Abstract
Calreticulin is a soluble endoplasmic reticulum protein comprising the major storage reservoir for inositol trisphosphate-releasable calcium. Although its highly conserved primary structure and a wide range of functions have been well described, less attention has been paid to its biosynthesis, particularly in human tissues. We report analyses of synthesis, proteolytic processing and glycosylation of human calreticulin. In both HL-60 and PLB-985 myeloid cell lines calreticulin was immunoprecipitated as a single 60-kD species without evidence of precursor forms. However, in vitro cell-free synthesis produced a 62-kD primary translation product, which in the presence of microsomal membranes, was processed by cotranslational signal peptide cleavage to a 60-kD species that comigrated with mature calreticulin produced in myeloid cells. Neither tunicamycin treatment of the cells nor endoglycosidase digestion of calreticulin resulted in any forms other than the 60-kD protein on sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis, suggesting that the potential site for N-glycosylation at asparagine-327 was unmodified. However, oxidative derivatization of carbohydrate components with digoxigenin showed that human calreticulin produced in either HL-60 cells or Sf9 insect cells is glycosylated, indicating that glycosylated and nonglycosylated human calreticulin have indistinguishable electrophoretic mobilities. Direct measurement by phenol-H2SO4 confirmed the presence of carbohydrate on recombinant human calreticulin. These data show that human myeloid calreticulin undergoes cotranslational signal peptide cleavage and posttranslational N-linked glycosylation. Although glycosylation of calreticulin has been shown in rat liver and bovine liver and brain, it has been reported to be lacking in other tissues including human lymphocytes.
CALRETICULIN is a high-capacity calcium-binding protein comprising the major intracellular storage reservoir for inositol-1,4,5 trisphosphate-releasable calcium in most nonmuscle cells.1-4 Its structure is highly conserved and its range of expression is extremely broad with respect to both species and cell type.1,5-7 Previously known by several other names (eg, high-affinity calcium binding protein, calregulin, ERp60, CRP55, CAB-63, CaBP3, calsequestrin-like protein), calreticulin has a single high-affinity and multiple low-affinity calcium binding sites.1,8 Although the calcium-binding properties of calreticulin have been known for some time, recent work in many systems has established a wide range of other functions not directly related to calcium storage, as well as localizations of the protein outside of the endoplasmic reticulum in keeping with some of its novel functions.3,4 9-11
Binding of calreticulin to a conserved cytoplasmic domain of integrin α subunits may be involved in regulation of cell shape and interaction with the extracellular matrix.12,13 Steroid hormone receptors bind DNA through a peptide domain very similar to the calreticulin-binding sequence of integrins and the association of calreticulin with these receptors thereby inhibits their binding to the promoter elements of steroid-responsive genes.14,15 Calreticulin is present in cytotoxic granules of T lymphocytes where it may regulate calcium-sensitive cytolysin activity.16 Whether calreticulin is a prominent target of autoimmune responses has been a subject of great interest in immunology.7,17-20 In cells infected with rubella virus calreticulin binds to viral RNA, thereby modulating viral replication.21,22 Calreticulin on the cell surface may serve as a lectin that triggers melanoma cell spreading.23 Binding of calreticulin to various coagulation factors interferes with blood clotting.24 We have shown that calreticulin functions as a molecular chaperone for newly synthesized myeloperoxidase in myeloid cells25 and a similar function has been recently reported for calreticulin in other cell systems.26
Despite calreticulin's broad range of functions and widespread expression, its biosynthesis and processing have received relatively little attention. The cDNA sequence predicts a typical N-terminal signal sequence and, depending on species, one or two sites of potential N-linked glycosylation.1,6,7,17,27 28 In the current work we cloned the cDNA for human myeloid cell calreticulin, developed a high-level expression system in insect cells, and generated a high-affinity polyclonal antibody against the purified recombinant protein. These reagents were then used to characterize calreticulin biosynthesis in cultured human myeloid cells and in cell-free systems, focusing particularly on the primary product of translation and its posttranslational modification by proteolysis and glycosylation.
MATERIALS AND METHODS
Calreticulin cloning, expression, purification, and antibody preparation.The cDNA for human myeloid calreticulin was cloned from a previously described HL-60 cell cDNA library in the λ-Zap bacteriophage vector (Stratagene, La Jolla, CA).29 The library was screened by hybridization with a 32P-labeled probe comprising 1,200 base pairs (bp) of the cDNA for rabbit skeletal muscle calreticulin6 kindly provided by Dr M. Michalak of the University of Alberta, Canada. Following plaque purification the pBluescript phagemids were excised and sequenced. The longest insert contained 1,891 bp including a 5′-untranslated region (53 bp), a 1,251-bp open reading frame and 587-bp 3′-untranslated region ending with the ATTAAA polyadenylation signal and a poly-A tract. The region of the starting methionine codon has an appropriate consensus sequence for a eukaryotic initiator site (CCGCCATGC). The predicted protein is 417 amino acids in length, which based on N-terminal sequencing of mature calreticulin,1,17,30,31 includes a 17-residue signal sequence. There is a single potential site for N-linked glycosylation at asparagine-327 (of the mature protein) within the motif NET. Comparing the sequence to the calreticulin cDNA initially reported as that of the human Ro/SS-A autoantigen,17 there are several polymorphisms, but the predicted amino acid sequence is identical. As previously noted the conservation of amino acid sequence among human, rabbit, rat, and murine calreticulins is 92% to 94%.1
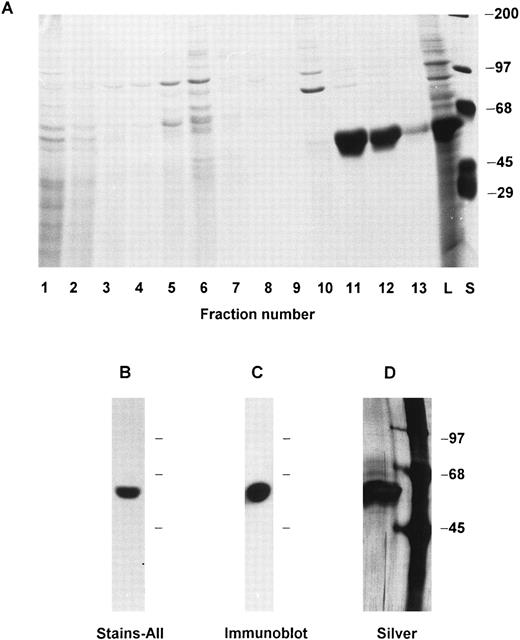
Recombinant calreticulin was expressed in the baculovirus system (Invitrogen Corp, San Diego, CA). The full-length cDNA was excised from pBluescript and cloned into the EcoRI site of the pVL1393 baculovirus transfer vector. Forward orientation of the insert was shown by restriction enzyme mapping. Spodoptera frugiperda Sf9 cells were cotransfected with the transfer vector and linearized baculovirus DNA according to the manufacturer's directions. Recombinant plaques were identified by microscopic visualization and carried through three cycles of plaque purification. Of eight clones selected for further study, three induced the synthesis in Sf9 cells of a prominent 60-kD protein (Fig 1A) that stained blue with Stains-All32,33 (Fig 1B) and was not present in Sf9 cells left uninfected or infected with wild-type baculovirus. The 60-kD protein was recognized in immunoblots by a polyclonal rabbit antibody prepared against a C-terminal human calreticulin peptide (kindly provided by Dr K.-H. Krause, University of Geneva; data not shown). The viral clone producing the largest amount of recombinant protein was optimized for multiplicity of infection (approximately 10:1) and time of cell culture (3 days). Large batches of infected Sf9 cells (usually 6 flasks of 225 cm2 with a total of about 109 cells) were cultured, procured by pipette disruption of the monolayer, collected at 500g for 6 minutes, washed in phosphate-buffered saline (PBS) pH 7.2 and resuspended in 10 to 20 mL of lysis buffer (relaxation buffer34 with 12.5 mmol/L EGTA, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), and 0.5% NP-40). The cells were then kept on ice for 10 minutes and vortexed for about 5 seconds once each minute. The lysate was cleared by centrifugation at 200g, 4°C for 6 minutes. The supernatant was subjected to chromatography on a DEAE-Sepharose (Pharmacia, Piscataway, NJ) column that was equilibrated and run at 4°C with 0.1 mol/L potassium phosphate buffer pH 7.1 plus 1 mmol/L EDTA, 0.02% NaN3 , and a linear gradient from 50 to 550 mmol/L NaCl. Fractions containing the 60-kD recombinant protein (see Fig 1A) were dialyzed against 20 mmol/L Tris-HCl pH 7.5, lyophilized, and dissolved in running buffer for chromatography on a Sephacryl S-300 (Pharmacia) column run at 4°C with 20 mmol/L Tris-HCl pH 7.5 plus 0.15 mol/L KCl. Fractions containing the recombinant protein were pooled, dialyzed against 10 mmol/L Tris-HCl pH 7.5, lyophilized, and stored at −80°C. The usual yield was ∼1 mg of protein per 108 Sf9 cells. Silver-stained polyacrylamide gels of the final preparation exhibited a single band at 60 kD (Fig 1D). Native human calreticulin was purified from HL-60 cells by the method of Krause et al.32
Recombinant calreticulin expression, characterization, purification, and antibody generation. (A) Lysate of Sf9 cells infected with recombinant baculovirus was chromatographed on DEAE-Sepharose and aliquots of the fractions were analyzed by 7.5% SDS-PAGE and Coomassie Blue staining. Recombinant calreticulin is seen as a prominent 60-kD band in the Sf9 cell lysate (lane marked L) and in column fractions 11 and 12. The lane marked S shows standards with molecular weights as indicated. (B) The same Sf9 cell lysate as in (A) was analyzed by 7.5% SDS-PAGE and staining with Stains-All. The prominent 60-kD band stained blue, whereas other bands stained faintly red and are not visible in the photograph. (C) Purified recombinant calreticulin was analyzed by 7.5% SDS-PAGE, transfer to nitrocellulose, and detection by immunoblotting using a 1:1,000 dilution of rabbit polyclonal antibody to the purified protein. (D) Purified recombinant calreticulin was analyzed by 7.5% SDS-PAGE and silver staining. The left lane is the purified protein and the right lane shows standards with molecular weights as indicated.
Recombinant calreticulin expression, characterization, purification, and antibody generation. (A) Lysate of Sf9 cells infected with recombinant baculovirus was chromatographed on DEAE-Sepharose and aliquots of the fractions were analyzed by 7.5% SDS-PAGE and Coomassie Blue staining. Recombinant calreticulin is seen as a prominent 60-kD band in the Sf9 cell lysate (lane marked L) and in column fractions 11 and 12. The lane marked S shows standards with molecular weights as indicated. (B) The same Sf9 cell lysate as in (A) was analyzed by 7.5% SDS-PAGE and staining with Stains-All. The prominent 60-kD band stained blue, whereas other bands stained faintly red and are not visible in the photograph. (C) Purified recombinant calreticulin was analyzed by 7.5% SDS-PAGE, transfer to nitrocellulose, and detection by immunoblotting using a 1:1,000 dilution of rabbit polyclonal antibody to the purified protein. (D) Purified recombinant calreticulin was analyzed by 7.5% SDS-PAGE and silver staining. The left lane is the purified protein and the right lane shows standards with molecular weights as indicated.
A polyclonal antibody (presently available from Affinity Bioreagents, Golden, CO) to the purified recombinant calreticulin was raised in a rabbit by subcutaneous injection every 3 to 4 weeks of about 50 μg of protein in incomplete Freund's adjuvant. The antiserum recognizes a single 60-kD species in immunoblots of lysates of Sf9 cells expressing calreticulin (Fig 1C), human neutrophils, and cultured human myeloid cell lines HL-60 and PLB-985. Immunoprecipitation of biosynthetically labeled cells yields a prominent 60 kD species and in some experiments a trace 90-kD protein (see Results).
Protein biosynthesis in cultured cell lines.The human myeloid leukemia cell lines HL-6035 and PLB-98536 were cultured and biosynthetic labeling was performed as we have reported in detail25,37 using a 30-minute preincubation in methionine-free medium followed by pulse-labeling with 35S-methionine (∼1,000 Ci/mmol; Amersham, Arlington Heights, IL). The duration of the pulse and subsequent chases with excess unlabeled methionine are indicated in individual protocols. Following labeling the cells were disrupted in antiprotease buffer and labeled proteins were immunoprecipitated with whole antisera to either calreticulin (above) or myeloperoxidase37 using our previously described protocol.25 Immunoprecipitates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and fluorography of the dried gels. In some experiments biosynthesis was performed in the presence of tunicamycin (Boehringer Mannheim, Indianapolis, IN) to inhibit protein glycosylation. Since prolonged exposure of myeloid cell lines to tunicamycin induces differentiation,38,39 the period of preincubation with the inhibitor was limited to 4 hours. In other studies the immunoprecipitated calreticulin and myeloperoxidase were treated at 37°C for 16 hours in Endo F buffer (0.1 mol/L sodium phosphate buffer pH 6.5 containing 50 mmol/L EDTA, 0.5% NP-40, 0.2% SDS) plus 1% β-mercaptoethanol with either endoglycosidase H or N-glycosidase F (Boehringer Mannheim) before gel electrophoresis as we have described.40
Cell-free biosynthesis.Cell-free protein synthesis was carried out in the rabbit reticulocyte lysate system (Promega, Madison, WI) in the presence of 35S-methionine according to the manufacturer's directions and our published technique.37 The reaction mixture included no RNA (control sample), poly-A–enriched RNA prepared from HL-60 cells using the FastTrack System (Invitrogen), or synthetic transcripts generated from the calreticulin cDNA in pBluescript using T3 polymerase and with polyadenylation and capping in the presence of 0.5 mmol/L G(5′ )ppp(3′ )G (Promega). In some experiments dog pancreatic microsomes (Promega) were added to study processing of the nascent proteins.
Digoxigenin labeling of glycoproteins.The glycosylation state of calreticulin was analyzed by covalent labeling of glycoproteins with digoxigenin41 using manufacturer's method A of the Glycan Detection Kit (Boehringer Mannheim). Fetuin (Sigma, St Louis, MO) was used as a control glycoprotein. Briefly, ∼1 μg of protein was dissolved in 60 μL of 0.1 mol/L sodium acetate buffer pH 5.5. Samples were oxidized by addition of 40 μL of sodium metaperiodate (3.3 mg/mL) and incubation at 22°C for 20 minutes in the dark. Excess periodate was quenched by addition of 40 μL of sodium disulfite (3.75 mg/mL) and incubation at 22°C for 5 minutes. The oxidized carbohydrates were then derivatized with digoxigenin by addition of 20 μL of digoxigenin-succinyl-ε-amidocaproic acid (in dimethylformamide) and incubation at 22°C for 1 hour. Samples were separated on SDS-PAGE, transferred to nitrocellulose (BA85; 0.45 μ, Midwest Scientific, Valley Park, MO) at 50 V for 4.5 hours, and digoxigenin-labeled proteins were detected by immunoblotting according to the Glycan Detection Kit protocol except that exposure to antidigoxigenin antibody was done overnight at 4°C. To determine whether digoxigenin labeling represented N-linked carbohydrate moieties the protein was incubated in Endo F buffer with or without one U of N-glycosidase F for 4.5 hours at 37°C before labeling with digoxigenin. To confirm that the 60-kD digoxigenin-labeled protein was calreticulin the samples were labeled with digoxigenin and then immunoprecipitated with anticalreticulin, separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with antibody to either calreticulin or digoxigenin.
Phenol-sulfuric acid quantitation of carbohydrate.The carbohydrate content of proteins was directly measured by the phenol-sulfuric acid method42 using standard curves run with dextrose. Fetuin and transferrin served as control glycoproteins and bovine serum albumin as a nonglycosylated protein (all from Sigma). Samples of 0.5 mg of protein in 0.5 mL of PBS were mixed with 0.5 mL aqueous phenol. Color was developed by rapid addition of 2.5 mL concentrated sulfuric acid, followed by incubation at 50°C for 15 minutes, cooling and determination of absorbance at 490 nm.
Replicate experiments.In all cases the experiments shown are representative of the results obtained in at least 3 separate studies.
RESULTS
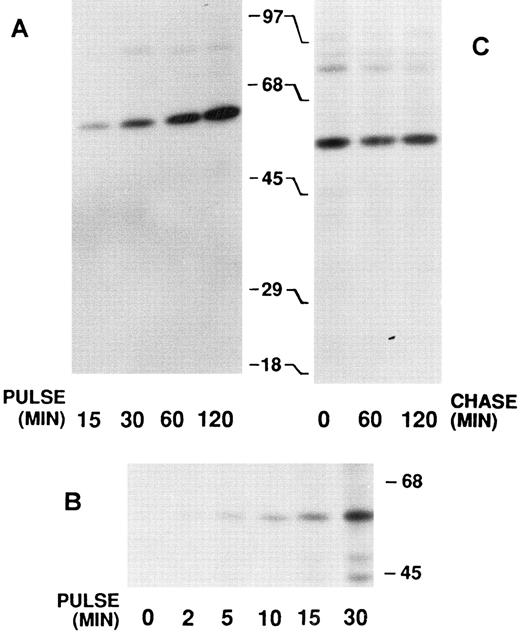
Biosynthesis of calreticulin by HL-60 cells.Cultured HL-60 myeloid cells were pulse-labeled with 35S-methionine and the products were immunoprecipitated with antiserum to recombinant human calreticulin and analyzed by SDS-PAGE. With labeling pulses of 15 minutes to 2 hours only a single 60-kD product was observed (Fig 2A). Similar results were obtained with PLB-985 cells (data not shown, but see Fig 4). In an attempt to detect an early precursor of the mature 60-kD species, pulses as short as 2 minutes were used (Fig 2B). However, only the 60-kD product was observed, suggesting that cleavage of the 17-amino acid signal peptide occurs cotranslationally. Further evidence for this and for the lack of any other posttranslational processing was the finding that postlabeling chases of up to 2 hours with cold methionine did not result in the appearance of any other forms of calreticulin, at least as detectable in the SDS-PAGE system (Fig 2C). In some anticalreticulin immunoprecipitates a 90-kD species was detected (eg, Fig 2A and C). We have recently shown that this coprecipitating protein is the apopro form of myeloperoxidase and that calreticulin functions as a chaperone for myeloperoxidase.25
Immunoprecipitates of biosynthetically labeled calreticulin from HL-60 cells. After 35S-methionine pulse labeling, the cells were disrupted and the labeled proteins immunoprecipitated with anticalreticulin. The precipitates were analyzed by 7.5% SDS-PAGE and fluorography. The locations of molecular weight markers are indicated. (A) Pulse labeling was performed for 15 to 120 minutes as indicated. (B) Pulse labeling was performed for 0 to 30 minutes as indicated. Long exposure of the film resulted in the appearance of unidentified lower molecular weight bands in the 30-minute pulse lane. (C) Pulse labeling was performed for 30 minutes and then followed by a chase with excess unlabeled methionine for 0 to 120 minutes as indicated.
Immunoprecipitates of biosynthetically labeled calreticulin from HL-60 cells. After 35S-methionine pulse labeling, the cells were disrupted and the labeled proteins immunoprecipitated with anticalreticulin. The precipitates were analyzed by 7.5% SDS-PAGE and fluorography. The locations of molecular weight markers are indicated. (A) Pulse labeling was performed for 15 to 120 minutes as indicated. (B) Pulse labeling was performed for 0 to 30 minutes as indicated. Long exposure of the film resulted in the appearance of unidentified lower molecular weight bands in the 30-minute pulse lane. (C) Pulse labeling was performed for 30 minutes and then followed by a chase with excess unlabeled methionine for 0 to 120 minutes as indicated.
Effect of tunicamycin on the biosynthesis of calreticulin and myeloperoxidase in HL-60 and PLB-985 cells. Biosynthesis using a 60-minute pulse label in either HL-60 (left) or PLB-985 (right) cells, immunoprecipitation with either anticalreticulin (CRT; upper panel) or antimyeloperoxidase (MPO; lower panel), SDS-PAGE, and fluorography were performed as in Fig 2 except that the cells were cultured in tunicamycin at the concentrations indicated. The apparent increased intensity of the calreticulin signal in PLB-985 cells treated with tunicamycin was not a consistent finding in other experiments.
Effect of tunicamycin on the biosynthesis of calreticulin and myeloperoxidase in HL-60 and PLB-985 cells. Biosynthesis using a 60-minute pulse label in either HL-60 (left) or PLB-985 (right) cells, immunoprecipitation with either anticalreticulin (CRT; upper panel) or antimyeloperoxidase (MPO; lower panel), SDS-PAGE, and fluorography were performed as in Fig 2 except that the cells were cultured in tunicamycin at the concentrations indicated. The apparent increased intensity of the calreticulin signal in PLB-985 cells treated with tunicamycin was not a consistent finding in other experiments.
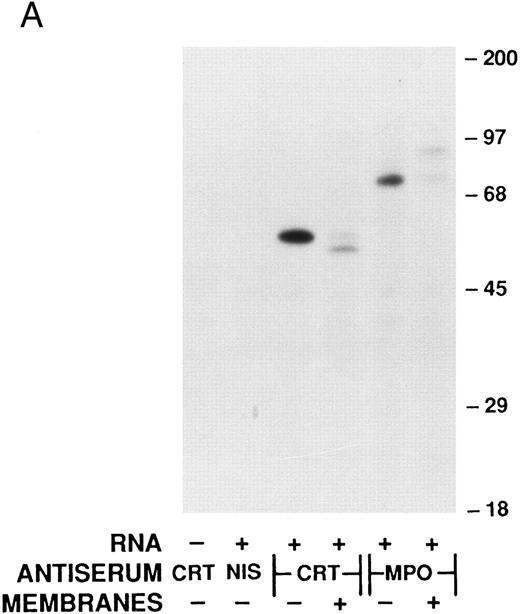
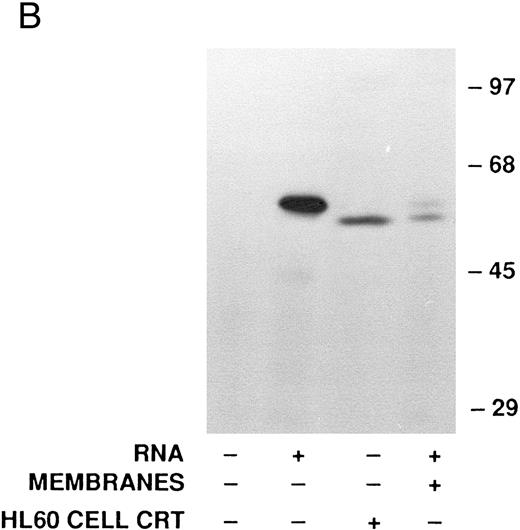
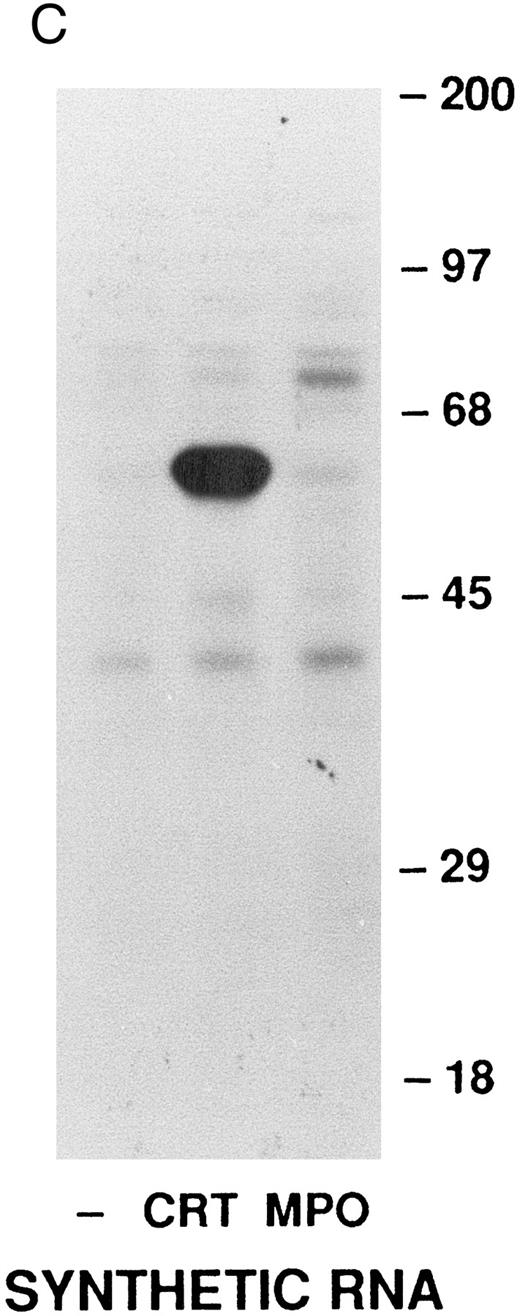
Cell-free synthesis of calreticulin.The in vitro rabbit reticulocyte lysate system was used to characterize the primary translation product of the calreticulin transcript. Since we have previously used this system to study myeloperoxidase synthesis,37 calreticulin and myeloperoxidase were analyzed in parallel. In the presence of HL-60 RNA the products of cell-free synthesis immunoprecipitated by antiserum to calreticulin or myeloperoxidase were detected as 62- or 80-kD proteins, respectively (Fig 3A). Nonimmune rabbit serum did not precipitate any detectable labeled proteins. When no RNA was present the anticalreticulin immunoprecipitates contained no labeled proteins. Dog pancreatic microsomes were added to the cell-free translation system to study processing of the primary translation products (Fig 3A). As previously reported37 myeloperoxidase was converted from an 80-kD to an 89-kD form (signal peptide cleavage and glycosylation). Under the same conditions the 62-kD calreticulin precursor was converted to a 60-kD form, presumably by signal peptide cleavage. To provide further evidence for this interpretation the products of cell-free translation with and without microsomal membranes were compared with mature calreticulin synthesized by HL-60 cells (Fig 3B). The HL-60 cell product comigrated with the 60-kD form processed by microsomal membranes in the cell-free system. When synthetic transcripts for calreticulin and myeloperoxidase were translated in the cell-free system (Fig 3C) the major products were 62- and 80-kD proteins, respectively, as when HL-60 RNA was used. It should be noted that Fig 3C shows, not immunoprecipitates, but total products of translation. Collectively, the data using the cell-free translation system indicate that the primary translation product of calreticulin mRNA is a 62-kD species, which in the presence of microsomal membranes is converted to the mature 60-kD protein by signal peptide cleavage.
Analysis of calreticulin biosynthesis in vitro. Cell-free protein synthesis was performed in the rabbit reticulocyte lysate system in the presence of 35S-methionine. Either immunoprecipitates (A and B) or total translation products (C) were analyzed by 7.5% SDS-PAGE and fluorography. The locations of molecular weight markers are indicated. (A) Reactions contained either no RNA (−) or poly-A–enriched RNA from HL-60 cells (+) and either no membranes (−) or dog pancreatic microsomes (+). The products of protein synthesis were immunoprecipitated with either nonimmune serum (NIS; from the rabbit subsequently immunized with calreticulin), anticalreticulin (CRT), or antimyeloperoxidase (MPO). (B) Reactions were as in (A) with (+) or without (−) HL-60 cell RNA and dog pancreatic microsomes. HL-60 cell calreticulin biosynthetically labeled as in Fig 2 was run (third lane from left) for comparison with the cell-free products. For all samples products of translation were immunoprecipitated with anticalreticulin.
(C) Reactions contained no RNA (−) or synthetic mRNA generated from plasmid templates of the cDNA for either calreticulin (CRT) or myeloperoxidase (MPO).
Analysis of calreticulin biosynthesis in vitro. Cell-free protein synthesis was performed in the rabbit reticulocyte lysate system in the presence of 35S-methionine. Either immunoprecipitates (A and B) or total translation products (C) were analyzed by 7.5% SDS-PAGE and fluorography. The locations of molecular weight markers are indicated. (A) Reactions contained either no RNA (−) or poly-A–enriched RNA from HL-60 cells (+) and either no membranes (−) or dog pancreatic microsomes (+). The products of protein synthesis were immunoprecipitated with either nonimmune serum (NIS; from the rabbit subsequently immunized with calreticulin), anticalreticulin (CRT), or antimyeloperoxidase (MPO). (B) Reactions were as in (A) with (+) or without (−) HL-60 cell RNA and dog pancreatic microsomes. HL-60 cell calreticulin biosynthetically labeled as in Fig 2 was run (third lane from left) for comparison with the cell-free products. For all samples products of translation were immunoprecipitated with anticalreticulin.
(C) Reactions contained no RNA (−) or synthetic mRNA generated from plasmid templates of the cDNA for either calreticulin (CRT) or myeloperoxidase (MPO).
Analysis of glycosylation of calreticulin.The cDNA for human calreticulin predicts a single potential site for N-linked glycosylation at asparagine-327. Whether calreticulin is indeed N-glycosylated appears to vary among species and tissues,1 30 so we sought to determine if human myeloid cell calreticulin is a glycoprotein. The growth of HL-60 or PLB-985 cells in tunicamycin at concentrations up to 10 μg/mL had no detectable effect on the electrophoretic mobility of the protein that was immunoprecipitated by anti-calreticulin; ie, only the usual 60-kD product was detected (Fig 4). That tunicamycin was, in fact, blocking glycosylation under these conditions was shown in parallel analyses of myeloperoxidase. In the presence of 3 to 10 μg/mL of tunicamycin the 89-kD myeloperoxidase precursor was replaced by the 80-kD nonglycosylated species (Fig 4).
When HL-60 cell calreticulin immunoprecipitates were treated with either endoglycosidase H or N-glycosidase F, there was no detectable effect on the electrophoretic mobility of the 60-kD mature calreticulin (Fig 5). In contrast, the same treatments of the myeloperoxidase immunoprecipitates converted the 89-kD precursor to an 80-kD species indicating that deglycosylation had occurred under the conditions used, as previously described.40
Effect of glycosidases on the electrophoretic mobility of calreticulin and myeloperoxidase synthesized in HL-60 cells. Biosynthesis using a 60-minute pulse label in HL-60 cells and immunoprecipitation with either anticalreticulin (CRT; lower panel) or antimyeloperoxidase (MPO; upper panel) were performed as in Figs 2 and 4. The immunoprecipitates were then incubated with Endo F buffer either alone (−/−) or plus the indicated amounts of endoglycosidase H (ENDO H) or N-glycosidase F (N-GLYC F ). The treated immunoprecipitates were then analyzed by 7.5% SDS-PAGE and fluorography.
Effect of glycosidases on the electrophoretic mobility of calreticulin and myeloperoxidase synthesized in HL-60 cells. Biosynthesis using a 60-minute pulse label in HL-60 cells and immunoprecipitation with either anticalreticulin (CRT; lower panel) or antimyeloperoxidase (MPO; upper panel) were performed as in Figs 2 and 4. The immunoprecipitates were then incubated with Endo F buffer either alone (−/−) or plus the indicated amounts of endoglycosidase H (ENDO H) or N-glycosidase F (N-GLYC F ). The treated immunoprecipitates were then analyzed by 7.5% SDS-PAGE and fluorography.
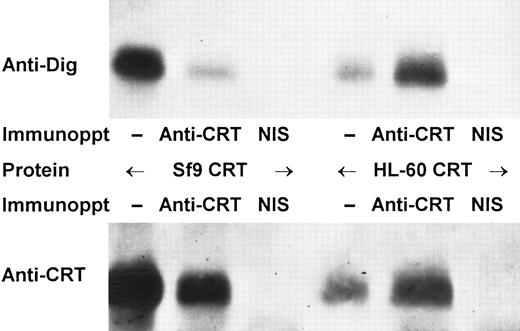
The foregoing results suggested that human myeloid cell calreticulin lacks N-linked carbohydrate side chains. However, the alternative explanation that glycosylated and nonglycosylated calreticulin exhibit nearly identical electrophoretic mobilities could not be excluded. Thus, we looked for direct evidence of glycosylation through oxidation and covalent derivatization of carbohydrate moieties with digoxigenin. Fetuin (control glycoprotein) and calreticulin purified from either HL-60 cells or Sf9 cells expressing the recombinant protein were oxidized, derivatized with digoxigenin, and then analyzed by SDS-PAGE and immunoblotting with antidigoxigenin antibody (Fig 6). In each case a labeled protein of the appropriate size (∼80 kD for fetuin; 60 kD for calreticulin) was detected. In contrast, no proteins were detected with antidigoxigenin if the proteins were treated with N-glycosidase F before oxidation and labeling (Fig 6). Parallel immunoblots with anticalreticulin antiserum showed 60-kD bands that comigrated with the calreticulin species detected by antidigoxigenin, but the anticalreticulin bands were unaffected by prior treatment of the proteins with N-glycosidase F (Fig 6). The identity of the digoxigenin-labeled 60-kD protein from either HL-60 or Sf9 cells was confirmed by immunoprecipitation with anticalreticulin antiserum (Fig 7). Precipitates obtained with immune, but not with non-immune serum contained a 60-kD protein recognized in immunoblots by both antidigoxigenin and anticalreticulin antibodies (Fig 7).
Immunoblot analysis of digoxigenin-labeled calreticulin. Fetuin (control glycoprotein) and purified calreticulin (CRT) from either HL-60 or Sf9 cells were incubated in Endo F buffer with (+) or without (−) N-glycosidase F (N-Glyc F ), then oxidized with periodate and derivatized with digoxigenin. The proteins were then analyzed by 7.5% SDS-PAGE, transfer to nitrocellulose and immunoblotting with either antidigoxigenin (upper panel) or anticalreticulin (lower panel). The blots are arranged as indicated with paired lanes (minus or plus N-glycosidase F ) for each of the three proteins.
Immunoblot analysis of digoxigenin-labeled calreticulin. Fetuin (control glycoprotein) and purified calreticulin (CRT) from either HL-60 or Sf9 cells were incubated in Endo F buffer with (+) or without (−) N-glycosidase F (N-Glyc F ), then oxidized with periodate and derivatized with digoxigenin. The proteins were then analyzed by 7.5% SDS-PAGE, transfer to nitrocellulose and immunoblotting with either antidigoxigenin (upper panel) or anticalreticulin (lower panel). The blots are arranged as indicated with paired lanes (minus or plus N-glycosidase F ) for each of the three proteins.
Immunoprecipitation of digoxigenin-labeled calreticulin. Purified calreticulin (CRT) from either Sf9 or HL-60 cells was derivatized with digoxigenin as in Fig 6 and then analyzed either without immunoprecipitation (−) or after immunoprecipitation with anticalreticulin or non-immune serum (NIS). Analysis was performed as in Fig 6 by immunoblotting with either antidigoxigenin (upper panel) or anticalreticulin (lower panel).
Immunoprecipitation of digoxigenin-labeled calreticulin. Purified calreticulin (CRT) from either Sf9 or HL-60 cells was derivatized with digoxigenin as in Fig 6 and then analyzed either without immunoprecipitation (−) or after immunoprecipitation with anticalreticulin or non-immune serum (NIS). Analysis was performed as in Fig 6 by immunoblotting with either antidigoxigenin (upper panel) or anticalreticulin (lower panel).
Finally, we determined the amount of carbohydrate present in highly purified (see Fig 1D) Sf9 cell recombinant human calreticulin using the phenol-sulfuric acid method (Table 1). Control glycoproteins fetuin and transferrin had readily measured levels of carbohydrate, whereas a nonglycosylated protein bovine serum albumin contained no detectable carbohydrate by this method. The mean value obtained for calreticulin was 23 μg of carbohydrate/mg of protein, similar to that reported for bovine liver calreticulin of 16 μg/mg protein.42 We were unable to obtain sufficient quantities of highly purified HL-60 cell calreticulin to permit accurate carbohydrate determinations.
Direct Measurement of Carbohydrate Content by the Phenol-Sulfuric Acid Method
| Protein . | Carbohydrate (μg/mg protein) . |
|---|---|
| Fetuin | 163 ± 2* |
| Transferrin | 36 ± 4 |
| Bovine serum albumin | Not detectable |
| Recombinant human calreticulin | 23 ± 6 |
| Protein . | Carbohydrate (μg/mg protein) . |
|---|---|
| Fetuin | 163 ± 2* |
| Transferrin | 36 ± 4 |
| Bovine serum albumin | Not detectable |
| Recombinant human calreticulin | 23 ± 6 |
Mean ± SEM; n = 3.
DISCUSSION
These studies address the mechanisms of synthesis and processing of human myeloid calreticulin. In two different human leukemia cell lines, HL-60 and PLB-985, a 60-kD species representing mature calreticulin was the only form detected. Neither short periods of pulse labeling nor long chases revealed any other electrophoretically detectable products. These observations indicated that either (1) no posttranslational processing occurred or (2) precursor and mature forms of calreticulin have very similar mobility under conditions of SDS-PAGE. Further experiments showed that for proteolytic processing the first alternative applied in that processing was cotranslational and that for glycosylation the second alternative was true.
In the cell-free reticulocyte lysate system either HL-60 mRNA or synthetic calreticulin transcripts primed synthesis of a 62-kD calreticulin primary translation product. In the presence of microsomal membranes the 62-kD protein was processed to a 60-kD species, presumably by cleavage of the signal peptide. The latter product comigrated in SDS-PAGE with the protein that was immunoprecipitated from HL-60 cells. These data indicate that during in vivo synthesis nascent calreticulin undergoes cotranslational proteolysis of the signal peptide. Previous work indicated that in vitro synthesis of calreticulin using rabbit mRNA as template produced a ∼57-kD product, compared with the 55- kD mature protein from muscle,6 whereas synthetic transcripts prepared from a human cDNA produced a 60-kD product that was processed to a smaller form in the presence of membranes.18
Among the standard approaches to analysis of glycosylation of proteins are inhibition of glycosylation by tunicamycin and deglycosylation by endoglycosidases. Our experimental design used myeloperoxidase, a known myeloid cell glycoprotein, to assure that tunicamycin was effectively blocking glycosylation and that endoglycosidase H and N-glycosidase F, enzymes expected to cleave either high-mannose or all N-linked carbohydrates, respectively, were functioning as predicted. Because there was no shift in the apparent size of calreticulin after treatment, neither the tunicamycin nor endoglycosidase studies provided any evidence for glycosylation of calreticulin, leading to the tentative conclusion that asparagine-327, despite its presence in a consensus sequence for glycosylation, was not modified in human myeloid cells. However, the electrophoretic mobility of calreticulin is known to be anomalous,1 the 46.5-kD mature protein showing SDS-PAGE migration compatible with a molecular size of 60 kD. This diminished mobility of calreticulin is likely a consequence of its unusual zonal structure with globular N-terminal and highly acidic C-terminal domains separated by a series of proline-rich repeats.1
Our experiments using covalent derivatization of glycoproteins with digoxigenin showed unequivocally that human calreticulin is, in fact, glycosylated when synthesized either in the myeloid HL-60 cell line or as a recombinant protein in Sf9 insect cells. Of course, the possibility that nonmyeloid human cells synthesize calreticulin that is not glycosylated cannot be excluded. That the baculovirus-insect cell expression system maintains the fidelity of glycosylation observed in HL-60 cells was further shown by direct chemical measurement of the carbohydrate content of purified recombinant calreticulin. Thus, Sf9 cells provide an excellent source of structurally authentic recombinant human calreticulin.
Previous studies of calreticulin glycosylation have applied a wide range of methods to various species and tissues. Thus, it has been reported that calreticulin is not glycosylated in rat43 or rabbit44 muscle, rabbit or chicken liver,42 or lymphoid cells from mouse45-47 or human.17 In contrast, evidence of calreticulin glycosylation was found in rat41,48 and bovine42,46 liver, bovine brain,30 and hamster ovary.49 Although the bovine calreticulin sequence indicates two potential sites for N-glycosylation, only asparagine-162 is glycosylated in brain tissue, whereas the 327 site homologous to the single potential site in human calreticulin is not modified.30 The only previous study of human calreticulin17 reported that there was no glycosylation, based on digestion with endoglycosidase H and SDS-PAGE, an approach which in our hands also failed to show glycosylation.
Interestingly, in two studies exploring the structure of calreticulin N-linked carbohydrates, different results were observed in different tissues. In bovine brain a high mannose structure of the form GlcNAc2Man4-9 was found30 as generally expected for endoplasmic reticulum resident proteins. In contrast, rat liver calreticulin contained a complex hybrid structure of the form GlcNAc7Man3Gal248 suggesting passage through the trans-Golgi before retrieval to endoplasmic reticulum. Although we did not assess carbohydrate structure, our quantitative data for recombinant calreticulin (23 μg/mg protein) are more in keeping with a high mannose structure (predicted carbohydrate content of 26 μg/mg for the most abundant species reported, GlcNAc2Man530) than the complex hybrid structure (predicted carbohydrate content of 48 μg/mg). Moreover, the lack of a detectable electrophoretic shift with deglycosylation is easier to explain for the high mannose structure (∼1.2 kD) than for the larger complex carbohydrate (∼2.2 kD). Thus, we suggest that human myeloid cell calreticulin contains a single N-linked high mannose carbohydrate moiety.
Although the biological role of calreticulin glycosylation is uncertain, the apparent variations among different cells and species lead us to speculate that tissue-specific functional properties of calreticulin could be regulated by the presence or absence of N-linked carbohydrate. The most likely mechanism for this would be through effects on the interaction of calreticulin with other proteins in the endoplasmic reticulum. Thus, the chaperone function of calreticulin25,26 or its potential calcium-signaling role11 may be controlled in part by the presence and specific configuration of carbohydrate moieties.
The studies presented here provide a basic characterization of the biosynthesis of calreticulin by human myeloid cells. The primary translation product is modified by both cotranslational signal peptide cleavage and posttranslational N-linked glycosylation. The experimental approaches as well as the cellular, molecular and immunologic tools developed will be useful for the further definition of the structure, function and regulation of calreticulin. Finally, our results point out the limitations of methods for analysis of glycoproteins that rely exclusively on the detection of differences in electrophoretic mobility. Especially for proteins that display anomalous migration in SDS-PAGE, the use of alternative methods, including oxidative derivatization with digoxigenin and direct chemical measurement of carbohydrate as we have employed, as well as lectin chromatography, biosynthetic carbohydrate labeling, gas chromatography and mass spectrometry, may be required.
ACKNOWLEDGMENT
The cloning of the human myeloid calreticulin cDNA was performed while Dr Clark was on sabbatical at the University of Geneva and the Fondation pour Recherches Médicales, Geneva, Switzerland. The many valuable contributions of Drs Daniel P. Lew, Werner Schlegel, and Karl-Heinz Krause of the University of Geneva are gratefully acknowledged. We thank Dr Marek Michalak of the University of Alberta, Canada for his generous gift of the rabbit calreticulin cDNA and for helpful suggestions on the manuscript.
Supported by Grants from the Medical Research Service of the Department of Veterans Affairs (Washington, DC) and the Fondation Zyma (Nyon, Switzerland).
Address reprint requests to Robert A. Clark, MD, Department of Medicine, University of Texas Health Science Center, 7703 Floyd Curl Dr, San Antonio, TX 78284-7870.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal