Abstract
Although a large amount of data is available on the effects of filgrastim (granulocyte colony-stimulating factor [G-CSF]) on the mobilization of stem cells in the circulation, data concerning its effects on bone marrow (BM) harvesting is scarce and controversial. We have designed a randomized trial comparing filgrastim-mobilized peripheral blood stem cell (PBSC) transplantation with filgrastim-primed autologous bone marrow transplantation (ABMT). Fifty-five patients affected by non-Hodgkin's (n = 38) or Hodgkin's (n = 17) lymphoma, selected for autologous transplantation over a 12-month period in a single institution, were randomized 2:1 to undergo BM or PB harvest/collection after priming for 3 days with filgrastim, 16 μg/kg body weight daily subcutaneously. BM priming with G-CSF allowed the harvest of a significantly higher number of mononuclear cells (MNC) (0.53 × 108/kg, range, 0.32 to 1.40), as compared with a historical control of unprimed BM harvests (0.43 × 108 MNC/kg, range, 0.15 to 0.72, P = .001). After high-dose ablative therapy, median time to neutrophil recovery above 0.5 × 109/L was 12 days for BM and 11 days for PB (P = .219); median time to platelet recovery above 20 × 109/L was 13 days for BM and 11 days for PB (P = .242). The same number of red blood cells, platelet transfusions, and posttransplant G-CSF doses were required in the two groups of patients. Less patients (50% v 70%) became febrile in the group transplanted with mobilized PB, but days of fever/patient and days on antibiotics were overlapping. The median time spent in the hospital after reinfusion was 16.5 and 15.5 days after primed BM and primed PB, respectively (P = .134). These data suggest that in patients with lymphoma submitted to autologous transplantation, the reinfusion of filgrastim-primed BM or filgrastim-mobilized PB leads to similar results, with an advantage of only 1 day in the neutrophil recovery and 1 day on the time spent in the hospital in favor of primed PB. Either option can be chosen on the basis of the availability of a surgery room or cell separator facilities and considering the patients' characteristics and wishes.
IN THE PAST few years, peripheral blood (PB) has been increasingly used as the primary source of stem cells (SC) for autologous transplantation. In this setting, priming (mobilizing) before PBSC collection with granulocyte colony-stimulating factor (G-CSF ), alone or in combination with cytotoxic drugs, is currently considered the best option to increase the number of circulating SC permitting an easier collection as compared with steady-state PB.1 Several uncontrolled analyses showed that G-CSF–primed autologous PBSC transplantation is advantageous over traditional (unprimed) bone marrow (BM) transplantation in shortening the time to neutrophil and platelet engraftment and the time spent in the hospital and in decreasing costs.2-4 Recently, this data has been confirmed in two randomized trials. Schmitz et al5 observed in lymphoma patients median recovery times of 11 (9 to 48) and 16 (8 to 52) days for neutrophils (PMN) and platelets (PLT), respectively, as opposed to 14 (9 to 25) and 23 (13 to 56) days, respectively after unprimed BM transplants. Beyer et al6 observed in germ cell tumor patients median recovery of 10 days for neutrophils and platelets, as opposed to 11 and 17 days, respectively after unprimed BM transplants. However, in the enthusiasm to move from BM to PBSC, an intermediate step has been overlooked: G-CSF–primed BM. In fact, it does not seem suitable to compare primed PBSC transplants with transplants performed with resting BM, known to have a high fraction of hematopoietic progenitors in the Go phase.7 This fraction can be recruited into cycle by in vivo priming with G-CSF8 and possibly allowing for a harvest rich enough in cells to engraft as quickly as those collected from primed PB.
These observations were the background for designing a randomized trial on the in vivo impact of G-CSF administration on BM cells harvesting (as well as on hematological recovery after high-dose ablative chemotherapy) as compared with PBSC collection, in patients selected for autologous transplantation. The study population included a homogeneous cohort of patients with non-Hodgkin's (NHL) and Hodgkin's (HL) lymphoma, seen in a single institution over a period of 12 months, who were randomized to undergo BM or PB harvest/collection after receiving the same dose and schedule of G-CSF. The results obtained in the two groups of patients were eventually compared with each other and with those of a historical control group previously autotransplanted with unprimed BM.
MATERIALS AND METHODS
From February 1995 through February 1996, 55 patients who were candidates for autologous transplantation entered the study. They were eligible if they had a diagnosis of intermediate or high-grade NHL or of high-risk HL, in first or higher complete remission or in partial remission. An age between 16 and 56, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1, an absolute neutrophil count (ANC) of ≥ 1.5 × 109/L, and a platelet count of ≥ 150 × 109/L were also required at the study entry. Exclusion criteria were the following: BM involvement by the disease at the time of harvest/collection; congestive heart failure (New York Heart Association Class III or IV); chronic renal failure; active chronic hepatitis; pregnancy; lactation and human immunodeficiency virus (HIV) infection. The study protocol was approved by the ethics committee of the University and General Hospitals of Udine, and all the patients gave informed consent before entering the study. Because results of PB priming were already known, we decided to obtain a high number of data on BM priming. Patients were stratified in the two study arms based on diagnosis (NHL or HL) and conditioning regime (BAVC or BEAM). Priming was performed with recombinant human G-CSF/filgrastim (Granulokine, Roche, Milan, Italy or Neupogen, Dompè-Biotec, Milan, Italy) at a daily dose of 16 μg/kg body weight (b.w.) given as a single afternoon subcutaneous injection. Patients randomized to undergo BM harvest were primed for 3 days and were harvested on day 4, 15 to 18 hours after the last injection of G-CSF. According to our previous experience,9,10 around 20 mL of BM/kg b.w. were harvested, with the target of harvesting at least 0.4 × 108/kg b.w. mononuclear cells (MNC), considered the minimal safe dose for autologous BM transplantation.11 Patients randomized to undergo PBSC collection were primed for 4 or 5 days, depending on the number of leukaphereses performed. Aphereses were started on day 4, 15 to 18 hours after the previous G-CSF injection and were performed on 2 or 3 consecutive days with the target of collecting at least 2 × 108/kg b.w. MNC and 2 × 106/kg b.w. CD34+ cells, considered the minimal safe doses for autologous PBSC transplantation.11 12
Laboratory studies and quality control of the graft.The harvested products (BM and PBSC) were processed on a Fenwall CS3000 separator (Baxter Health Care Products, Deerfield, IL), cryopreserved in 20% dimethyl sulfoxide (DMSO) with 50% autologous plasma and kept at −196°C. Total nucleated cells (TNC), MNC, and CD34+ cells were assayed from day 1 to day 6, and colony-forming units granulocyte-macrophage (CFU-GM) on day 1 (before G-CSF administration) and day 4 (before BM harvest or first apheresis) on the PB. All of the same parameters were also assayed on day 1 and on day 4 on a BM aspirate of 5 mL and on the leukaphereses products. TNC and MNC were determined using an automated cell counter (Cell Dyn 3000, Abbott, Santa Clara, CA) with an incorporated high resolution flow cytometer. Besides traditional forward and perpendicular light scattering, the Cell Dyn 3000 technology, referred to as M.A.P.S.S. (multiangle polarized scatter separation) introduces two additional dimensions to differentiate WBC without cytochemical staining or monoclonal tagging. The PB and BM aspirate differentials were performed simultaneously with the counter and on a light microscope: only lymphocytes and monocytes were included in the MNC. Day 14 CFU-GM were detected by plating in duplicate 105 light density cells in a total volume of 1 mL of IMDM medium (Iscove's modified Dulbecco's medium, GIBCO, Grand Island, NY) brought to 25% with fetal calf serum (Stem Cell Corp, Vancouver, Canada) and supplemented with 0.8% methylcellulose and G-CSF, GM-CSF, and interleukin-3 (IL-3) (all at a final concentration of 50 ng/mL) in 35-mm tissue culture dishes.
Daily PB CD34+ cell counts and day 1 and day 4 BM CD34+ cell counts were determined by flow cytometry. Samples obtained from PB or BM were simultaneously incubated at 4°C for 30 minutes with the phycoerythrin-conjugated monoclonal antibody HPCA2 (CD34) and the fluorescein-labeled monoclonal antibody HLE-1 (CD45) or with an irrelevant double-labeled isotype matched control antibody (all from Becton Dickinson, Milan, Italy). At the end of incubation, red blood cells were lysed using the fluorescence-activated cell sorting (FACS)-lysing solution (Becton Dickinson). At least 30,000 events were acquired for each sample. Immunofluorescence analysis was performed using a five parameter FACScan (Becton Dickinson, San Jose, CA) equipped with an argon-laser tuned at 488 nm. A side scatter (SSC) versus CD45 fluorescence dot plot was used to discriminate between the lymphohematopoietic cell population and erythrocytes and debris. The CD34+ cells were analyzed in a fluorescence versus SSC dot plot. Only cells with low SSC were counted as CD34+ cells.
Conditioning, supportive care, and clinical monitoring.The conditioning regimen used for the NHL was a modified BAVC protocol,10,13 which consists of: BCNU 200 mg/m2 intravenously (IV) on day −4, Cytarabine 150 mg/m2 IV every 12 hours on days −5, −4, −3, −2, Etoposide (VP-16) 150 mg/m2 IV every 12 hours on days −5, −4, −3, −2, Cyclophosphamide 45 mg/kg b.w. IV on days −5, −4, −3, −2. For the HL, the conditioning regimen was the BEAM,13 which consisted of: BCNU 300 mg/m2 IV on day −6, Cytarabine 400 mg/m2 IV on days −5, −4, −3, −2, Etoposide 200 mg/m2 IV on days −5, −4, −3, −2, Melphalan 150 mg/m2 IV on day −1.
The cryopreserved BM or PB were thawed and reinfused on day 0, at least 24 hours after completion of chemotherapy (CHT). The physician team of our BM unit was not told about the source of SC reinfused to patients. After transplant, all patients received subcutaneous filgrastim at a daily dose of 5 μg/kg b.w. starting on day +4 and up to a neutrophil count in excess of 1 × 10/9L for 3 consecutive days, as described elsewhere.13
Patients were treated in private rooms with reverse isolation and a diet low in bacterial and fungal content. Antimicrobical, antimycotic, and antiviral profilaxis consisted in trimethoprim-sulphamethoxazole or ciprofloxacin, itraconazole, nystatin or amphotericin suspensions, and acyclovir, respectively. Parenteral antibiotics were started after the onset of fever ≥38°C during neutropenia and maintained until the patient was afebrile for at least 3 consecutive days, or at least for 5 days. First-line intravenous antibiotic therapy included the association of teicoplanin and ceftazidime. Platelet transfusions (single-donor thromboaphereses) were scheduled to be given if the platelet count fell below 15 × 109/L; packed red blood cells were transfused to maintain a hemoglobin (Hb) level ≥80 g/L. Complete blood counts and vital signs were monitored daily during the hospital stay. Further posttransplants analyses were performed as necessary. Blood chemistry for liver and renal function were assayed regularly.
Historical control group.A historical group of 43 patients (matched for diagnosis and clinical characteristics) autotransplanted with unprimed BM was used as control. Exept for data on CD34+ cells and CFU-GM for this group of patients, the same data as for the randomized patients was available.
Study end points.The objectives of this study were to compare the two groups of transplanted patients (primed BM, primed PB) for the time to neutrophil recovery (number of days from reinfusion until the first of 3 consecutive days with an ANC of 0.5 × 109/L or more), the time to an unsupported platelet count of 20 × 109/L or more, the number of blood product transfusions, the number of posttransplant G-CSF doses, the percentage of febrile patients, the number of days of fever/patient, and the duration of the hospital stay (the number of days from reinfusion to the date of discharge from the hospital).
Statistical analysis.Time-dependent variables were compared between the groups using the log rank test. The Mann Whitney U test was employed to compare quantitative data. Differences with P values less than .05 were accepted as statistically significant.
RESULTS
Patients.A total of 36 of 55 randomized patients who entered the study were assigned to the primed BM group, while 19 were assigned to the primed PB group. The patients' characteristics are shown in Table 1. There were no major differences between the two groups in terms of sex, age, diagnosis, treatment history, and disease status at the time of harvest/collection. Conditioning regimen and supportive care were the same in both groups. As far as antecedent therapy in particular, first-line CHT consisted in the ALL0288 regimen14 for the lymphoblastic lymphomas (4 cases), in the F-MACHOP regimen15 for the other NHL (34 cases), and in the ABVD regimen for the HL (17 cases). When required, second-line treatments consisted in the MOPP regimen for HL and in various salvage regimens for NHL. The harvest/collection was performed at a median of 3 months (range, 1 to 9) from the end of chemotherapy. The transplant was performed at a median of 3 months (range, 0.5 to 10) from harvest and of 12 months (range, 7 to 24) from diagnosis.
Patient Characteristics
| . | G-CSF–Primed BM . | G-CSF–Primed PB . |
|---|---|---|
| No. of patients | 36 | 19 |
| Sex (M/F) | 23/13 | 10/9 |
| Age yr median (range) | 32 (16-56) | 41 (22-55) |
| Diagnosis | ||
| NHL | 25 | 13 |
| HL | 11 | 6 |
| Lines of treatment | ||
| 1 | 32 | 16 |
| ≥2 | 4 | 3 |
| RT (subdiaphrag.) | 8 (1) | 4 (2) |
| Status at H/C | ||
| CR (1st or 2nd) | 18 | 10 |
| PR | 18 | 9 |
| . | G-CSF–Primed BM . | G-CSF–Primed PB . |
|---|---|---|
| No. of patients | 36 | 19 |
| Sex (M/F) | 23/13 | 10/9 |
| Age yr median (range) | 32 (16-56) | 41 (22-55) |
| Diagnosis | ||
| NHL | 25 | 13 |
| HL | 11 | 6 |
| Lines of treatment | ||
| 1 | 32 | 16 |
| ≥2 | 4 | 3 |
| RT (subdiaphrag.) | 8 (1) | 4 (2) |
| Status at H/C | ||
| CR (1st or 2nd) | 18 | 10 |
| PR | 18 | 9 |
Abbreviations: RT, radiotherapy; H/C, harvest/collection; CR, complete remission; PR, partial remission.
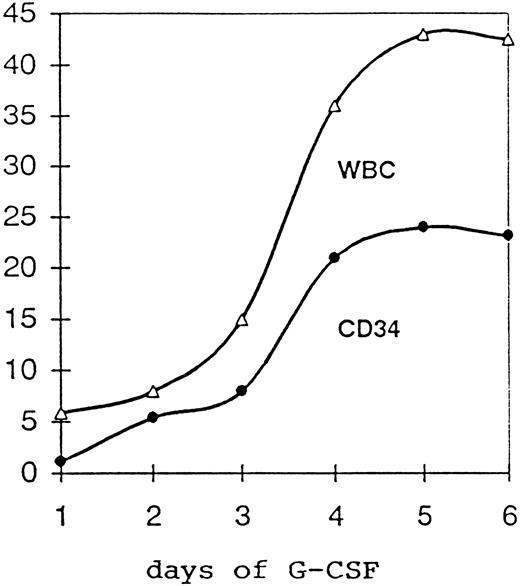
Effects of priming on PB and BM.In general, G-CSF was well tolerated, and side effects were mild to moderate. Bone pain was the most frequent side effect (80% of the patients) and was well controlled by paracetamol. No case required the reduction and/or suspension of the cytokine administration. Figure 1 shows the median concentration of WBC × 109/L and CD34+ cells/μL in the PB of the 19 patients randomized to PB collection over the 5-day period of G-CSF treatment. Considering all 55 randomized patients, the administration of the growth factor gave an increase in the PB TNC, from a median of 5.1 × 109/L (2.2 to 13.8) before G-CSF to a median of 29.7 × 109/L (12.2 to 75.0) after 3 days of G-CSF and before the first apheresis. At the same time points, PB MNC increased from a median of 1.6 × 109/L (0.7 to 5.4) to a median of 4.0 × 109/L (1.2 to 16.3), PB CD34+ cells increased 10-fold over baseline values, from a median of 0.87/μL (0.1 to 13.2) to a median of 8.5/μL (0.32 to 163), and PB CFU-GM increased from a median of 10/mL (0 to 224) to a median of 100/mL (1 to 4,300). All of these increments were highly significant (Table 2).
Median concentration of WBC × 109/L and CD34+ cells/μL in the PB of 19 patients receiving G-CSF 16 μg/kg/b.w. for 5 days.
Median concentration of WBC × 109/L and CD34+ cells/μL in the PB of 19 patients receiving G-CSF 16 μg/kg/b.w. for 5 days.
Effect of G-CSF Priming on PB
| . | Day +1 Before G-CSF . | Day +4 Before BM Harvest or 1st Apheresis . | P . |
|---|---|---|---|
| TNC × 109/L | |||
| Mean + SD | 6.0 ± 2.8 | 35.7 ± 15.4 | |
| Median (range) | 5.1 (2.2-13.8) | 29.7 (12.2-75.0) | <.0001 |
| MNC × 109/L | |||
| Mean ± SD | 1.9 ± 1.0 | 4.9 ± 3.1 | |
| Median (range) | 1.6 (0.7-5.4) | 4.0 (1.2-16.3) | <.0001 |
| CD 34+ cells/μL | |||
| Mean ± SD | 1.6 ± 2.1 | 15.7 ± 24.2 | |
| Median (range) | 0.87 (0.1-13.2) | 8.5 (0.32-163) | <.0001 |
| CFU-GM/mL | |||
| Mean ± SD | 20 ± 43 | 395 ± 729 | |
| Median (range) | 10 (0-224) | 100 (1-4,300) | <.0007 |
| . | Day +1 Before G-CSF . | Day +4 Before BM Harvest or 1st Apheresis . | P . |
|---|---|---|---|
| TNC × 109/L | |||
| Mean + SD | 6.0 ± 2.8 | 35.7 ± 15.4 | |
| Median (range) | 5.1 (2.2-13.8) | 29.7 (12.2-75.0) | <.0001 |
| MNC × 109/L | |||
| Mean ± SD | 1.9 ± 1.0 | 4.9 ± 3.1 | |
| Median (range) | 1.6 (0.7-5.4) | 4.0 (1.2-16.3) | <.0001 |
| CD 34+ cells/μL | |||
| Mean ± SD | 1.6 ± 2.1 | 15.7 ± 24.2 | |
| Median (range) | 0.87 (0.1-13.2) | 8.5 (0.32-163) | <.0001 |
| CFU-GM/mL | |||
| Mean ± SD | 20 ± 43 | 395 ± 729 | |
| Median (range) | 10 (0-224) | 100 (1-4,300) | <.0007 |
Table 3 shows the effects of the 3-day priming on BM. TNC increased from 21.8 × 109/L (4.2 to 48.8) to 37.8 × 109/L (16 to 159), MNC from 7.3 × 109/L (1.3 to 19.6) to 10.2 × 109/L (2.4 to 40), CD34+ cells from 47.5/μL (1.3 to 924) to 79.8/μL (6.8 to 857), and CFU-GM from 83/mL (70 to 3,800) to 3220/mL (60 to 36,000). Again, all of the increments were highly significant.
Effect of G-CSF Priming on BM
| . | Day +1 Before G-CSF . | Day +4 Before BM Harvest or 1st Apheresis . | P . |
|---|---|---|---|
| TNC × 109/L | |||
| Mean ± SD | 22.9 ± 10.6 | 43.5 ± 23.6 | |
| Median (range) | 21.8 (4.2-48.8) | 37.8 (16-159) | <.0001 |
| MNC × 109/L | |||
| Mean ± SD | 7.5 ± 4.0 | 12.4 ± 8.8 | |
| Median (range) | 7.3 (1.3-19.6) | 10.2 (2.4-40.0) | .0006 |
| CD 34+ cells/μL | |||
| Mean ± SD | 91.6 ± 147.6 | 148.5 ± 192.2 | |
| Median (range) | 47.5 (1.3-924) | 79.8 (6.8-857) | .0080 |
| CFU-GM/mL | |||
| Mean ± SD | 285 ± 573 | 607 ± 875 | |
| Median (range) | 83 (70-3,800) | 3,220 (60-36,000) | .0060 |
| . | Day +1 Before G-CSF . | Day +4 Before BM Harvest or 1st Apheresis . | P . |
|---|---|---|---|
| TNC × 109/L | |||
| Mean ± SD | 22.9 ± 10.6 | 43.5 ± 23.6 | |
| Median (range) | 21.8 (4.2-48.8) | 37.8 (16-159) | <.0001 |
| MNC × 109/L | |||
| Mean ± SD | 7.5 ± 4.0 | 12.4 ± 8.8 | |
| Median (range) | 7.3 (1.3-19.6) | 10.2 (2.4-40.0) | .0006 |
| CD 34+ cells/μL | |||
| Mean ± SD | 91.6 ± 147.6 | 148.5 ± 192.2 | |
| Median (range) | 47.5 (1.3-924) | 79.8 (6.8-857) | .0080 |
| CFU-GM/mL | |||
| Mean ± SD | 285 ± 573 | 607 ± 875 | |
| Median (range) | 83 (70-3,800) | 3,220 (60-36,000) | .0060 |
Quality of the grafts.Only 3 of the 19 patients randomized to PB collection underwent two PB aphereses, while all of the others were submitted to three procedures (median volume of blood processed: 28.0 L/patient, range, 18.8 to 34.0). The leukapheresis products contained a median of 6.1 MNC × 108/kg b.w. (range, 2.1 to 13.0), a median of 3.3 CD34+ cells × 106/kg b.w. (range, 0.44 to 12.7) and a median of 11.7 CFU-GM × 104/kg b.w. (range, 1.9 to 57.3). While the target of ≥ 2 × 108 MNC/kg b.w. was reached in all of the patients, in four of 19 (21%), the number of CD34+ cells collected was inferior to the target number of 2 × 106/kg b.w. The BM grafts of the other 36 G-CSF–primed patients contained a median of 0.53 MNC × 108/kg b.w. (range, 0.32 to 1.40), a median of 0.6 CD34+ cells × 106/kg b.w. (range, 0.16 to 2.3) and a median of 2.3 CFU-GM × 104/kg b.w. (range, 0.26 to 12.3). In only 1 of 36 patients, the target of 0.4 × 108 MNC/kg b.w. was not reached.
The BM harvests from patients primed with G-CSF were compared with those of the historical control group of unprimed BM. Despite the same volume of harvested BM/kg b.w. (a median of 21 mL/kg, range, 15 to 31 in historical group v a median of 18.4, range, 12 to 30 in primed BM, P = .138) a significantly higher number of MNC/kg was obtained after G-CSF priming as compared with the historical group (a median of 0.53, range, 0.32 to 1.40 v a median of 0.43, range, 0.15 to 0.72, P = .001). The difference became even more significant if the number of MNC/kg/L were compared in the two groups (median 0.40, range, 0.23 to 0.85 in primed group v median 0.28, range, 0.11 to 0.52, in historical unprimed group, P = .0002). Only data on MNC could be compared because data on CD34+ cells and CFU-GM are lacking for the historical control group.
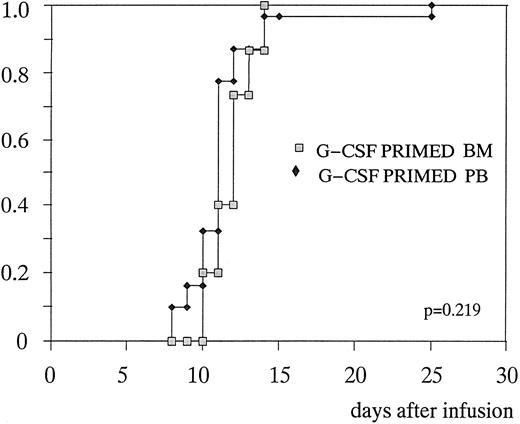
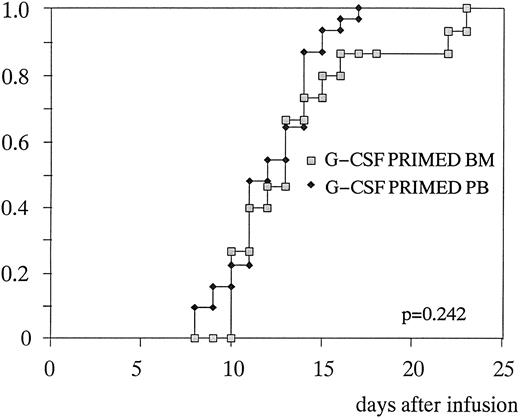
Hematopoietic recovery.Table 4 shows the comparison between the patients randomized to receive primed BM or primed PB. Median number of days to 0.5 × 109/L PMN was 12 (10 to 14) and 11 (8 to 25), respectively (P = .219) (Fig 2). Median number of days to unsupported platelets greater than 20 × 109/L was 13 (10 to 23) and 11 (7 to 14), respectively (P = .242) (Fig 3). The same number of transfusions of blood units (n = 2) and platelet aphereses (n = 3), as well as the same number of posttransplant G-CSF doses (10 v 9, respectively, P = .151) were required in the two groups. There was no statistical difference in the percentage of febrile patients, days of fever/patient, and days on antibiotics, although a trend in favor of infectious episodes of lesser gravity was observed in the group of patients transplanted with primed PB. These last patients were eventually discharged from the hospital 1 day in advance: 15.5 (11 to 22) versus 16.5 (13 to 22), P = .134. The only significant difference between the two groups was the median time to achieve a platelet count ≥50 × 109/L (16 days in primed BM v 14 days in primed PB, P = .011).
Transplant-Related Data: Comparison Between G-CSF–Primed BM and G-CSF–Primed PB
| . | G-CSF–Primed BM . | G-CSF–Primed PB . | P . |
|---|---|---|---|
| Days to | |||
| PMN ≥ 0.5 × 109/L median (range) | 12 (10-14) | 11 (8-25) | .219 |
| PMN ≥ 1.0 × 109/L median (range) | 13 (10-16) | 11 (8-26) | .117 |
| PLT ≥ 20 × 109/L median (range) | 13 (10-23) | 11 (7-14) | .242 |
| PLT ≥ 50 × 109/L median (range) | 16 (10-30) | 14 (9-26) | .011 |
| Blood units transfused median (range) | 2 (0-6) | 2 (0-6) | .749 |
| PLT apheresis transfused median (range) | 3 (1-6) | 3 (2-7) | .205 |
| Posttransplant G-CSF doses median (range) | 10 (3-16) | 9 (6-11) | .151 |
| % of febrile patients | 70 | 50 | .320 |
| Days of fever/patient median (range) | 2.5 (1-6) | 3 (1-9) | .165 |
| Days of antibiotic median (range) | 7 (6-10) | 8 (3-17) | .746 |
| Days of hospitalization median (range) | 16.5 (13-22) | 15.5 (11-22) | .134 |
| . | G-CSF–Primed BM . | G-CSF–Primed PB . | P . |
|---|---|---|---|
| Days to | |||
| PMN ≥ 0.5 × 109/L median (range) | 12 (10-14) | 11 (8-25) | .219 |
| PMN ≥ 1.0 × 109/L median (range) | 13 (10-16) | 11 (8-26) | .117 |
| PLT ≥ 20 × 109/L median (range) | 13 (10-23) | 11 (7-14) | .242 |
| PLT ≥ 50 × 109/L median (range) | 16 (10-30) | 14 (9-26) | .011 |
| Blood units transfused median (range) | 2 (0-6) | 2 (0-6) | .749 |
| PLT apheresis transfused median (range) | 3 (1-6) | 3 (2-7) | .205 |
| Posttransplant G-CSF doses median (range) | 10 (3-16) | 9 (6-11) | .151 |
| % of febrile patients | 70 | 50 | .320 |
| Days of fever/patient median (range) | 2.5 (1-6) | 3 (1-9) | .165 |
| Days of antibiotic median (range) | 7 (6-10) | 8 (3-17) | .746 |
| Days of hospitalization median (range) | 16.5 (13-22) | 15.5 (11-22) | .134 |
Time to to neutrophil recovery ≥ 0.5 × 109/L after G-CSF–primed BM or G-CSF primed PB transplantation.
Time to to neutrophil recovery ≥ 0.5 × 109/L after G-CSF–primed BM or G-CSF primed PB transplantation.
Time to achieve platelets ≥ 20 × 109/L after G-CSF–primed BM or G-CSF–primed PB transplantation.
Time to achieve platelets ≥ 20 × 109/L after G-CSF–primed BM or G-CSF–primed PB transplantation.
It must be emphasized that patients transplanted with a number of cells lower than the desired target did not present differences in the engraftment kinetic or in the incidence and gravity of infectious episodes (four patients in the primed PB group and one in the primed BM group).
DISCUSSION
A large amount of data is available on the effects of G-CSF on the mobilization of stem and progenitor cells in the circulation,2-4 while data concerning its effects on BM harvesting is scarce and controversial, although its effects on BM are well known (increase in marrow cellularity, in the myeloid: erythyroid ratio and amplification of the myeloid compartment).16 Few brief and nonrandomized studies are available on the impact of G-CSF priming on BM transplants. Johnsen et al17 in 1992 and Hansen et al18 in 1995 showed that pretreatment with G-CSF before marrow harvest does not significantly accelerate myeloid engraftment when compared with historical controls of unprimed BM.
More recently Janssen et al19 and Lowenthal et al20 supported the idea that the engraftment with primed BM is as rapid as with PBSC and superior to unprimed BM. Although a possible concern was the decrease in the number of CFU-GM/mL of BM after G-CSF priming, as reported in a previous study on cancer patients,21 when designing our protocol, we had hypothesized that G-CSF could increase not only the numbers of TNC and MNC/mL of BM, but also those of CFU-GM and CD34+ cells. Moreover, we thought that this could translate into a faster kinetic of engraftment after high-dose CHT. We chose as the mobilizing protocol the one already well established in Seattle4 (16 μg/kg b.w. daily, subcutaneously, for 5 days) and performed the BM harvest or the first apheresis about 15 to 18 hours after the third dose of the growth factor. This protocol was very effective in the mobilization in the PB of CD34+ cells (increased by a factor of 10, with a peak reached on day +5) and CFU-GM (increased by a factor of 20 with the peak on the same day). A total of 16 of 19 patients required three aphereses to collect the desired amount of progenitors. The number of MNC, CD34+ cells, and CFU-GM/kg b.w. collected was in line with previous reports,2-4 and well above the minimal required dose which is considered safe for transplantation,11,12 although all of our patients were pretreated with at least six cycles of CHT. The same parameters as above were studied on the BM of all the 55 randomized patients before and after three doses of G-CSF. Our data showed that the G-CSF priming caused a significant increase in the number of MNC, CD34+ cells (with their percentage increasing only from 0.6% to 1%) and CFU-GM/mL of BM. This allowed us to harvest, in a very short period of time (less than 40 minutes), the desired volume of marrow, significantly improving the yield of MNC harvested/kg of patients b.w. and /L of marrow harvested, as compared with the historical group of unprimed BM. Only MNC could be compared, as data on CD34+ cells and CFU-GM were not available in the historical group. However, having observed a faster engraftment kinetic in primed BM transplantation, it is likely that the increase of MNC had to be associated with a simultaneous increase of CD34+ cells and of CFU-GM. Schmitz et al5 and Beyer et al6 in randomized studies compared the engraftment after primed PB and unprimed BM transplantation, confirming the superiority of PB. In their study, median days to PMN recovery above 0.5 × 109/L, median days to platelet recovery above 20 × 109/L and median days spent in hospital were 11, 16, and 17 for primed PBSC transplantation and 14, 23, and 24 for unprimed ABMT, respectively. Our data is in line with their results, but shows a faster platelet recovery, which can be due to different patients' characteristics and/or regimens of CHT or conditioning regimens. Nevertheless, it must be emphasized that our historical unprimed BM showed faster hematological recovery with respect to the data reported by Schmitz et al5 and Beyer et al6 (13 days to neutrophil > 0.5 × 109/L, 15 days to platelets > 20 × 109/L and 22 days of hospital stay, data not shown). Very interesting was the comparison between primed PB and primed BM. In fact, the engraftment kinetic of primed BM was only slightly slower than in PB. The only significant difference between the two groups was the median time to platelets > 50 × 109/L (16 v 14 days in BM and PB, respectively, P = .011). However, it must be mentioned that even if statistically significant, a difference of only 2 days in the platelet recovey is likely not to be biologically or clinically relevant.
The impact of primed BM on PLT engraftment must be stressed because a delay in PLT recovery with the use of growth factors after the reinfusion of relatively low numbers (≤2.5 × 106/kg b.w.) of G-CSF mobilized CD34+ cells was recently described. It was speculated that “the adverse effect of GF could reflect the ability of these cytokines to influence cells of intermediate lineage that have the potential to become either neutrophils or platelets. Alternatively the negative effect may be due to increased consumption of PLT by activated myeloid cells.”22
Our study showed the same kinetics of PLT engraftment between primed BM and primed PB (even in patients transplanted with less than 2.5 × 106/kg b.w. PB CD34+ cells), indicating that primed BM still retains a number of PLT precursors able to rapidly mature into terminal cells (almost 4 days faster than resting BM precursors; P = .006, our data, not shown).
Another explanation for the fast engraftment observed with primed BM was its possible contamination with PB. To this purpose, it must be pointed out that the amount of marrow aspirated on day 1 and day 4 to perform the biological studies was only 5 mL, decreasing to the minimum the possibility of massive PB contamination. Moreover, even assuming that the BM harvests were heavily contamined by PB, this does not change the efficacy and the purpose of priming BM before harvesting.
In conclusion, our study confirms the efficacy of G-CSF (at the described dose and schedule) as a mobilizing agent for the collection of PB stem and progenitor cells, even in pretreated patients; it confirms the superiority, in terms of hematological recovery, of PBSC as compared with unprimed BM; it shows, in a randomized study, that the BM can be efficiently primed with G-CSF before harvest, and this produces a more rapid engraftment as compared with unprimed BM; and finally, it underlines that there is little difference, in terms of engraftment kinetic, between primed BM and primed PB, allowing to choose between the two options based on the availability of a surgery room or cell separator facilities and on patients' characteristics and wishes. Even when considering the whole procedure (priming, harvesting, and reinfusion) from the economical point of view, primed BM is probably as cheap (or as expensive) as primed PB. In fact, the claimed economical superiority of PBSC transplantation is mainly due to the shorter hospital stay after reinfusion as opposed to unprimed BM.23 In our study, the cost of G-CSF (priming plus postreinfusion) is slightly in favor of BM; the cost of hospitalization is still slightly in favor of PB; and the cost of 1 hour in the surgery room is the same or even less than two or three aphereses and following cryopreservation.
The future possible applications of primed BM, other than in the obvious autologous setting, could be in the fields of allogeneic and unrelated BM transplantation where a higher number of CD34+ and a decreased number of T cells is needed; and, possibly, in the field of allogeneic BMT in children. In fact, priming the donor with G-CSF could allow for a shorter anesthesia, could avoid the insertion of a central venous catheter, and permit the harvest of a smaller amount of BM, possibly avoiding previous autologous blood banking.
Supported by AIL, 30 ORE PER LA VITA and by Progetto Sangue, Istituto Superiore di Sanità, Rome, Italy.
Address reprint requests to Daniela Damiani, MD, Division of Hematology, University Hospital, P.le S. Maria della Misericordia, 33100 Udine, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal