chromosomes 6, 12, and 16.6 The best studied and most common of these translocations is T(12; 15) in which c-myc on chr 15 is illegitimately recombined to an Ig heavy chain switch region sequence on chr 12, usually switch α (Sα)or switch μ (Sμ). This results in a dysregulation of c-myc transcription.7 As the most prevalent T(12; 15) breaksite in the Ig bearing chromosome 12 is Ig switch α (Sα)6,8 we were able to develop a nested polymerase chain reaction (PCR) strategy that detects many of the T(12; 15) recombinations linking the Ig Sα or Sμ sequences to c-myc.9,10 Nested PCR can be effectively used in place of cytogenetics to determine the presence of T(12; 15) chromosomal translocations in DNA isolated from various tissues. IgSα/c-myc recombinations are frequently found in lymphoid tissues of Peyer's patches, intestines, and spleen in both normal and pristane-treated mice.5 One week after the injection of pristane, IgSα/c-myc rearrangements can be found in mesenteric-oil granuloma tissues in increased frequency. Together, this provides strong evidence that the c-myc oncogenic mutation can originate in B cells before pristane administration and before the oil granuloma is formed.
In 1966, Takakura et al11 showed that daily injections of cortisol inhibited paraffin oil induction of PCTs. We have previously reported that the nonsteroidal anti-inflammatory agent indomethacin (INDO) administered continuously in the drinking water (20 μg/mL) strongly inhibited the development of PCT formation induced by the i.p. injection of l mL pristane in BALB/cAnPt mice.12 INDO treatment did not produce striking histological changes in peritoneal oil granuloma formation. The biochemical mechanism of INDO inhibition of PCT formation is not yet established, but the most likely biochemical targets of this inhibitor are the cyclooxygenase (prostaglandin synthase) enzymes, COX-1, and COX-2,13-15 and this suggests prostaglandins may be the important effectors in the PCT inhibitory process.
In the present study we have tested the effects of INDO treatment under more challenging conditions than were previously used12: first, by using the more effective three-dose schedule of pristane administration where pristane is given in three 0.5-mL doses on days 0, 60, and 120; second, by using a BALB/c congenic strain, C.D2 I/P, in which 60% to 70% of the mice develop PCTs within 300 days,1 (also this paper); third, by extending the period of INDO treatment to 300 days. We show that INDO treatment effectively inhibits plasmacytoma induction by pristane under these more rigorous conditions. In addition, we also now show that INDO inhibited the induction of PCTs by the implantation of plastic (Lucite) discs. This model system of plasmacytoma induction has permitted us to attempt to define the biological step(s) in PCT development that are sensitive to INDO inhibition.
MATERIALS AND METHODS
Mice.BALB/c.DBA/2 Idh1-Pep3 (C.D2 I/P) mice16 at N20 F14+ were used in most of the experiments. The mice were fed Purina Mouse Chow (5001) (PMI Feeds, St Louis, MO) and acidified water ad libitum. All mice were raised in a conventional AAALAC approved barrier protected colony at PerImmune, Inc (Rockville, MD) under NCI contract NO1-CB-21075. The experiments were performed under Animal Use Protocol No. 190 at PerImmune, Inc.
Indomethacin.INDO was purchased from Sigma Chemical Co (St Louis, MO) and put into solution in absolute ethanol and then diluted in the sterile drinking water at 10 to 20 μg/mL. The water bottles were changed twice weekly.
PCT diagnosis.PCTs were diagnosed by finding 10 or more characteristic plasma cells in cytofuged preparations of ascites. In mice where there were less than 50 PCT cells/slide, a confirmatory smear was usually obtained. Ascitic fluid was examined every 21 days.
Pristane.Pristane 99% pure was purchased from Aldrich Chemical Co (Milwaukee, WI) or 95% pure pristane from Sigma Chemical Co. The standard induction schedule used three injections of 0.5 mL pristane given on days 0, 60, and 120. In more recent experiments shown here we have found a dose of 0.2 mL pristane to produce fewer side effects and to be highly effective.
Plastic discs.The 2 × 21 mm plastic discs were punched out from polycarbonate sheets (Lexan; General Electric, Pittsfield, MA).
PCR.Mesenteric tissue containing oil granuloma was separated from the intestine, then removed in a block, and cut into five fragments for each mouse. Each of the fragments was sieved through a disposable mesh screen, and the cell suspension was immediately treated with Proteinase K in a lysis buffer solution. DNA was extracted with phenol followed by chloroform, then precipitated in ethanol and resuspended over several days in Tris-EDTA. Working dilutions were made in water, and each amplification used 0.5 μg DNA. For this method of DNA isolation from oil granuloma tissue, we estimate that the limits of detection require approximately 400 copies of any translocated sequence. Depending on whether the cells are diploid or tetraploid, this would require from 200 to 400 cells.
Most of the nested PCR amplification procedure has been previously described.9,10 Essentially, 3- to 5-primer pair sets were used for each sample to locate potential Ig/c-myc recombinations on chr 12+. In addition, a modified Sα, Sμ consensus primer set 995 D/B was used in the confirmatory experiments. This primer set has four annealing sites in Sα and several in Sε and Sγ genes. Approximately 3 kb of Sμ has not yet been sequenced because of its highly repetitive nature, and it may contain annealing sites for 995D/B. These primers have no perfect annealing sites in Sμ, but contain 38 sites allowing 3 or 4 mismatches at least 7 nucleotides away from their 3′ ends. There are no inverted annealing sites at this stringency. This allows a broader range of sequences to which it can anneal than the switch region specific primers. The following previously described specific primer pairs were used for IgH/c-myc: m,α 1a/1b; m,α 2a/2b, m3a/3b, m,α 4a/4b, m,α 5a/5b, m6a/6b.17 The sequence of 995D is 5′-agctcattccagctcagctcagcct-3′, and 995B is 5′-agctcagctcagcctarcccagctc-3′. For most of the samples no attempt was made to systematically identify the Ig/c-myc recombination site on the 15− chromosome.
Reproducibility and uniqueness of the Ig/c-myc recombination.Since this laboratory studies many PCTs, a log book of all Ig/c-myc recombinations is maintained. Most are unique by having a specific break site in c-myc and an Igh-switch sequence, overlaps or insertion sequence at the break site. When an identical Ig/c-myc junction sequence is encountered in two different mice in a single experiment or in experiments performed on different days, it is considered to be a contaminant in the second isolation and is discarded. Contaminations of this sort are encountered rarely. To ensure reproducibility, each unique junction sequence was isolated twice from the same DNA source, except in those cases where multiple mesenteric fragments from the same mouse yielded identical sequences. Many of the confirmations were also performed with the 995 D/B consensus primer pair.
RESULTS
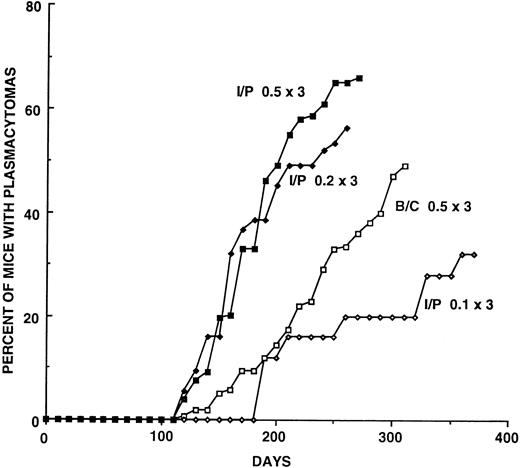
Plasmacytoma induction in BALB/cAnPt and BALB/c.DBA/2-Idh1-Pep3 (C.D2 I/P) strains.BALB/cAn mice have been the most susceptible inbred strain in which to induce PCTs by i.p. pristane.18 The incidence of PCTs induced in our subline of BALB/cAnPt has fluctuated and declined over the last 8 years for undetermined reasons1; we suspect a silent mutation affecting susceptibility may have occurred in the colony. This is not without precedent as the BALB/cJ strain is partially resistant to PCT induction.19 During the testing of BALB/c.DBA/2 congenic strains of mice for susceptibility or resistance to plasmacytoma induction by i.p. pristane, the C.D2 I/P strain that carries a 30-centimorgan (cM) segment of DBA/2 chromatin from chromosome 1 was found to be hypersusceptible to PCT induction.1 This finding has been greatly extended in the present study. When three 0.5-mL injections of pristane were given, more than 60% of the mice developed PCTs by 300 days, usually with relatively short latent periods. A summary of induction experiments performed over the last 5 years is shown in Fig 1 and Table 1. Seventy-three percent (range 67% to 81%) of C.D2 I/P mice develop PCTs by day 270, while only 36% of BALB/cAnPt (range 33% to 40%) develop PCTs by this time point. Using the day 270 incidence, the median latent periods were at 170 days for C.D2 I/P and 215 days for BALB/cAnPt. These differences indicate C.D2 I/P mice are more susceptible to PCT induction than BALB/cAnPt, and the consistency of their response makes C.D2 I/P an excellent strain for testing quantitative differences in responses to potenital inhibitors such as INDO.
Plasmacytoma induction curves that compare (a) responses of C.D2 I/P (I/P) and BALB/cAnPt (B/C) mice to three doses of 0.5 mL pristane given on days 0, 60, and 120; and (b) responses of C.D2 I/P mice to three different dose regimens of pristane. The numbers of mice and the percentages at 25-day intervals are given in Table 1. Note that C.D2 I/P mice develop a higher incidence of PCTs more rapidly than BALB/c.
Plasmacytoma induction curves that compare (a) responses of C.D2 I/P (I/P) and BALB/cAnPt (B/C) mice to three doses of 0.5 mL pristane given on days 0, 60, and 120; and (b) responses of C.D2 I/P mice to three different dose regimens of pristane. The numbers of mice and the percentages at 25-day intervals are given in Table 1. Note that C.D2 I/P mice develop a higher incidence of PCTs more rapidly than BALB/c.
Incidence of Plasmacytomas in Mice Given 3 Doses of Pristane on Days 0, 60, 120
| Strain . | No. Exps . | No. Mice . | Pristane Dose . | Percent of Mice With PCTs on Day: . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | 125 . | 150 . | 175 . | 200 . | 225 . | 250 . | 275 . | 300 . |
| BALB/c | 7 | 275 | 0.5 × 3 | 0.7 | 5.1 | 9.4 | 14.5 | 22.0 | 33.0 | 36 | 47 |
| C.D2-I/P | 5 | 196 | 0.5 × 3 | 4.0 | 19.7 | 33 | 49 | 58 | 65 | 66 | 66 |
| C.D2-I/P | 5 | 106 | 0.2 × 3 | 5.6 | 16 | 32 | 38.6 | 49 | 52.7 | 56.5 | 60.4 |
| C.D2-I/P | 1 | 25 | 0.1 × 3 | 0 | 0 | 0 | 12 | 16 | 16 | 20 | 20 |
| Strain . | No. Exps . | No. Mice . | Pristane Dose . | Percent of Mice With PCTs on Day: . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | 125 . | 150 . | 175 . | 200 . | 225 . | 250 . | 275 . | 300 . |
| BALB/c | 7 | 275 | 0.5 × 3 | 0.7 | 5.1 | 9.4 | 14.5 | 22.0 | 33.0 | 36 | 47 |
| C.D2-I/P | 5 | 196 | 0.5 × 3 | 4.0 | 19.7 | 33 | 49 | 58 | 65 | 66 | 66 |
| C.D2-I/P | 5 | 106 | 0.2 × 3 | 5.6 | 16 | 32 | 38.6 | 49 | 52.7 | 56.5 | 60.4 |
| C.D2-I/P | 1 | 25 | 0.1 × 3 | 0 | 0 | 0 | 12 | 16 | 16 | 20 | 20 |
Incidence of Plasmacytomas in Indomethacin Treated Mice
| Exp. . | Pristane Dose . | INDO Dose μg/mL (days given) . | No. Mice . | Percent of Mice With PCTs on Day: . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | 150 . | 175 . | 200 . | 225 . | 250 . | 275 . | 300 . |
| 1 | 0.5 × 3 | 0 | 40F | 15 | 15 | 30 | 55 | — | — | — |
| 1A | 0.5 × 3 | 20 (D0-219) | 37F | 0 | 0 | 0 | 0 | — | — | — |
| 2 | 0.5 × 3 | 0 | 25M, F | 12 | 16 | 36 | 36 | 56 | 76 | 76 |
| 2A | 0.5 × 3 | 20 (D0-D70) | 24M, F | 4 | 12.5 | 29.2 | 41.7 | 41.7 | 70.8 | 70.8 |
| 2B | 0.5 × 3 | 20 (D0-295) | 12M | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2C | 0.5 × 3 | 20 (D.50-295) | 24M, F | 0 | 4 | 8.3 | 12.5 | 16.6 | 25 | 25 |
| 2D | 0.5 × 3 | 20 (D70-295) | 23M, F | 0 | 17.3 | 21.8 | 21.8 | 21.8 | 30.2 | 30.2 |
| 3 | 0.2 × 3 | 0 | 35M, F | 8.5 | 17.2 | 17.2 | 34.2 | 34.2 | 40 | 43 |
| 3A | 0.2 × 3 | 20, 10 (D0-310) | 36M, F | 0 | 0 | 0 | 5.5 | 5.5 | 5.5 | 5.5 |
| 3B | 0.2 × 3 | 10 (D0-310) | 36M, F | 0 | 2.7 | 2.7 | 5.5 | 5.5 | 5.5 | 5.5 |
| 3C | 0.2 × 3 | 20, 10 (D60-310) | 36M, F | 2.7 | 5.5 | 5.5 | 11.5 | 17.4 | 24 | 27.6 |
| 4 | DISC | 0 | 23M, F | 0 | 4.3 | 4.3 | 4.3 | 4.3 | 4.3 | 30.3* |
| 4A | DISC | 20 D0-361 | 23F | 0 | 0 | 0 | 0 | 0 | 0** | |
| Exp. . | Pristane Dose . | INDO Dose μg/mL (days given) . | No. Mice . | Percent of Mice With PCTs on Day: . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | 150 . | 175 . | 200 . | 225 . | 250 . | 275 . | 300 . |
| 1 | 0.5 × 3 | 0 | 40F | 15 | 15 | 30 | 55 | — | — | — |
| 1A | 0.5 × 3 | 20 (D0-219) | 37F | 0 | 0 | 0 | 0 | — | — | — |
| 2 | 0.5 × 3 | 0 | 25M, F | 12 | 16 | 36 | 36 | 56 | 76 | 76 |
| 2A | 0.5 × 3 | 20 (D0-D70) | 24M, F | 4 | 12.5 | 29.2 | 41.7 | 41.7 | 70.8 | 70.8 |
| 2B | 0.5 × 3 | 20 (D0-295) | 12M | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2C | 0.5 × 3 | 20 (D.50-295) | 24M, F | 0 | 4 | 8.3 | 12.5 | 16.6 | 25 | 25 |
| 2D | 0.5 × 3 | 20 (D70-295) | 23M, F | 0 | 17.3 | 21.8 | 21.8 | 21.8 | 30.2 | 30.2 |
| 3 | 0.2 × 3 | 0 | 35M, F | 8.5 | 17.2 | 17.2 | 34.2 | 34.2 | 40 | 43 |
| 3A | 0.2 × 3 | 20, 10 (D0-310) | 36M, F | 0 | 0 | 0 | 5.5 | 5.5 | 5.5 | 5.5 |
| 3B | 0.2 × 3 | 10 (D0-310) | 36M, F | 0 | 2.7 | 2.7 | 5.5 | 5.5 | 5.5 | 5.5 |
| 3C | 0.2 × 3 | 20, 10 (D60-310) | 36M, F | 2.7 | 5.5 | 5.5 | 11.5 | 17.4 | 24 | 27.6 |
| 4 | DISC | 0 | 23M, F | 0 | 4.3 | 4.3 | 4.3 | 4.3 | 4.3 | 30.3* |
| 4A | DISC | 20 D0-361 | 23F | 0 | 0 | 0 | 0 | 0 | 0** | |
Summary of statistical analysis. At each time point the proportions of animals with PCTs were compared using Fisher's exact test (reported results are for two-sided test). There was no significant difference ( P > .4) between the tumor rates in groups 2 and 2A. At the 5% significance level ( P < .05), the tumor rates for groups 2B and 2C were significantly lower than that for group 2 from day 200 on, and the tumor rate for group 2C was significantly lower than that for group 2 from day 225 on. At the 0.5% significance level ( P < .005), the tumor rates for groups 2B and 2C were significantly lower than that for group C from day 225 on, and the tumor rate for group 2D was significantly lower than that for group 2 from day 275 on. There were no significant differences in tumor rates among groups 2B, 2C and 2D (although this is due in large part to the small sample size in group 2B). There was no sustained significant difference between groups 3 and 3C (the tumor rate in group 3C was significantly lower, with P = .038, at 225 days only). At the 5% significance level, the tumor rate for group 3A was significantly lower than that for group 3 from 175 days on, and the rate for group 3B was significantly lower than that for group 3 from 225 days on. At the 0.5% significance level, the tumor rates for both groups 3A and 3B were significantly lower than that for group 3 from 225 days on. There were no significant differences between groups 3A and 3B. The tumor rates for groups 3A and 3B were significantly lower than that for group 3C ( P < .05) from day 275 on.
Abbreviations: M, male; F, female.
At day 361, 38.9% of the mice had PCTs.
Mice autopsied at days 361, 397.
Lowering the dose of pristane to 0.2 mL per injection (total dose of 0.6 mL) reduced the incidence of PCTs in C.D2 I/P from 66% to 56% in 270 days. This dose of pristane, however, produces much less accumulation of oil granuloma tissue on the diaphragm, omentum, and retroperitoneal tissues, and the mice develop far fewer complications from excessive OG formation. The latent periods up to day 200 were very similar to those obtained with the 0.5-mL dose; thereafter the PCTs developed at a slower rate, indicating these tumors had longer latent periods.
Inhibition of plasmacytomagenesis in C.D2 I/P mice by INDO.The first experiment carried in C.D2 I/P mice was performed to determine if continuous INDO treatment could be effective in mice given the 0.5-mL injections of pristane. The mice tolerated INDO well until the 200th day. Further control mice were rapidly developing PCTs (Table 2, Exp. 1). It was then decided to terminate the experiment at day 219 and obtain tissue sections from all of the undiagnosed mice. By day 219, 20 pristane control mice (50%) had developed PCTs, as determined by cytofuge smears. On day 219, 27 of the control mice were terminated and their peritoneal tissues were examined histologically for plasmacytomas; two additional mice were found to have PCTs that were not positive by cytofuge smears, bringing the total to 55%. Four had six or more foci indicating a developing PCT. In contrast, none of the INDO-treated mice had developed a PCT before day 219. These mice all had well-developed mesenteric oil granulomas, and of the 37 INDO-treated mice autopsied on day 219, one mouse was found to have a developed PCT, and evidence of early plasmacytoma formation was found in 2 other mice that each had 5 foci. These results indicated INDO could probably be maintained for 200 days or more and that continuous INDO treatment prevented the development of PCTs in C.D2 I/P mice.
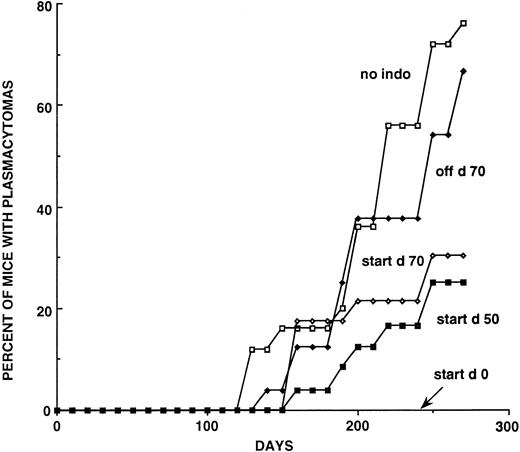
A second experiment explored the effects of INDO given for various intervals in mice that received three 0.5-mL injections of pristane (Table 1, Fig 2). The mice on continuous INDO did not develop PCTs by day 295, while 76% of the pristane injected nontreated controls developed PCTs. The female mice on continuous INDO treatment developed weight loss, possibly from an unexplained intercurrent infection or an intolerance to INDO and were eliminated from the experiment, leaving only 12 male mice in this group. None of the males developed a PCT by day 295 when the experiment was terminated. When INDO was administered for the first 70 days after the first injection of pristane, the incidence of PCTs was 66%, and there was a slight delay in the appearance of the PCTs developing after 200 days (Fig 2). When the beginning of INDO treatment was delayed until the 50th or 70th day, the number of PCTs at day 295 was 25% and 30%, respectively, ie, approximately 40% to 50% reduction in the incidence of PCTs (Fig 2).
Induction curves showing the effects of different INDO treatment schedules on PCT formation in C.D2I/P mice. The groups labeled “start” were begun on the day indicated and maintained thereafter on INDO. The Off D70 group was treated for the first 70 days and then discontinued. All INDO-treated mice received the 20 μg/mL dose. There were originally 24 mice in the continuous treatment group, but the female mice died for unexplained reasons and this group contained only 12 mice. See Table 2 for numbers of mice and percentages of PCTs at 28-day intervals from day 125 to day 300.
Induction curves showing the effects of different INDO treatment schedules on PCT formation in C.D2I/P mice. The groups labeled “start” were begun on the day indicated and maintained thereafter on INDO. The Off D70 group was treated for the first 70 days and then discontinued. All INDO-treated mice received the 20 μg/mL dose. There were originally 24 mice in the continuous treatment group, but the female mice died for unexplained reasons and this group contained only 12 mice. See Table 2 for numbers of mice and percentages of PCTs at 28-day intervals from day 125 to day 300.
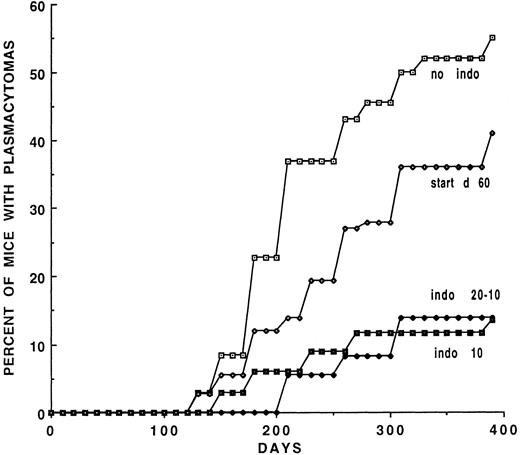
Experiment 3 (Table 2, Fig 3) examined the long-term effects of 10 μg/mL of INDO in the drinking water on PCT development. All mice were given 0.2-mL doses of pristane instead of the 0.5-mL dose. In group 3A (Table 2) the mice were given 20 μg/mL INDO for 2 weeks and then switched to 10 μg/mL; group 3B received 10 μg/mL INDO continuously. The PCT incidence was reduced from 55% in the controls to 13.5% and 13.8% in the two groups, respectively, at day 400. Group 3C mice were begun on INDO 20 μg/mL on day 60 for 2 weeks and switched to 10 μg/mL. The appearance of the PCTs was delayed and reduced from 55% in the controls to 41% in the treated mice. The mice were observed for 395 days (Fig 3) during which time the inhibition of PCT was sustained in the groups treated continuously. This dose of pristane while being effective did not completely reduce the incidence of PCTs.
Induction curves for mice treated continuously with 10 μg/mL of INDO. Two of the groups, VI-start d60 and Indo 20-10, were begun on INDO at 20 μg/mL for 2 weeks and then switched to the 10 μg/mL dose. This lower dose of INDO is less effective than the 20 μg/mL dose.
Induction curves for mice treated continuously with 10 μg/mL of INDO. Two of the groups, VI-start d60 and Indo 20-10, were begun on INDO at 20 μg/mL for 2 weeks and then switched to the 10 μg/mL dose. This lower dose of INDO is less effective than the 20 μg/mL dose.
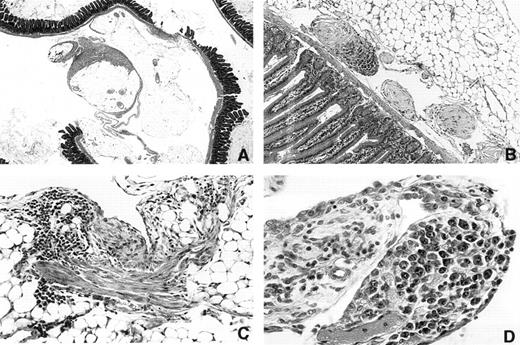
Inhibition of plastic disc induced PCT formation by INDO.When it was clear that mice could be maintained on INDO in the drinking water for more than 300 days, it became possible to determine if INDO could inhibit induction by plastic discs. This was of special interest because on-going histological studies of mice bearing i.p. discs indicated that this kind of foreign body produced a very different pathological response than was seen with oils. Essentially, there was no accumulative granuloma deposition on mesenteric surfaces, but instead the discs induced a patchy fibroplastic reaction (Fig 4A and C) that varied in amount from mouse to mouse. Occasional polyp-like structures containing collagenous material was seen near the mesenteric attachment sites (Fig 4B). The omentum was frequently adherent to the disc and appeared thickened. Most plastic discs were found to be adherent to the peritoneal connective tissues of the abdominal wall or omentum. An occasional disc was found in the retroperitoneum. In a few mice the disc was unattached. Varying numbers of adhesions of disc to intestine, omentum, abdominal fat, or abdominal wall were found. The discs themselves were also covered by a tenacious fibrous capsule. Adhesions of the discs to the omentum often contained blood vessels. It was suspected that in some of the mice this connective tissue bridge was broken as the surfaces of some of the disc contained piled-up necrotic tissue. The PCTs, however, did not appear to develop in the dense fibroplastic tissue as they were found either in the omental connective tisues (Fig 4D) or were first detected as multiple growths attached to or within small fibrotic polyp-like structures (Fig 4B).
Photomicrographs of peritoneal surfaces in mice carrying Lucite 21× 2 mm plastic discs. (A) 3616 1.5 × 2× Day 315. Section through mesenteric fat and intestine showing patchy fibrous deposition on mesothelial surfaces of mesenteric fat. Note that much of the surface is not covered. (B) 3173 20 × 10× Day 214. Region near the mesenteric attachment site containing three polyp-like structures. The two light staining polyps contain fibrinoid tissue covered with mesothelium. The third polyp is invaded by plasmacytoma cells. (C) 3164 20 × 5× Day 214. A patch of fibrous tissue on a fat surface on the left. The tissue contains numerous lymphocytes. (D) 3333 20 × 5× Day 258 Vascularized polyp-like structure arising from the omentum that contains a focus of atypical plasma cells. This was the only plasma cell lesion found in this mouse.
Photomicrographs of peritoneal surfaces in mice carrying Lucite 21× 2 mm plastic discs. (A) 3616 1.5 × 2× Day 315. Section through mesenteric fat and intestine showing patchy fibrous deposition on mesothelial surfaces of mesenteric fat. Note that much of the surface is not covered. (B) 3173 20 × 10× Day 214. Region near the mesenteric attachment site containing three polyp-like structures. The two light staining polyps contain fibrinoid tissue covered with mesothelium. The third polyp is invaded by plasmacytoma cells. (C) 3164 20 × 5× Day 214. A patch of fibrous tissue on a fat surface on the left. The tissue contains numerous lymphocytes. (D) 3333 20 × 5× Day 258 Vascularized polyp-like structure arising from the omentum that contains a focus of atypical plasma cells. This was the only plasma cell lesion found in this mouse.
Accordingly, 23 C.D2 I/P control mice were implanted with 21 × 2-mm Lucite discs and another 23 were given discs and treated continuously with 20 μg/mL INDO in the drinking water. By day 289, seven of the controls had developed PCTs that were diagnosed by cytofuge smears and confirmed histologically. At day 361 two more controls developed PCTs bringing the total incidence to 39%. Of the 23 mice given INDO continuously, 7 were autopsied at day 361 to 3633 and 15 were autopsied and examined histologically between days 393 and 397, and no PCTs were found.
t(12; 15) [Ig/c-myc] recombinations.Oil granuloma/mesenteric tissues from INDO-treated or control mice at days 30 to 31 and 82 to 89 were examined by nested PCR amplification for the presence of illegitimate Ig/c-myc recombinations (Tables 3 and 4). The entire intestinal mesentery, which contains most of the OG was removed, and each was divided into 5 fragments from which DNA was isolated. A summary of the experiments is shown in Table 3, and the junction sites are listed in Table 4. As may be seen from these results (Table 3), the number of Ig/c-myc isolations was similar in both the control (alcohol-H2O, H2O groups) and indomethacin-treated mice, indicating that indomethacin treatment has not appeared to inhibit the development of the recombinants or the ability of the cells containing them to propagate in the oil granuloma tissue. In three mice (column 2) two different junction fragments were isolated, indicating the presence of two clones in each of these mice, and in another six mice (indicated in column 4) the same junction fragment was isolated from different mesenteric fragments of the same mouse. The latter phenomenon could be due to spread of cells from the same clone into different mesenteric sectors or to contamination by ascites cells during the isolation. When this was encountered, the mouse was scored (Table 3) as having only a single Ig/c-myc recombination.
Isolation of Ig/c-myc Junction Fragments From Indomethacin Treated Mice
| Experiment3-150 . | No. Mice . | Treatment . | Day . | No. Ig/c-myc Recomb. . | |
|---|---|---|---|---|---|
| . | . | . | . | No. Mice . | Total . |
| 1 | 10 | Alcohol-H2O | 30-31 | 4 | 4 |
| 1 | 10 | Indomethacin3-151 | 30-31 | 5 | 5 |
| 2 | 10 | Alcohol-H2O | 82-89 | 4 | 4 |
| 2 | 10 | H2O | 82-89 | 1 | 2 |
| 2 | 10 | Indomethacin3-151 | 82-89 | 5 | 7 |
| Experiment3-150 . | No. Mice . | Treatment . | Day . | No. Ig/c-myc Recomb. . | |
|---|---|---|---|---|---|
| . | . | . | . | No. Mice . | Total . |
| 1 | 10 | Alcohol-H2O | 30-31 | 4 | 4 |
| 1 | 10 | Indomethacin3-151 | 30-31 | 5 | 5 |
| 2 | 10 | Alcohol-H2O | 82-89 | 4 | 4 |
| 2 | 10 | H2O | 82-89 | 1 | 2 |
| 2 | 10 | Indomethacin3-151 | 82-89 | 5 | 7 |
Mice injected with 0.5 mL pristane on d0 in experiment 1 and d0, d60 in experiment 2.
Starting at d0.
Ig/c-myc Junction Sequences From Control and Indomethacin Treated Mice
| Day . | Mouse No. . | Treatment . | Mesenteric Fragment4-150 . | Ig Sα (chr 12)‡ . | Junction4-151 . | c-myc (chr 15)‡ (5′-3′) . |
|---|---|---|---|---|---|---|
| . | . | . | . | (3′-5′) MUSIALPHA . | . | From Exon 1 Pos. 1 . |
| 30 | 20 | Alc | S6, S10 | 5,108 | A | 527 |
| 5,108 | T | 526 | ||||
| 30 | 8 | Alc | S29 | 4,080 | 247 | |
| 30 | 11 | Alc | S33, S34, S35 | 5,636 | gagac | 475 |
| 30 | 16 | Alc | S39A | 3,693 | 689 | |
| 30 | 4 | Indo | T34 | 3,681 | TC | 414 |
| 30 | 17 | Indo | S1, S3 | 2,433 | 600 | |
| 30 | 14 | Indo | T5A, T5B | 499 (Sμ) MUSIGCD09‡ | G | 967 |
| 30 | 1 | Indo | T13 | 2,919 | 973 | |
| 30 | 9 | Indo | T19 | 824 (Sμ) MUSIGHMY‡ | 837 | |
| 82 | 9ρ | H2O | X32 | 5,148 | G | 1,039 |
| X33A | 46 (Sμ) MUSIGCD09‡ | 521 | ||||
| 82 | 28 | Alc | X48 | 4,409 | AA | 922 |
| 87 | 21 | Alc | Y13 | 5,432 | A | 1,392 |
| 89 | 13 | Alc | Z29A, Z29B | 4,385 | ag | 567 |
| 4,382 | G | 567 | ||||
| 89 | 16 | Alc | Z26B, Z29 | 5,426 | CCTAG | 114 |
| 87 | 30ρ | Indo | Z32 | 4,090 | C | 422 |
| Z34 | 4,460 | ga | 680 | |||
| 87 | 25 | Indo | Y9 | 3,521 | T | 508 |
| 89 | 27 | Indo | Y19 | 3,954 | 977 | |
| 89 | 15ρ | Indo | Z16 | 4,172 | T | 1,054 |
| Z19 | 3,570 | 1,013 | ||||
| 89 | 11 | Indo | Z43, Z43A | 4,243 | CG | 799 |
| Day . | Mouse No. . | Treatment . | Mesenteric Fragment4-150 . | Ig Sα (chr 12)‡ . | Junction4-151 . | c-myc (chr 15)‡ (5′-3′) . |
|---|---|---|---|---|---|---|
| . | . | . | . | (3′-5′) MUSIALPHA . | . | From Exon 1 Pos. 1 . |
| 30 | 20 | Alc | S6, S10 | 5,108 | A | 527 |
| 5,108 | T | 526 | ||||
| 30 | 8 | Alc | S29 | 4,080 | 247 | |
| 30 | 11 | Alc | S33, S34, S35 | 5,636 | gagac | 475 |
| 30 | 16 | Alc | S39A | 3,693 | 689 | |
| 30 | 4 | Indo | T34 | 3,681 | TC | 414 |
| 30 | 17 | Indo | S1, S3 | 2,433 | 600 | |
| 30 | 14 | Indo | T5A, T5B | 499 (Sμ) MUSIGCD09‡ | G | 967 |
| 30 | 1 | Indo | T13 | 2,919 | 973 | |
| 30 | 9 | Indo | T19 | 824 (Sμ) MUSIGHMY‡ | 837 | |
| 82 | 9ρ | H2O | X32 | 5,148 | G | 1,039 |
| X33A | 46 (Sμ) MUSIGCD09‡ | 521 | ||||
| 82 | 28 | Alc | X48 | 4,409 | AA | 922 |
| 87 | 21 | Alc | Y13 | 5,432 | A | 1,392 |
| 89 | 13 | Alc | Z29A, Z29B | 4,385 | ag | 567 |
| 4,382 | G | 567 | ||||
| 89 | 16 | Alc | Z26B, Z29 | 5,426 | CCTAG | 114 |
| 87 | 30ρ | Indo | Z32 | 4,090 | C | 422 |
| Z34 | 4,460 | ga | 680 | |||
| 87 | 25 | Indo | Y9 | 3,521 | T | 508 |
| 89 | 27 | Indo | Y19 | 3,954 | 977 | |
| 89 | 15ρ | Indo | Z16 | 4,172 | T | 1,054 |
| Z19 | 3,570 | 1,013 | ||||
| 89 | 11 | Indo | Z43, Z43A | 4,243 | CG | 799 |
Fragments on the same line contain similar junctions isolated from different sectors. For each mouse there were 5 mesenteric fragments: the letter = randomized series, the numeral = the fragment. Thus in mouse #20, 2 separate fragments were isolated (S6, S10) that had the same junction sequence (except for overlaps which do not reflect significant differences).
The Ig and myc sequences at the junction shared common bases are capitalized while contained inserts are lower case.
Reference sequences from Genbank.
ρ Two different junction sequences were identified from these mice.
In Table 4 the location of the individual junctional breakpoints in Sα, Sγ, or Sμ and c-myc are shown. Because the breakpoints in the switch region and c-myc are stochastic and vary in each t(12; 15) translocation, the junction site becomes a clonal marker. The identified breakpoints are indicated by the underlined numbers in columns 5 and 7.
DISCUSSION
We previously showed that INDO inhibited plasmacytomagenesis initiated in BALB/c mice by a single 1-mL dose of pristane a regimen that produced only a 40% yield of PCTs.12 The basic findings in this study are, first, that INDO can inhibit plasmacytomagenesis initiated by the highly effective three-dose schedule of pristane administration.20 Further, this was accomplished in C.D2 I/P strain in which 60% to 70% of the mice develop PCTs by day 300 and the median latent period is approximately 170 days. Continuous administration of INDO almost completely inhibits PCT development in C.D2 I/P. Together, these results make it possible to evaluate the effects of different INDO treatment schedules on PCT incidence.
When INDO was administered for the first 70 days, only a minimal reduction of PCTs was seen, along with a slight delay in latent periods. This suggests that during this 70-day period clones of potential PCT cells had partially developed in the presence of INDO. Removal of INDO allowed these cells to progress towards greater autonomy, which indicates the more sensitive period for INDO is probably later in the course of the latent period (50 days to the time of appearance of the first PCTs). In those mice begun on INDO treatment on day 50 or 70, some of the PCT precursor cells had advanced to a point where indomethacin was no longer effective, ie, had attained a sufficient size in these mice. These cells were able to progress during INDO treatment and accounted for PCTs that did develop in these INDO treated groups. This result further substantiates previous observations that INDO treatment does not affect the late-stages of PCT development including the establishment in transplant of primary PCT cells in pristane-conditioned mice.12 Thus, beginning indomethacin at a later time point prevented the development of B cells that entered the oil granuloma after the 50- or 70-day period. Taken together these results and those previously reported12 indicate that INDO is effective for only a certain phase of PCT development, and this appears to be between the time the B cells arrive in the oil granuloma and before they begin to grow progressively.
INDO was also found for the first time to inhibit PCT induction by plastic discs. This is remarkable because the reactive tissue that forms in response to the plastic object is very different from the macrophage/neutrophil rich oil granuloma and consists for the most part as a fibroplasia on contacted peritoneal surfaces. Histopathological studies (M.P., unpublished observations, December 1996) indicate the earliest evidence of plasma cell proliferative lesions occurs in the omentum. This is a well-vascularized tissue, which contains in the mouse a specialized lymphoid tissue, the milky spots, or omental lymphoid organ.21-24 During i.p. immune responses plasma cell formation occurs in these lymphoid tissues. Fibroblasts are a well-known source of prostaglandins.25 Some of the early plasma cell lesions are associated with the connective tissues of the omentum. In others no preneoplastic lesions were found and developed PCTs were located in polyp-like lesions in the mesentery.
The biological mechanism of INDO inhibition is not yet firmly established, but a number of observations in other systems strongly suggest how INDO may be working. First, INDO is a powerful inhibitor of cyclooxygenase enzymes COX-1 and COX-213,15 whose primary function is the synthesis of prostaglandins and thromboxanes from arachidonic acid. This strongly implies that prostaglandins are actively produced in the oil granuloma and are key effector molecules in plasmacytomagenesis. The oil granuloma has been found to contain substantial ambient levels of PGE2 from analyses of peritoneal lavages.26
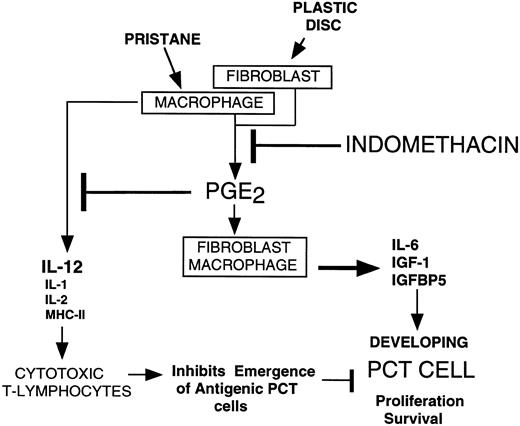
The effects of prostaglandin E2 have been extensively studied in B and T lymphocytes. The initial step is the binding to EP (PGE) receptors, recently shown to be present on B lymphocytes and plasma cells27,28 and T lymphocytes.29 Binding of PGE2 to the EP receptor activates one of the trimeric G proteins30 and can lead to the activation of adenyl cyclase and elevation of intracellular cAMP levels.29 In general, the findings in B cells indicate that PGE2 under specifically defined conditions may stimulate plasma cell differentiation,31 but usually inhibits B-cell proliferation.32-35 At present there is little evidence to support the hypothesis that PGE2 directly stimulates proliferation of B lymphocytes or plasma cells. The major biological effects of PGE2 are ascribed to the inhibition of cytotoxic T lymphocytes or the augmentation of macrophage functions. In T cells PGE2 inhibits interleukin-2 (IL-2) production in TH1 cells,36-39 probably inhibits TH0 to TH1 development and subsequent IL-2 and interferon-γ (IFN-γ) production.40 PGE2 downregulates MHC-II antigen expression,41,42 IL-1 expression,43 and downregulates IL-12 production44 45 by macrophages and monocytes. Hypothetically, the net effect of PGE2 is to strongly inhibit the formation of IL-2 and IFN-γ producing T cells, thus paralyzing the formation and proliferation of CD8+ T cells and the acquisition of cytotoxic T lymphocytes (CTL) (Fig 5).
Hypothetical scheme depicting how INDO inhibits plasmacytomagenesis. Pristane stimulates macrophages and plastic discs stimulate fibroblasts to produce PGE2 that acts in an autocrine fashion to cause these cells how to produce IL-6, IGF-1, and IGFBP5 that stimulate developing PCT cells to survive and proliferate. PGE2 inhibits production of macrophage factors IL-12, IL-1, IL-2, and MHCII (see text) that stimulate cytotoxic T lymphocyte formation. This potentially generates an immunologically permissive environment for antigenic plasma cells to escape immunological surveillance. Proof that this is a factor in plasmacytomagenesis is not yet available.
Hypothetical scheme depicting how INDO inhibits plasmacytomagenesis. Pristane stimulates macrophages and plastic discs stimulate fibroblasts to produce PGE2 that acts in an autocrine fashion to cause these cells how to produce IL-6, IGF-1, and IGFBP5 that stimulate developing PCT cells to survive and proliferate. PGE2 inhibits production of macrophage factors IL-12, IL-1, IL-2, and MHCII (see text) that stimulate cytotoxic T lymphocyte formation. This potentially generates an immunologically permissive environment for antigenic plasma cells to escape immunological surveillance. Proof that this is a factor in plasmacytomagenesis is not yet available.
Two effects of these inhibitions may be to increase the number of TH2 cells, which potentially could supply growth factors for B cells and plasma cells40 and to paralyze the formation of CTLs, which normally might play a role in controlling the increasing emergence of B-cell clones. Evidence for the accumulation of C.D4+ T cells in the oil granuloma that peaks around 80 days has been described.46 47
The different agents that induce chronic peritoneal granulomas (oil granuloma, or the fibroplasia from plastic discs) produce different morphological microenvironments and different amounts of reactive tissues. A potentially important microenvironmental component is the formation of a suitable adhesive surface onto which a pre-PCT cell might attach. Several candidates for this function are macrophages, fibroblasts, and mesothelial cells. Close physical contacts of PCT cells with mesothelial cells have been shown to be a requirement for adaptation of primary PCTs to tissue culture.48 A working hypothesis in our laboratory is that the reason why the multiple-dose regimen produces a greater yield of PCTs is that it is continuously generating new microenvironmental niches for candidate pre-PCT cells to develop. Based on the findings with the T(12; 15) translocations, one may speculate these cells are being continuously generated.
The plastic discs produced little reactive tissue on mesenteric surfaces and a variable amount in the omentum, hence has a reduced ability to recruit cells for PCT development. Consequently, the longer latent periods for disc induced plasmacytomagenesis. Nonetheless, the fibroplasia may provide appropriate niches for preplasmacytoma cells to lodge and progress. More investigation of this model is needed.
The macrophages of the oil granuloma are known to be a rich source of IL-6,26,49 a survival factor, inhibitor of apoptosis and possibly a growth factor for plasma and PCT cells.50-52 Shacter et al49 have shown that stimulated macrophages increase the production of IL-6.26 Another important and relevant macrophage growth factor is the insulin-like growth factor-1 (IGF-1) and the associated IGF binding proteins (IGFBPs), in particular IGFBP5. IGFs are produced in many tissues and cells including macrophages53 and fibroblasts,54 but probably not B cells.53 IGFs are actively bound to one of six known IGFBPs.55 IGFBP5 binds to the extracellular matrix and is stored and concentrated on these fibers. Matrix metalloproteinases and serine proteases in interstitial fluids degrade the IGFBP5 and release IGF-1.54 IGFBP5 is produced by fibroblasts54 and osteoblasts.56 IGF-1 has been found to modestly stimulate proliferation of human myeloma cell lines57-59 and B cells.56 Further, human myeloma cell lines and one mouse PCT line60 express IGF-1 receptors.57,60 Relevant to the present study PGE2 stimulates IGF-1 production by macrophages61 and regulates IGFBP5 formation in osteoblasts.62 63 These findings suggest that IGF-1 produced by macrophages and other cells in the oil granuloma, including fibroblasts could play a critical role in the survival of developing PCTs in the mouse and that lowering PGE2 production by indomethacin may limit the availability of IGF-1 and IGFBP5. We are currently working on this hypothesis.
Moreover, PGE2 stimulates IL-6 production by mouse peritoneal macrophages.26,49 Also, it is known that the oil granuloma produces transforming growth factor-β, which is known in other tumor cell systems to play a negative role in tumor cells, PCT cells, like many other tumor cells, may develop resistance to these negative influences.64,65 Bcl-2 is active in human myeloma cells but curiously inactive in mouse PCT cells. However, bcl-XL has been found in mouse PCT cells.66 The regulation of bcl-XL has not been studied in plasmacytoma development in oil granuloma tissues.
The formation of the 12; 15 translocation does not appear to be affected by indomethacin, as we detected equal numbers of DNAs with illegitimate Ig/c-myc recombinations in the oil granuloma tissue at 30 and 80 days after the injection of pristane in INDO-treated and in control mice. One can conclude that potential precursor cells, ie, those containing the illegitimate Ig/c-myc recombinations, are in the intestinal mesenteries and oil granuloma of INDO-treated mice. The sensitivity of the nested PCR amplification employed in these studies probably requires that a given B-cell clone would have undergone sufficient proliferation to provide enough DNA for detection.67 It is estimated that each mesenteric fragment that yielded an Ig/c-myc recombination contained at least 200 to 400 cells with that recombination, suggesting these cells had proliferated in the pristane stimulated oil granuloma.
The general picture that emerges from these studies is that B cells determined to differentiate into plasma cells enter the oil granuloma where they are confronted with a variety of positive and negative factors that impact on their survival and proliferation. It would appear that cells able to resist the negative effects of the environment and respond to the positive ones would be able to survive and proliferate. This process probably has many steps. Continuous INDO blocks the process. Critical, however, to the development of plasmacytoma are the special properties of the c-myc deregulated cell, which must also go through this process.
ACKNOWLEDGMENT
We are most grateful to Dr Robert Tarone, NCI, NIH, for the statistical evaluation of the data in Table 2. We thank Dr Emily Shacter (Food and Drug Administration, Center for Biologics, Bethesda, MD) for reading the manuscript and making many thoughtful suggestions. Special thanks to Mary Millison for the preparation of the manuscript.
Address reprint request to Michael Potter, MD, Laboratory of Genetics, National Cancer Institute, National Institutes of Health, Bldg 37, Room 2B04, 37 Convent Dr MSC4255, Bethesda, MD 20892-4255.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal