Abstract
A 75-year-old woman taking the nonsteroidal anti-inflammatory drug diclofenac (DCF ) presented with acute Coombs-positive hemolytic anemia and subsequently developed renal failure. A drug-dependent antibody specific for red blood cells (RBCs) could not be demonstrated by in vitro testing with DCF. However, her serum was found to contain an IgM antibody that reacted strongly with RBCs in the presence of urine from any of four subjects who had ingested DCF. The active substance in urine was isolated, subjected to high-performance liquid chromatographic (HPLC) analysis, and found to be a glucuronide conjugate of a known DCF metabolite, 4′-hydroxydiclofenac (4′-OH DCF ). Negative results were obtained with four other DCF metabolites. Two 4′-OH DCF glucuronides were synthesized in vitro using a liver microsomal system. One promoted agglutination of normal RBCs by the patient's serum and was identified as the glucuronide ester of 4′-OH DCF by proton nuclear magnetic resonance (NMR) analysis. Studies with a panel of RBCs showed that the patient's antibody reacted preferentially with the e antigen of the Rh system. Acute immune hemolytic anemia in this patient appears to have been caused by sensitization to DCF modified by 4′ hydroxylation and glucuronidation. This is the first reported example of immune cytopenia caused by sensitivity to a glucuronide conjugate of a drug metabolite. Since glucuronidation is a common pathway of drug metabolism, studies of the frequency with which glucuronide derivatives of primary medications cause immune cytopenia seem warranted.
MANY DIFFERENT DRUGS are capable of inducing immune hemolytic anemia.1-3 The underlying pathogenesis of this side effect of drug treatment is not fully understood, but at least three different mechanisms appear to be involved.3,4 Some drugs, such as methyldopa and procainamide, induce autoantibodies that bind to red blood cells (RBCs) in the absence of drug and may perpetuate hemolysis for a period after the drug is discontinued.2,3 Others such as penicillin and cephalothin bind covalently to cell membrane proteins and act as haptens to induce antibodies specific for the drug-protein complex.3-5 Hemolysis caused by these two mechanisms is usually mild and may even be subclinical.
A third type of drug-induced immune hemolysis, usually more severe and sometimes fatal, is characterized by antibodies that bind to normal erythrocytes only when the sensitizing drug is present in the fluid phase.3,6 At one time, it was thought that this type of antibody reacts with its cellular target(s) in the form of “immune complexes” consisting of antibody bound to the drug or to drug-protein complexes.2,7 However, the putative immune complexes have never been convincingly demonstrated, and it is now generally thought that the provocative drugs interact noncovalently with certain membrane proteins to form combinatorial epitopes or induce conformational changes for which the antibodies are specific.3,8 This type of antibody can sometimes be identified by showing that it binds to RBCs in the presence of the sensitizing drug,1,3 but negative results are often obtained.1,7 In several studies, positive reactions were obtained using serum9,10 or urine11 from a person taking the implicated medication as the source of “drug.” These observations suggested that drug metabolites may be important in pathogenesis and provided a potential explanation for the failure in some cases to detect antibodies using the primary medication.
Diclofenac (DCF ), a widely used nonsteroidal anti-inflammatory drug, is one of the medications implicated as a cause of immune hemolytic anemia.11-13 In one instance, it was found that the patient's serum contained Igs that reacted with normal RBCs in the presence of urine from an individual taking DCF, but the active substance, presumably a DCF metabolite, was not identified.11 We recently encountered a patient who developed severe immune hemolytic anemia while taking DCF. No drug-dependent antibodies could be demonstrated using DCF, but the patient's serum contained antibodies that reacted with normal RBCs in the presence of urine containing DCF metabolites. Because little is known about the structural properties of metabolites that may cause this type of drug sensitivity, we performed further studies to characterize the substance responsible.
SUBJECT AND METHODS
Case Report
A 75-year-old woman presented at the hospital complaining of dark urine, abdominal pain, and jaundice of several days' duration. She had been taking DCF intermittently for about 2 years for symptoms of osteoarthritis. She took two 75-mg tablets approximately 2 weeks before admission and one tablet 24 hours before the onset of symptoms. Her other medications were thyroxin and diltiazem. Laboratory studies upon admission demonstrated a hemoglobin level of 9.0 g/dL, normal white blood cell count and differential, and normal platelet count. Nucleated RBCs and spherocytes were identified in the peripheral blood smear. Hemoglobinuria was present. The lactate dehydrogenase level was 12,067 IU/L, bilirubin 4.3/0.7 (total/direct) mg/dL, and creatinine 1.9 g/dL. A direct antiglobulin test was strongly positive using anti-IgG (H + L chain) and anti-C3 reagents. The reticulocyte level was 3.5% 2 days after admission and increased to 7.9% the next day. The patient became anuric on the second hospital day and serum creatinine increased to 4.4 mg/dL. Renal failure was controlled by seven hemodialysis treatments over the next 2 weeks. Urine outflow gradually improved, renal function was regained, and the patient was discharged after 25 days with normal hematologic values. She was cautioned to avoid re-exposure to DCF and has been well since discharge.
Reagents
DCF, bovine uridine 5′-diphosphoglucuronyltransferase, uridine diphosphoglucuronic acid (UDPGA), bovine β-glucuronidase, bovine serum albumin (BSA), and dimethylsulfoxide-d6 were purchased from Sigma Chemical Co (St Louis, MO). Fluorescein isothiocyanate conjugated, affinity-purified F(ab′ )2 goat anti–human IgG Fc and phycoerythrin-conjugated F(ab′ )2 donkey anti–human IgM Fc μ were purchased from Jackson Immunoresearch Laboratory (West Grove, PA). Five known metabolites of DCF were kindly provided by Ciba-Geigy (Basel, Switzerland). High-performance liquid chromatography (HPLC)-grade acetonitrile and trifluoroacetic acid (TFA) were purchased from Aldrich Chemical Co (Milwaukee, WI).
Serologic Studies
Agglutination.Twenty microliters of patient serum was added to 20 μL of a 4% suspension of washed group O RBCs in phosphate-buffered (0.01 mol/L) isotonic saline (PBS), pH 7.4, and 20 μL PBS containing 1% BSA (PBS-BSA) with or without drug at a concentration of 1.0 mg/mL. In some studies, 20 μL urine neutralized to pH 7.0 was added as the source of “drug.” The mixture was incubated at room temperature for 30 minutes, RBCs were sedimented by rapid centrifugation and gently resuspended by shaking, and agglutination was graded (0 to 4+) by inspection. An agglutination score was also assigned.14 The mixture was then washed four times with PBS-BSA containing drug at the same concentration as the primary mixture or urine diluted 1:10 in PBS-BSA. After the fourth wash, an antiglobulin test was performed using a reagent specific for human IgG heavy and light chains (Immucor Inc, Norcross, GA).
Flow cytometry.Ten microliters of patient serum was incubated with 10 μL of a 2% suspension of washed group O RBCs and 10 μL of a solution containing drug in PBS-BSA or urine adjusted to pH 7.0. After incubation for 1 hour at room temperature, the RBCs were washed twice with PBS-BSA containing drug at the same concentration as in the primary mixture. The washed RBCs were suspended in 15 μL PBS-BSA, also containing drug, and 15 μL antibody, either anti–IgG Fc or anti–IgM μ, labeled with a fluorescent probe (1:80 dilution) was then added. An aliquot of the reaction mixture was diluted in 0.5 mL saline immediately before analysis by flow cytometry (FACScan; Becton Dickinson, Franklin Lakes, NJ). Fluorescence intensity was recorded in the logarithmic mode.
Preparation of Rat Liver Microsomes
Fractions containing microsomes were isolated from the livers of Sprague-Dawley rats as described by Radominski-Pyrek et al.15 All procedures were performed between 0° and 4°C. Frozen rat livers were thawed at 4°C in homogenizing buffer (0.25 mol/L sucrose, 1 mmol/L EDTA, 5 mmol/L Tris, pH 7.6). Each liver was blotted dry and weighed in 10 mL cold homogenizing buffer. The volume of buffer was adjusted to four times the weight of the livers, and they were minced and homogenized separately. The pooled homogenates were centrifuged at 5,000g for 15 minutes. The supernatants were collected and centrifuged at 17,000g for 30 minutes. The supernatants were again collected, and their content of microsomes was pelleted at 100,000g for 1 hour. The pelleted microsomes were washed once in cold homogenizing buffer and resuspended in 1 mL storage solution (0.25 mol/L sucrose and 5 mmol/L Tris, pH 7.5) for each liver. Aliquots were then frozen at −80°C until used. On average, the microsomal preparations contained 40 mg protein/mL determined by the bicinchoninic acid method.16
Microsomal Glucuronidation of DCF and 4′ Hydroxydiclofenac
Rat liver microsomes (0.1 mL containing 4 mg protein) were added to 0.25 mL Tris buffer (200 mmol/L Tris and 20 mmol/L MgCl2 , pH 7.4) and 0.1 mL of a solution containing DCF or 4′ hydroxydiclofenac (4′-OH DCF ) (1 mg/mL in 5% BSA with 25 mmol/L D-saccharic 1,4 lactone). After a 15-minute preincubation at 37°C, 50 μL 30-mmol/L solution of UDPGA in water was added. Aliquots (50 μL) were removed immediately and at various times thereafter and were added to 100 μL acetonitrile. After centrifugation to remove protein, the supernatants were stored at −20°C pending HPLC analysis.
Bovine glucuronyl transferase (lyophilized microsomal preparation from bovine liver) (10 mg in 0.25 mL Tris buffer, pH 7.4) was added to a 0.1-mL solution of DCF or 4′-OH DCF (1 mg/mL in 5% BSA with 25 mmol/L D-saccharic 1,4 lactone). After incubation for 15 minutes at 37°C, 50 μL 30-mmol/L solution of UDPGA in water was added. Immediately and at various times thereafter, 50-μL aliquots were removed, added to 0.1 mL acetonitrile, centrifuged to remove protein, and frozen at −20°C pending HPLC analysis.
HPLC Analysis
A Beckman system gold model 126 pump and 167 variable-wavelength dual-channel detector set at 254 and 275 nm were used. A 5-μm C18 guard column from Vydac (Hesperia, CA) was used as the precolumn. The column was a 5-μm C18 column (4.6 mm × 250 mm), also from Vydac. Solvent A consisted of 0.1% TFA in deionized distilled water. Solvent B consisted of 0.08% TFA in HPLC-grade acetonitrile. The flow rate was 1 mL/min. The program for a 30-minute procedure was as follows: 2 minutes at isocratic conditions using 95% solvent A and 5% solvent B; a linear gradient over the next 20 minutes to a mixture of 15% solvent A and 85% solvent B; and an isocratic flush at 5% solvent A and 95% solvent B for 3 minutes. The program ended with a 5-minute re-equilibration at 95% solvent A and 5% solvent B. Peaks of interest were collected, frozen at −80°C, and lyophilized.
Nuclear Magnetic Resonance Analysis
Nuclear magnetic resonance (NMR) spectra were analyzed on a Bruker (Billerica, MA) AC 300, 300-MHz device using samples dissolved in dimethylsulfoxide-d6.
Isolation of DCF Metabolites From Urine
Urinary metabolites were isolated by passing 15 mL of a 24-hour urine collection from an individual taking DCF (75 mg three times daily) through a 600-mg Maxi-clean solid-phase (C18) extraction cartridge (Alltech, Deerfield, IL). The cartridge was first prepared by washing with acetonitrile and then with water. After the urine was passed through the cartridge, it was washed with 5% acetonitrile in water. Urinary metabolites were then extracted with 4 mL acetonitrile. The effluent was frozen at −80°C and lyophilized. The residue was reconstituted in PBS-BSA for serologic testing and HPLC analysis.
RESULTS
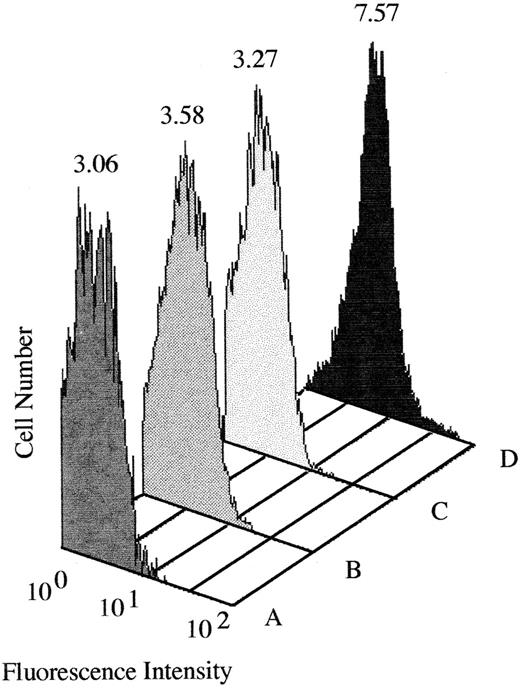
An IgM antibody that required a presumptive DCF metabolite for binding to RBCs was identified in the patient's serum.The patient's serum obtained shortly after admission to the hospital reacted weakly with normal RBCs in the indirect antiglobulin test. An acid eluate (Elu-kit; Gamma Biologicals, Houston, TX) prepared from the patient's RBCs showed negative reactions. No reaction of the serum or eluate with RBCs in the presence of DCF at a concentration as high as 1 mg/mL could be demonstrated. Negative reactions were also obtained with thyroxine and diltiazem. However, strong positive agglutination reactions were obtained using urine from four different individuals who were taking DCF as the source of “drug” in the reaction mixture. Negative reactions were obtained with urine from three persons not taking DCF (Table 1). The strength of these reactions was not increased by antiglobulin testing, and they were abolished by pretreatment of serum with 2-mercaptoethanol (data not shown). By flow cytometry, it was found that the presumptive drug-dependent antibody was of the IgM class (Fig 1). RBCs pretreated with DCF or with urine containing DCF metabolites and then washed failed to react with the patient's serum. These findings suggested that the serum contained an IgM antibody that required a metabolite of DCF present in urine for its reaction with RBCs.
Urine From Subjects Taking DCF Promotes Agglutination of RBCs by Serum From the Patient
| Subject No. . | DCF Treatment . | Agglutination Titer . | Agglutination Score . |
|---|---|---|---|
| 1 | Yes | 8 | 33 |
| 2 | Yes | 4 | 18 |
| 3 | Yes | 16 | 40 |
| 4 | Yes | 8 | 29 |
| 5 | No | 0 | 0 |
| 6 | No | 0 | 0 |
| 7 | No | 0 | 0 |
| Subject No. . | DCF Treatment . | Agglutination Titer . | Agglutination Score . |
|---|---|---|---|
| 1 | Yes | 8 | 33 |
| 2 | Yes | 4 | 18 |
| 3 | Yes | 16 | 40 |
| 4 | Yes | 8 | 29 |
| 5 | No | 0 | 0 |
| 6 | No | 0 | 0 |
| 7 | No | 0 | 0 |
Binding of IgM antibody to normal RBCs incubated with (A) normal plasma and PBS, (B) normal plasma and urine from a subject taking DCF, (C) patient plasma and PBS, and (D) patient plasma and urine from a subject taking DCF. Mean fluorescence intensity is indicated above each histogram. Significant amounts of IgM became bound to target RBCs only in the presence of patient plasma and urine from a subject taking DCF (D). No antibody binding was detected with an IgG-specific probe (data not shown).
Binding of IgM antibody to normal RBCs incubated with (A) normal plasma and PBS, (B) normal plasma and urine from a subject taking DCF, (C) patient plasma and PBS, and (D) patient plasma and urine from a subject taking DCF. Mean fluorescence intensity is indicated above each histogram. Significant amounts of IgM became bound to target RBCs only in the presence of patient plasma and urine from a subject taking DCF (D). No antibody binding was detected with an IgG-specific probe (data not shown).
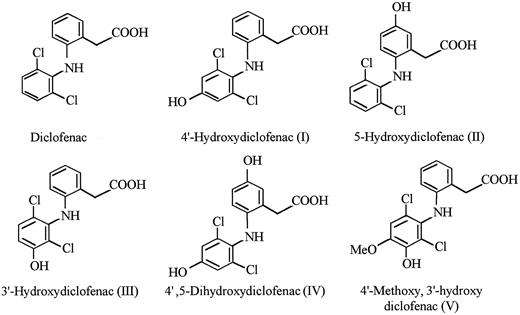
Known DCF metabolites failed to promote significant binding of the antibody to RBCs.The structure of DCF and five known DCF metabolites supplied by the manufacturer are shown in Fig 2. Each of these compounds, dissolved in PBS-BSA at a concentration of 1.0 mg/mL, was tested for the ability to promote binding of the patient's antibody to RBCs using direct agglutination. All reactions were negative, except for very weak (+/−) reactions obtained with metabolite I (4′-OH DCF ).
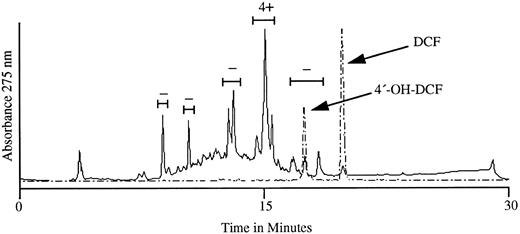
The active substance in urine was recovered in a single HPLC peak.We next subjected aromatic compounds isolated from the urine of a person taking DCF to HPLC analysis. HPLC profiles obtained with the urinary extract and with DCF and 4′-OH DCF standards are shown in Fig 3. When the substances in the four major peaks and the peak with an elution profile identical to that of 4′-OH DCF were tested with patient serum against normal RBCs, the fraction with a retention time of 15.1 minutes showed a strongly positive (4+) reaction in the agglutination assay, and negative reactions were obtained with the other four peaks. The active peak did not correlate with 4′-OH DCF, which eluted at 17.6 minutes.
HPLC profile of metabolites recovered from the urine of a normal subject taking DCF (———) and of DCF and 4′-OH DCF standards assayed separately (– – – –). The five bracketed fractions were tested for the ability to promote agglutination of normal RBCs in the presence of serum from our patient. As shown above each bracket, only the fraction eluting at ∼15.1 minutes caused RBC agglutination (4+). Negative results were obtained when material in each peak was tested with normal plasma (not shown).
HPLC profile of metabolites recovered from the urine of a normal subject taking DCF (———) and of DCF and 4′-OH DCF standards assayed separately (– – – –). The five bracketed fractions were tested for the ability to promote agglutination of normal RBCs in the presence of serum from our patient. As shown above each bracket, only the fraction eluting at ∼15.1 minutes caused RBC agglutination (4+). Negative results were obtained when material in each peak was tested with normal plasma (not shown).
The active substance was identified as the glucuronide ester of 4′-OH DCF.The metabolism of DCF in animals and man has been extensively studied. In addition to metabolites formed by hydroxylation and hydroxymethylation of the aromatic rings (Fig 2), both oxidized and unmodified DCF can be conjugated to glucuronic acid or tauric acid at the carboxyl residue.17 In man, conjugates of the hydroxylated metabolites predominate in the urine and consist mainly of glucuronides.18 We found that treatment of the active peak isolated from urine with bovine β-glucuronidase caused a complete loss of agglutinating activity and led to the formation of a substance with an elution profile identical to that of 4′-OH DCF on HPLC analysis (data not shown). This suggested that the active substance was a glucuronide conjugate of 4′-OH DCF.
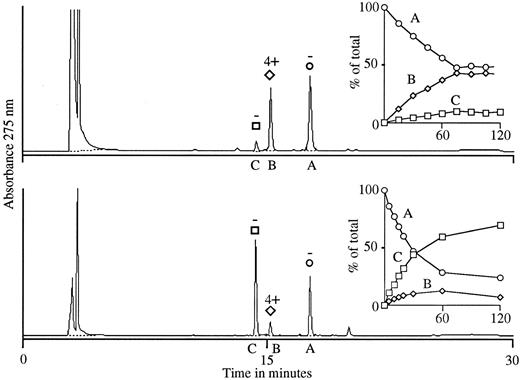
We then prepared glucuronide conjugates of 4′-OH DCF and DCF using rat and bovine liver microsomes and isolated the reaction products by HPLC. With 4′-OH DCF, two products were formed in each system (B and C; Fig 4), only one of which (product B) promoted the binding of antibody to normal target RBCs. The active substance (product B) had a retention time of 15.1 minutes on HPLC (Fig 4), identical to that of the active substance in urine (Fig 3). By NMR analysis, it is possible to characterize the chemical shift of the hydrogen atom of the C1 carbon of glucuronide and determine whether C1 is conjugated through an ether or ester linkage.15,19 With the inactive glucuronide (product C; Fig 4), the shift of the C1 hydrogen occurred at 4.9 ppm, indicating an ether (4′-OH) linkage, whereas with the active one (product B), it occurred at 5.4 ppm (data not shown), indicating an ester linkage through the carboxyl group of 4′-OH DCF.15 19 These findings indicate that the active product (B) obtained by microsomal synthesis is the glucuronide ester of 4′-OH DCF, and that the inactive substance (C) is the 4′ glucuronide ether.
Glucuronidation of 4′-OH DCF using UDPGA with rat liver microsomes (top) and bovine glucuronyl transferases (bottom). In each system, two products were formed (B and C) from starting material (A) over a 90-minute interval (insets). Product B was synthesized preferentially by rat liver microsomes, and product C by bovine glucuronyl transferases. The HPLC elution profile of substances B and C (90-minute samples) is shown in the larger graphs, and the ability of each substance to promote agglutination of normal RBCs by antibody from our patient is indicated above the respective peaks. Only material isolated from peak B was active in the agglutination assay.
Glucuronidation of 4′-OH DCF using UDPGA with rat liver microsomes (top) and bovine glucuronyl transferases (bottom). In each system, two products were formed (B and C) from starting material (A) over a 90-minute interval (insets). Product B was synthesized preferentially by rat liver microsomes, and product C by bovine glucuronyl transferases. The HPLC elution profile of substances B and C (90-minute samples) is shown in the larger graphs, and the ability of each substance to promote agglutination of normal RBCs by antibody from our patient is indicated above the respective peaks. Only material isolated from peak B was active in the agglutination assay.
The lowest concentration of 4′-OH DCF glucuronide that produced 4+ agglutination reactions with RBCs incubated with patient serum was 0.02 mg/mL (0.043 mmol/L). As noted, the five DCF metabolites shown in Fig 2 failed to promote a positive reaction even at a concentration 75 times greater (1.0 mg/mL, 3.3 mmol/L). DCF glucuronide, synthesized with rat liver microsomes using DCF as substrate, also failed to promote antibody binding at a concentration of 0.05 mg/mL (data not shown). Thus, both 4′ hydroxylation and conjugation to glucuronide at the carboxyl residue are necessary to create the active compound.
The antibody was found to have relative specificity for the antigen of Rh.Using urine as the source of the active DCF metabolite, positive (4+) agglutination reactions were obtained with RBCs of the following phenotypes: I neg, Bombay, Ko, Jk(a− b−), Ge: -1, -2, -3, MkMk, Lu (a− b−), P-P1 -Pk-, P−, Fy(a− b−), and Tc(a− b− c+). However, negative reactions were obtained with RBC typing Rhnull , DCw, and D--. Extremely weak reactions were obtained with R2R2 and r″r″ cells, but strong reactions were obtained with RBCs of other Rh phenotypes. The patient's phenotype was determined to be ccdee. These findings indicate that the antibody has relative specificity for the e antigen of the product of the RhCE gene.
DISCUSSION
Our patient developed antibody-mediated hemolysis that led to acute renal failure while taking the nonsteroidal anti-inflammatory drug DCF. With supportive care and discontinuation of DCF, she recovered in about 4 weeks. Her course was typical of the more severe (third) type of drug-induced immune hemolytic anemia (see Introduction). The finding of an IgM antibody in her serum that bound to RBCs only in the presence of the 4′ hydroxylated glucuronide ester of DCF provides strong evidence that the hemolysis was a result of sensitivity to this DCF metabolite.
Many drugs have been implicated as causes of acute immune hemolytic anemia.1-3 In some cases, antibodies reactive with normal RBCs in the presence of the suspected drug have been demonstrated by in vitro studies. Drugs identified in this way as causative agents include nomifensine,20 chlorpropamide,21 ceftriaxone,22 and quinidine.23 However, often, negative reactions are obtained with in vitro testing.7 In 1984, Salama et al9 described two patients who developed immune hemolytic anemia while taking the thiazide diuretic buthiazide and the antidepressant medication nomifensine, respectively. Drug-dependent antibodies reactive with RBCs could not be demonstrated using the primary drugs, but positive reactions were obtained using serum from persons taking these drugs as a source of drug “metabolites.” This appears to be the first documentation of drug-induced immune hemolytic anemia caused by apparent sensitivity to a drug metabolite. On the basis of studies with serum or urine, drug metabolites were subsequently implicated as causes of immune hemolytic anemia in patients taking amphotericin B,10 ibuprofen,24 and cianidanol.25 Two patients with immune hemolytic anemia associated with DCF exposure had IgG antibodies that reacted with RBCs in the presence of urine from an individual taking this drug.11 However, the active substance in DCF urine was not identified. In a study of antibodies from 35 patients who developed immune hemolytic anemia while taking nomifensine, Salama et al20 found that about one third reacted with RBCs in the presence of nomifensine itself, seven others reacted in the presence of one or more of three nomifensine metabolites modified by hydroxylation or hydroxymethylation, and 12 appeared to require an unidentified urinary metabolite of nomifensine for binding to RBCs. Because of the high incidence of immune hemolytic anemia associated with its use, nomifensine was later withdrawn from the market. Drug metabolites have also been implicated as causes of immune thrombocytopenia26-28 and neutropenia,29 and it has been speculated that metabolites formed in vivo may induce immune cytopenias more often than the unmodified primary drugs.8
Our studies implicate a metabolite of DCF, namely the glucuronide ester of 4′-OH DCF, as the cause of immune hemolytic anemia in our patient. To the best of our knowledge, this is the first demonstration of immune cytopenia apparently caused by sensitivity to a glucuronide conjugate of a drug. However, the possibility that glucuronides can cause sensitization appears not to have been investigated extensively. Since many drugs are conjugated to glucuronide in vivo,30 further studies of this possibility seem warranted.
It is of interest that the glucuronide conjugate of unmodified DCF was inactive in in vitro assays. Thus, both 4′ hydroxylation and conjugation with glucuronide at the carboxyl residue are required to convert DCF to a compound that promotes binding of the antibody from our patient to erythrocytes. Previous studies of drug-induced immune thrombocytopenia have shown that individual antibody specificities often are exquisitely sensitive to seemingly minor changes in polycyclic structures that promote their binding to a cellular target.31 32
We were able to demonstrate the antibody from our patient using urine from a person taking DCF as a source of “drug,” and this simple approach should probably be tried first in screening patients with immune cytopenia for possible sensitization to a drug metabolite. If the results are negative, aromatic compounds can be extracted from the urine (see the Methods) and concentrated for repeat testing. We were also able to synthesize the active metabolite with rat liver microsomes using the appropriate substrates. It is possible that the latter technique may yield a wider range of metabolites than are found in a urinary extract, and this approach may be useful for identifying the compound responsible for sensitization in some cases.
The quantity of serum available from our patient was insufficient to permit immunoprecipitation and other studies to identify the target protein(s) for which it is specific. However, its reactions against a conventional RBC panel and against RBCs of rare phenotypes indicated that it has relative specificity for the e antigen of the product of the RhCE gene. Preference for a component of the Rh complex has been described previously in immune hemolytic anemia induced by nonsteroidal anti-inflammatory drugs.13,33,34 As proposed by Mueller-Eckhardt and Salama,8 it is likely that the provocative drug binds to the CE protein to form a determinant consisting partly of the drug metabolite and partly of the e epitope in such cases.
NOTE ADDED IN PROOF
While this manuscript was under review, Salama A, et al (Br J Haematol 95:640, 1996) reported 15 additional cases of immune hemolytic anemia related to diclofenac (17 total including 2 cases from ref 11). Of the 17 patient samples, 13 reacted with DCF urine, DCF, and DCF-treated RBCs. Four reacted only with DCF urine.
ACKNOWLEDGMENT
We are indebted to Frank Laib, Department of Biochemistry, Medical College of Wisconsin, for assistance with NMR studies; to Dr Richard Mansfield, Dr Edward Barylak, and Ellen Heim, Holy Family Memorial Medical Center, Manitowoc, WI, for referral of the patient for study; and to the Word Processing Department of The Blood Center of Southeastern Wisconsin for manuscript preparation.
Supported by Grant No. HL13629 and Training Grant No. HL07205 from the National Heart, Lung, and Blood Institute.
Address reprint requests to D. Bougie, PhD, Blood Research Institute, The Blood Center of Southeastern Wisconsin Inc, PO Box 2178, Milwaukee, WI 53201-2178.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal