Abstract
We have investigated the leukocyte-repopulating-predictive value of granulocyte-macrophage colony-forming unit (CFU-GM) analyses in ex vivo–expanded versus fresh murine bone marrow (BM) grafts. After the transplantation of graded numbers of normal BM cells (from 15 to 5 × 103 CFU-GMs/mice), a dose-dependent increase in the recipient leukocytes was observed between the first and third weeks posttransplantation. During these stages, increases in the graft size of 100-fold improved the leukocyte counts up to 30-fold and shortened the leukopenia period by 5 to 11 days, depending on the leukocyte threshold considered. To investigate whether similar correlations could be established using ex vivo–expanded samples, the size of the CFU-GM population was maximized by means of the preactivation of the BM with 5-fluorouracil (9-day 5FU-BM), followed by 3 days of incubation with interleukin-1 plus stem cell factor. Under these conditions, the CFU-GM content of the ex vivo–expanded grafts was 73-fold higher than that observed in equivalent femoral fractions of normal fresh BM. When equivalent fractions of both graft types were transplanted, an improved leukocyte recovery was observed in mice transfused with the expanded grafts. However, the leukocyte values obtained after the transplantation of the ex vivo–expanded samples were not as high as expected, based on the number of transplanted CFU-GMs. Analyses performed during the second week posttransplantation showed that, in comparison with normal fresh BM, ex vivo–expanded grafts containing 6 to 50 times more CFU-GMs were required to generate a similar number of leukocytes. These results were confirmed in both the peripheral blood leukocytes and the myeloid Gr1+ cells, when similar numbers of CFU-GMs were transfused in the fresh and the ex vivo–expanded BM. The possibility that the preactivation of the ex vivo–expanded grafts with 5FU had a role in this effect was ruled out, because the leukocyte repopulation capacity of fresh 5FU-treated BM was as high as that observed in normal fresh BM which contained a similar number of CFU-GMs. Neither by extending the ex vivo incubation period nor by using other hematopoietic growth factor combinations was the functional capacity of the expanded grafts improved. The results presented in this study are consistent with the belief that ex vivo expansion procedures will be a useful tool for improving the hematologic recovery of patients who receive hematopoietic transplants. However, our data indicate that predicting the leukocyte repopulating capacity of ex vivo–expanded grafts according to correlations established with numbers of fresh CFU-GMs can lead to overestimations of their function, and therefore to unexpected and delayed hematopoietic engraftments.
THE EX VIVO EXPANSION of hematopoietic grafts is a novel strategy in hematopoietic transplantation that is rapidly progressing in both the basic and clinical aspects.1 Recent studies have shown the interest of using the ex vivo expansion in gene-marking and gene-therapy protocols2-5 and have suggested the possibility of using these procedures for purging strategies6 and for modulating the immunoresponse of the graft.7
In addition to the above strategies, earlier preclinical studies have shown an improved hematologic recovery, as well as an increased survival rate, in myelosupressed animals transplanted with ex vivo–expanded versus fresh hematopoietic samples.8-12 According to these observations, one of the obvious objectives for using ex vivo–expanded grafts is to minimize both the duration and the severity of the aplasia in myelosupressed recipients. Moreover, the efficiency of protocols based on the harvesting of small hematopoietic grafts for expanding and then transplanting the ex vivo–expanded samples is currently being explored in cancer patients as a way of limiting the transfusion of contaminating tumoral cells without affecting the speed of the hematopoietic engraftment.13 14
Despite all the information currently available regarding the transplantation of ex vivo–expanded grafts, the limitations that can be inherent in this type of bone marrow (BM) manipulation are still not well defined. For instance, the fate that the true stem cells follow during the different ex vivo expansion protocols is a critical question that is still open to debate.10-12 15-20 In this respect, our data have shown that a moderate restriction in the number and/or longevity of the self-renewing stem cells takes place during the expansion of the short-term repopulating progenitors (manuscript submitted).
Also of significance is the fact that most of the current protocols that aim to improve the short-term repopulating capacity of ex vivo–expanded grafts are focused on the amplification of the granulocyte-macrophage colony-forming unit (CFU-GM) population. Nevertheless, there is still no conclusive data revealing the extent to which the expansion of this progenitor accounts for the improved hematologic recovery generally associated to the transplantation of ex vivo–expanded samples. Moreover, although good correlations between the speed of the hematologic recovery and the number of transplanted CFU-GMs have already been observed after the transplantation of conventional hematopoietic grafts,21-23 the question of whether or not the CFU-GM content in ex vivo–expanded grafts can predict the hematologic recovery of these recipients is unknown.
The results presented in this study show important differences in the leukocyte repopulating capacity of fresh and ex vivo–expanded BM grafts when both are defined by the same CFU-GM content. The relevance of these observations to predicting the hematologic recovery of recipients transplanted with ex vivo–expanded grafts is discussed.
MATERIALS AND METHODS
Mice.Twelve- to 14-week-old (C57Bl/6JxDBA/2J)F1 mice were used throughout. Breeding pairs, originally obtained from the Jackson Laboratory (Bar Harbor, ME), were bred at the CIEMAT Animal Facility and allowed food and water ad libitum. Where indicated, donor animals were given one intravenous (IV) injection of 150 mg/kg of 5-fluorouracil (5FU; Roche, Basel, Switzerland).
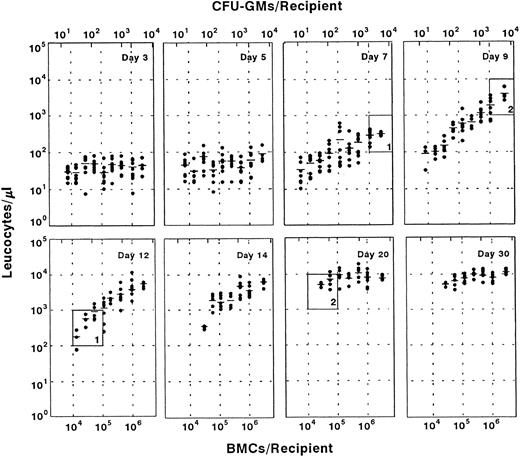
Influence of the transplantation of graded numbers of normal fresh BM cells on the leukocyte recovery of myeloablated recipients. The upper part of each diagram illustrates the number of transplanted CFU-GMs. Each point represents the leukocyte count of one recipient. A total number of eight mice was initially included in each group, with the exception of the group transplanted with 3 × 106 cells, which consisted of four mice. Windows 1 and 2 show the influence of the graft size on the period required for achieving representative numbers of leukocytes (see text).
Influence of the transplantation of graded numbers of normal fresh BM cells on the leukocyte recovery of myeloablated recipients. The upper part of each diagram illustrates the number of transplanted CFU-GMs. Each point represents the leukocyte count of one recipient. A total number of eight mice was initially included in each group, with the exception of the group transplanted with 3 × 106 cells, which consisted of four mice. Windows 1 and 2 show the influence of the graft size on the period required for achieving representative numbers of leukocytes (see text).
Expansion of BM in suspension cultures.BM cells were flushed from the femora of at least three 5FU-treated donor animals, suspended in Iscove's modified Dulbecco's medium (IMDM; GIBCO Laboratories, Grand Island, NY) supplemented with 20% fetal bovine serum (FBS; GIBCO Laboratories) and hematopoietic growth factors (HGFs) and then incubated at 37°C and 5%CO2 in air, at a density of 2 × 106 cells/mL. The HGFs considered in this study included murine recombinant interleukin-3 (mrIL-3) (2% medium conditioned by Chinese hamster ovary cells transfected with an expression plasmid containing a cDNA encoding for the murine IL-3; this concentration rendered plateau numbers of CFU-GMs in semisolid cultures; kindly provided by Genetics Institute, Cambridge, MA), human (h) rIL-6 (200 ng/mL of nonglycosilated purified hrIL-6; specific activity, 2 × 107 U/mL; kindly provided by Pharmacia, Madrid, SP), mr stem cell factor (mrSCF ) (4 U/mL of medium conditioned by COS cells transfected with an expression plasmid containing a cDNA encoding the murine soluble SCF; kindly provided by Genetics Institute), and 5 ng/mL hrIL-1α (purified hrIL1α; specific activity 6.7 × 107 U/mg; Genzyme Corp, Cambridge, MA).
CFU-GM assay.An appropriate number of BM cells were resuspended in IMDM supplemented with 25% horse serum (HS; GIBCO Laboratories) and 10% Wehi-3b Conditioned Medium, mixed with Bactoagar (0.3% final concentration; Difco Laboratories, Detroit, MI) and seeded into 35-mm plastic tissue culture dishes (Nunc, Roskilde, Denmark). Colonies were scored after 7 days of incubation at 37°C in a 95% humidified atmosphere with 5% CO2 in air.
Peripheral blood cell counts.Peripheral blood leukocytes from individual mice were enumerated by using a hemocytometer counting chamber. For conventional analysis, blood samples of 30 μL were collected from a fine incision in the lateral tail vein of mice.
Irradiation.Recipient mice were total body irradiated with a fractionated dose of 10 Gy (two doses of 5 Gy spaced 4 hours apart; dose rate 1.03 Gy/min) using a Philips MG 324 X-ray equipment (Philips, Hamburg, Germany), at 300 kV, 10 mA. Earlier studies have revealed the myeloablative properties of this irradiation regimen.24
Flow cytometry analysis.For analyzing the peripheral blood Gr-1 cells, 50 μL of blood was incubated with fluorescein isothiocyanate (FITC)-conjugated rat-antimouse Gr-1 antibody (Pharmingen, San Diego, CA) for 30 minutes at 4°C. Thereafter, red blood cells were lysed by adding 2.5 mL of the lysis solution (0.155 mol/L NH4Cl + 0.01 mol/L KHCO3 + 10−4 EDTA), and cells were washed and analyzed on an Epics ELITE ESP flow cytometer (Coulter, Hialeah, FL).
Statistics.Data are presented as the mean ± standard error of the mean. The significance of differences between groups was determined using the two-tailed Student's t-test.
RESULTS AND DISCUSSION
The transplantation of increasing numbers of normal BM cells does not prevent the leukocyte nadir, although it reduces the period of leukopenia in a predictive manner.As a first approach to understanding the theoretical repopulation benefits which could be expected from the transplantation of ex vivo–expanded grafts, a close follow-up of mice transplanted with graded numbers of normal fresh BM (range, 8 × 103 to 3 × 106 cells/mouse) was performed. Earlier studies have shown that it is below a threshold of 5 × 106 cells/mouse when the best correlations between the graft size and the hematologic recovery are seen.23 Our observations in Fig 1 first show that the transplantation of increasing numbers of BM cells does not improve the leukocyte numbers of recipients during the first 5 days posttransplantation. Given that all the cellular elements which are present in a normal BM were transplanted, one could presume that ex vivo–expanded protocols (at least those resulting in short-term repopulating cell expansions lower than 400-fold) have a limited capacity to ameliorate the first days of posttransplantation leukopenia.
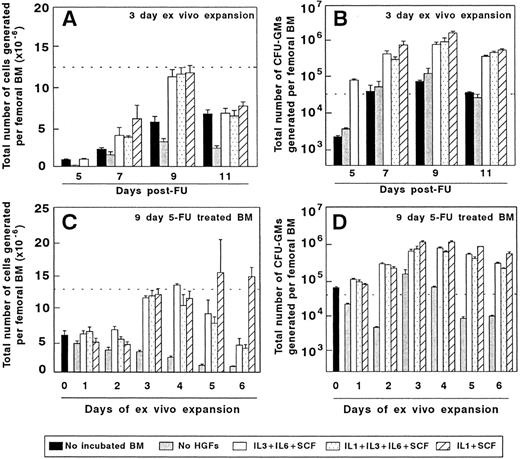
Ex vivo expansion of BM cells from mice treated with 5FU. The two upper diagrams represent the cellularity (A) and the number of CFU-GMs (B) of BM samples obtained at different days after 5FU administration and incubated for 3 days in vitro. The lower part of the figure represents the influence of the incubation period on the cellularity (C) and CFU-GM numbers (D) generated by BM samples obtained 9 days after 5FU treatment. The dotted line represents the cellularity (A and C) and the number of CFU-GMs (B and D) generated by the femoral BM of normal untreated mice. The figure represents the results obtained in three independent experiments.
Ex vivo expansion of BM cells from mice treated with 5FU. The two upper diagrams represent the cellularity (A) and the number of CFU-GMs (B) of BM samples obtained at different days after 5FU administration and incubated for 3 days in vitro. The lower part of the figure represents the influence of the incubation period on the cellularity (C) and CFU-GM numbers (D) generated by BM samples obtained 9 days after 5FU treatment. The dotted line represents the cellularity (A and C) and the number of CFU-GMs (B and D) generated by the femoral BM of normal untreated mice. The figure represents the results obtained in three independent experiments.
In contrast to this observation, a cell-dose–dependent increase in the number of leukocytes was noted during the second week posttransplantation, and more significantly at 9 and 12 days posttransplantation. At these stages, increases in the graft size of 100-fold raised the leukocyte values up to 30-fold (P < .05). In terms of days of aplasia, this increase in the graft size reduced the period of leucopenia by 5 to 11 days, depending on the leukocyte threshold considered (5 days for thresholds of 102 to 103 leukocytes/μL and 11 days for thresholds of 103 to 104 leukocytes/μL, see windows 1 and 2 in Fig 1).
When considering the number of CFU-GMs present in the transplanted grafts, our data indicate that the correlation with leukocyte numbers takes place at least within the range of 15 to 5 × 103 CFU-GMs/mouse (about 6 × 102 to 2 × 105 CFU-GMs/kg body weight). Also in humans a correlation between the number of transplanted CFU-GMs and the hematologic recovery of recipients has been observed in grafts containing <1.5 to 5 × 105 CFU-GMs/kg body weight.25 Although it could be assumed that similar correlations to those observed with fresh BM could also take place with ex vivo–expanded grafts, no data have already been presented in this respect.
The ex vivo expansion of 5FU pre-activated BM markedly increase the total number of CFU-GMs with respect to equivalent femoral fractions of normal fresh BM.Before determining whether the leukocyte recovery of recipients transplanted with ex vivo–expanded grafts is predicted by the number of transplanted CFU-GMs, a set of experiments was performed aiming to maximize the number of CFU-GMs that can be generated by the femoral BM of a donor mouse.
Figure 2A and B show the total number of hematopoietic cells and CFU-GMs produced as a result of a 3-day–ex vivo expansion of 5FU preactivated BM, using three different combinations of HGFs. Irrespective of the stimulatory conditions, grafts collected 9 days after the administration of 5FU were the most efficient for generating high numbers of hematopoietic cells and CFU-GMs. Data in Fig 2C show that the highest number of hematopoietic cells was obtained during the third to fifth days of expansion of 9-day 5FU-treated grafts, with the third or fourth days of culture being the optimal period for obtaining high numbers of CFU-GMs (Fig 2D).
Given that the IL-1/SCF was the HGF combination which rendered the highest number of CFU-GMs, grafts obtained from 9d 5FU-treated mice and expanded for 3 days with IL-1/SCF (9d 5FU + 3d IL-1/SCF BM) were used for the subsequent experiments. On average, the CFU-GM content of these expanded grafts was 73-fold higher than that obtained in equivalent femoral fractions of normal fresh BM. These results are consistent with data from Muench et al,26 who first showed the efficiency of the IL-1/SCF stimulation for expanding a diversity of hematopoietic progenitors in Δ-cultures. Moreover, our experiments indicate that in all tested stimulatory conditions, harvesting the BM at the ninth day post-5FU administration and maintaining the BM for only 3 days in culture generates the highest numbers of CFU-GMs.
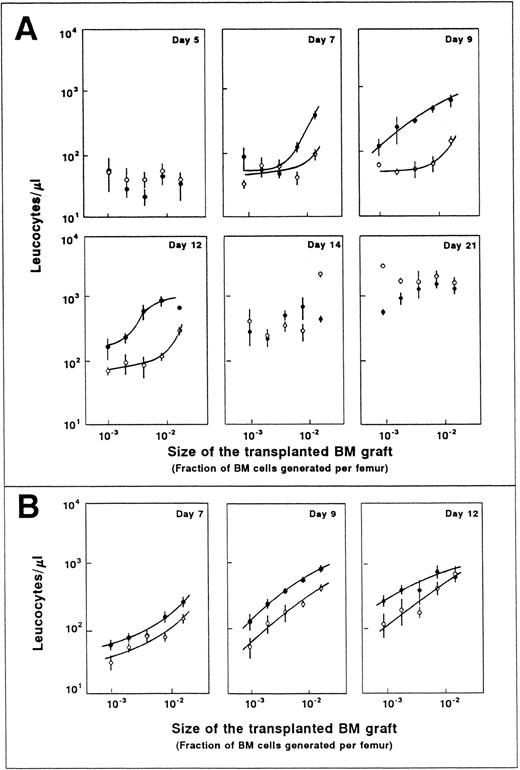
Analysis of the leukocyte recovery of mice transplanted with equivalent femoral fractions of normal fresh (○) and ex vivo–expanded (9d 5FU + 3d IL-1/SCF; •) BM. The figure represents the results obtained in two independent experiments (A and B). Each point represents the mean and standard error corresponding to four individually analyzed recipients.
Analysis of the leukocyte recovery of mice transplanted with equivalent femoral fractions of normal fresh (○) and ex vivo–expanded (9d 5FU + 3d IL-1/SCF; •) BM. The figure represents the results obtained in two independent experiments (A and B). Each point represents the mean and standard error corresponding to four individually analyzed recipients.
The transplantation of ex vivo-expanded BM significantly improves the leukocyte recovery of recipients with respect to mice transplanted with equivalent femoral fractions of normal unmanipulated BM grafts.To determine the extent to which the BM manipulation improved the recovery of the recipient leukocytes, equivalent femoral fractions of normal fresh and ex vivo–expanded (9d 5FU + 3d IL-1/SCF ) BM were transplanted into myeloablated mice. This was performed by determining the number of cells generated by one femoral BM, both in the normal fresh and in the ex vivo–expanded samples. An appropiate number of cells were then transplanted, so that both groups of recipients received the same fraction of one femoral BM (range, 10−3 to 2 × 10−2).
As it was indicated in Fig 2C, the cellularity corresponding to one femoral fresh BM and one femoral 9d 5FU + 3d IL-1/SCF expanded BM was very similar, although a higher content of CFU-GMs was present in the ex vivo–expanded versus the normal BM samples (105-fold and 54-fold, in experiments A and B from Fig 3). As expected from the results obtained in Fig 1, the transplantation of ex vivo–expanded grafts did not modify the leukocyte kinetics of recipients during the first days posttransplantation. These data reinforce the idea that expansion strategies resulting in CFU-GM increments in the range of 100-fold will hardly prevent the leukocyte nadir that is produced soon after the hematopoietic transplantation.
At the seventh day posttransplantation, a significant increase in leukocyte numbers became apparent in some of the groups transplanted with the expanded BM, something that was evident in almost all groups of recipients at 9 and 12 days posttransplantation. At these times, differences in leukocyte numbers between both groups of recipients were up to eightfold in experiment A and threefold in experiment B. In terms of days of aplasia, our data showed that the ex vivo–expanded grafts shortened the leukocyte recovery of the recipients (by an average of 5 and 2 days in experiments A and B, respectively) in comparison with animals transplanted with equivalent femoral fractions of unmanipulated normal BM.
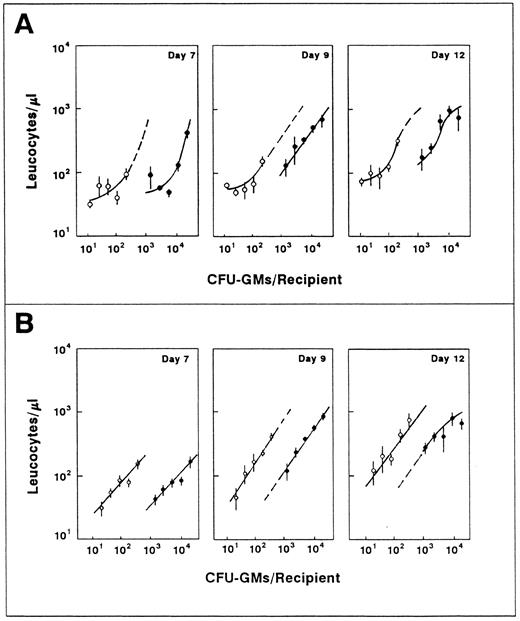
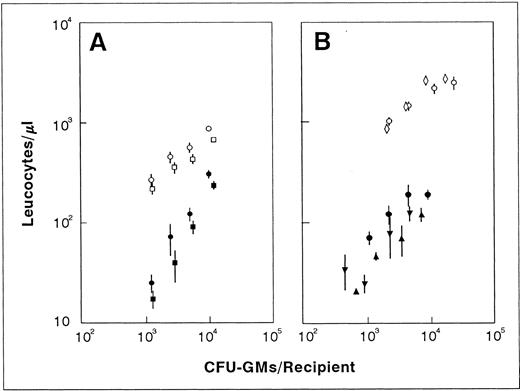
Influence of the number of transplanted CFU-GMs on the leukocyte recovery of recipients transfused with normal fresh (○) and ex vivo–expanded BM (•). The figure represents the leukocyte values shown in Fig 3 plotted against the number of CFU-GMs present in each graft type. For further details see footnote in Fig 3.
Influence of the number of transplanted CFU-GMs on the leukocyte recovery of recipients transfused with normal fresh (○) and ex vivo–expanded BM (•). The figure represents the leukocyte values shown in Fig 3 plotted against the number of CFU-GMs present in each graft type. For further details see footnote in Fig 3.
Although a number of laboratories, including our own, have previously shown an improved hematologic recovery in experimental animals transplanted with ex vivo–expanded versus fresh BM cells,9-12 the results presented in this report reveal that the beneficial effects associated to the BM expansion are applicable to a wide range of graft sizes. Moreover, our data indicate that, as was the case with fresh BM grafts, the leukocyte recovery of recipients transplanted with ex vivo–expanded samples is dependent on the size of the transplanted graft, at least within the range analyzed.
Predicting the functional capacity of ex vivo–expanded BM grafts according to correlations established with fresh CFU-GMs overestimates their capacity to mediate short-term leukocyte recovery.To understand whether the improved short-term repopulating capacity associated with the BM manipulation was a direct consequence of their increased content in CFU-GMs, the leukocyte values represented in Fig 3 were correlated to the number of CFU-GMs present in both the normal fresh and the ex vivo–expanded BM. As shown in Fig 4, the leukocyte values obtained after the transplantation of the ex vivo–expanded grafts were not as high as expected, based on their content in CFU-GMs. In fact, differences as high as eightfold between the predicted and the actual values of leukocytes were estimated during the second week posttransplantation. It is also significanct that, in comparison to normal fresh BM, the expanded grafts required 6 to 50 times more CFU-GMs to generate a similar number of leukocytes.
Taking into account that a very different range of CFU-GMs was present in the normal and the ex vivo–expanded BM samples, analogous experiments to those presented in Figs 3 and 4 were conducted, although in this case similar numbers of CFU-GMs were transfused in both graft types. The analysis of recipients at the ninth day posttransplantation (Fig 5A) confirmed the lower functional capacity of the expanded grafts when compared with fresh BM samples that contained a similar number of CFU-GMs. To rule out the possibility that changes in the ratio of the peripheral blood lymphocytes were affecting our conclusions, the proportion of Gr1+ cells was determined in all blood samples. As shown in Fig 5A, these analyses confirmed the above observations for the peripheral blood granulocytes.
Analysis of the leukocyte recovery of recipients transplanted with fresh (empty points) and ex vivo–expanded BM grafts (filled points) containing similar numbers of CFU-GMs. In experiment A, total leukocyte counts (circles) and Gr1+ cells (squares) are shown. Experiment B represents the leukocyte values of mice transplanted with fresh BM from normal (○) or 9d 5FU-treated mice (⋄) and with 3d (•), 5d (▾), and 7d (▴) ex vivo–expanded grafts from 9d 5FU-treated mice. Each point represents the mean and standard error corresponding to four recipients individually analyzed 9 days after transplantation.
Analysis of the leukocyte recovery of recipients transplanted with fresh (empty points) and ex vivo–expanded BM grafts (filled points) containing similar numbers of CFU-GMs. In experiment A, total leukocyte counts (circles) and Gr1+ cells (squares) are shown. Experiment B represents the leukocyte values of mice transplanted with fresh BM from normal (○) or 9d 5FU-treated mice (⋄) and with 3d (•), 5d (▾), and 7d (▴) ex vivo–expanded grafts from 9d 5FU-treated mice. Each point represents the mean and standard error corresponding to four recipients individually analyzed 9 days after transplantation.
To determine whether the 5FU pretreatment of the ex vivo–expanded grafts had induced an engraftment defect in the precursor cells, as has been already proposed for samples transplanted into unconditioned recipients,27 one further experiment was performed. In this case, in addition to the expanded (9d 5FU + 3d IL-1/SCF ) and the normal fresh BM, another graft type consisting of fresh BM from 9d 5FU-treated mice was transplanted. As shown in Fig 5B, the fresh 5FU-treated BM was as efficient as a normal fresh BM which contained similar numbers of CFU-GMs, thus suggesting that the reduced functionality of the ex vivo–expanded grafts was a consequence of the ex vivo expansion process per se.
To test whether the efficiency of the expanded population could be improved by extending the ex vivo expansion period, and thus the differentiation status of the hematopoietic sample, grafts that had been incubated for 3 days and also for 5 and 7 days were transplanted in this experiment. As shown in Fig 5B, the extension of the incubation period did not improve the leukocyte repopulating capacity of grafts which had similar CFU-GM numbers, but rather impaired it.
Finally, to rule out a specific negative effect mediated by the expansion with SCF/IL-1, further groups of mice were transplanted with grafts that had been expanded for 3 days with SCF/IL-3, SCF/IL-3/IL-6, SCF/IL-1/IL-3/IL-6, and SCF/IL-1. The leukocyte counts of these recipients at the ninth day posttransplantation evidenced that none of these expanded samples were more efficient than the IL-1/SCF expanded grafts (not shown).
According to these data it can be deduced that proliferative or engraftment28-30 deficiencies of the CFU-GM population may account for the relatively impaired functionality observed in ex vivo–expanded grafts, when compared with fresh BM samples containing a similar number of CFU-GMs. Alternatively, it must be considered that changes in other non–CFU-GM populations may account for the reduced repopulation efficiency observed in expanded versus fresh samples which contain a similar number of CFU-GMs. To discern between both possibilites, further experiments involving the transplantation of pure populations of fresh and expanded CFU-GMs should be performed.
The results obtained in our study are consistent with earlier studies revealing the capacity of ex vivo expansion procedures for improving the hematologic recovery of recipients. However, this research indicates that predicting the functional capacity of ex vivo–expanded grafts by means of correlations already established with fresh CFU-GMs can lead to important overestimations in their leukocyte repopulating function. According to this observation, it is suggested that unexpected deficiencies of engraftment could be produced in those cases in which conventional numbers of CFU-GMs are considered for the transplantation ex vivo–expanded grafts.
ACKNOWLEDGMENT
The authors thank Dr M. Lamana for careful reading of the manuscript and helpful discussions; C. Esteban and S. Garcı́a for excellent technical collaboration; I. Ormán for expert assistance with the flow cytometry; and J. Martinez for careful maintenance of the animals.
Supported in part by a grant of Comisión Interministerial de Ciencia y Tecnologı́a (SAF 95-1548-C02-01).
Address reprint requests to Juan A. Bueren, PhD, Molecular and Cell Biology Unit, CIEMAT, Avenida Complutense 22, 28040 Madrid, Spain.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal