Abstract
Flt3 ligand (FL) has been proposed as a possible modulator of early hematopoietic cell growth. The purpose of this study was to analyze the impact of FL on ex vivo expansion of hematopoietic cells obtained from adult donors. We sought to precisely identify hematopoietic populations responsive to FL and to quantitate the ability of FL to enhance the survival and/or proliferation of early hematopoietic precursors in a stroma-free culture system. Towards that end, four CD34+ subsets were isolated and their response to FL was characterized. In methylcellulose, FL significantly increased colony formation by CD34+ CD38dim cells but not CD34+ CD38+ cells. In suspension culture, the enhancement of cell expansion by FL was 10 times greater with the CD34+ CD38dim fraction than the CD34+ CD38+ fraction. FL stimulated the generation of colony-forming unit–granulocyte-macrophage (CFU-GM) from the CD34+CD38dim fraction by 14.5- ± 5.6-fold. To determine if CD34+ CD38dim cells responded uniformly to FL, the population was subdivided into a CD34+ CD38dim CD33dim HLA-DR+ (HLA-DR+) fraction and a CD34+ CD38dim CD33dim HLA-DRdim (HLA-DRdim) fraction. FL was far more effective at stimulating cell and progenitor growth from the HLA-DR+ fraction. To determine if FL enhanced or depleted the number of precommitted cells in expansion culture, CD34+ CD38dim and HLA-DR+ fractions were incubated in liquid culture and analyzed by flow cytometry. Inclusion of FL enhanced the absolute number of primitive CD34+ CD33dim cells and CD34+ HLA-DRdim cells after 5 to 12 days of cultivation. To confirm immunophenotypic data, the effect of FL on long-term culture-initiating cells (LTCIC) was determined. After 2 weeks of incubation of CD34+ CD38dim or HLA-DR+ cultures, LTCIC recoveries were significantly higher with FL in 5 of 6 trials (P < .05). For HLA-DR+ cells, LTCIC recoveries averaged 214% ± 87% of input with FL and 24% ± 16% without FL. In contrast, HLA-DRdim LTCIC could not be maintained in stroma-free culture. We conclude that less than 10% of CD34+ cells respond vigorously to FL and that those cells are contained within the HLA-DR+ fraction. FL stimulates the expansion of total cells, CD34+ cells, and CFU-GM and enhances the pool of early CD34+ CD33dim cells, CD34+ HLA-DRdim cells, and LTCIC. These data indicate that it is possible to expand hematopoietic progenitors from adult donors without losing precursors from the precommitted cell pool.
EX VIVO EXPANSION of hematopoietic precursors for transplantation is an area of intense investigation. The potential benefits of such studies include accelerated engraftment, reduced risk of infection, smaller stem cell harvests, and improved effectiveness of genetically modified stem cells.1,2 In murine models, the effects of expansion on subsequent engraftment are controversial.3-8 Human culture systems using interleukin-3 (IL-3) and c-kit ligand (KL) generally maintain at least some primitive cells.9-16 However, achieving a net expansion of early cells has proven elusive, although some laboratories have reported encouraging results.17-19 With the recent discovery of FLT3 and its ligand (FL),20-26 investigators have re-examined the possibility of expanding stem cells in vitro.
Studies of murine cells,27-29 human cord blood,16,30 fetal liver,31 and cadaveric marrow32,33 have shown that FL does indeed stimulate early hematopoietic cells. However, the response of early cells from adult donors has not been characterized as well. In addition, the extent to which late precursors respond to FL is not clear, because recent data suggest that FLT3 is present on both CD34bright and CD34dim cells.34 Finally, it is not clear how growth in FL might affect the number of primitive cells in culture. The response of the earliest precursors is a major concern, because stimulation by FL might promote their loss through activation, growth, and differentiation. Elegant single-cell and small-scale experiments have suggested that FL can support renewal of long-term culture-initiating cells (LTCIC) from cadaveric marrow.32 33 However, it is important to extend those investigations to cells collected from living donors.
Given that background, we initiated a study to determine how FL modulated the proliferative and maturational responses of cells collected from adult donors. This study focused on three issues. First, using four CD34+ subsets, we defined the populations most responsive to FL stimulation. Special emphasis was placed on determining cellular responses to combined exposure to FL and KL, about which little is known. Second, we determined the extent to which FL could enhance cell and progenitor expansion. Third, we defined the capacity of FL to modulate the number of primitive cells during ex vivo expansion. The results presented here show that FL-responsive cells are primarily found in CD34+ CD38dim and CD34+ CD38dim CD33dim HLA-DR+ populations. Inclusion of FL stimulates the production of committed progenitors and significantly enhances the number of primitive cells as measured by immunophenotype and long-term culture assay.
MATERIALS AND METHODS
Collection of Cells
This study was approved by the American Red Cross Committee for the Protection of Human Subjects. Aliquots of mobilized peripheral blood stem cells were obtained after granulocyte colony-stimulating factor mobilization with or without chemotherapy. Bone marrow cells (collected from healthy allogeneic donors or autologous donors under treatment for lymphoma or breast cancer) were retrieved from Fenwal bone marrow collection kits after filtration of the harvested product.
Flow Cytometric Isolation of Primitive Hematopoietic Precursors
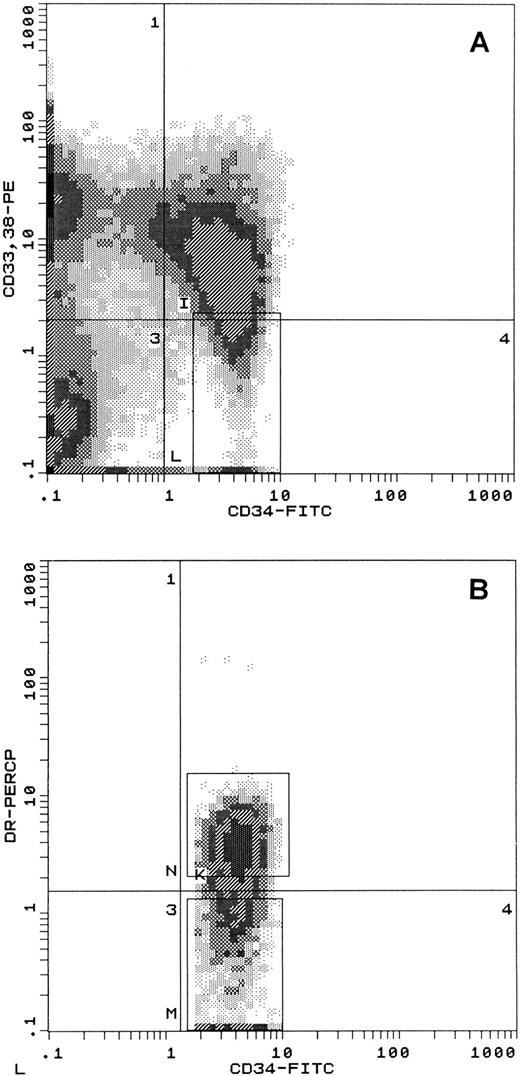
Bone marrow or mobilized blood cells were separated on Ficoll-Paque (Pharmacia, Piscataway, NJ) and the resulting mononuclear cells (MNC) were enriched for CD34+ cells using magnetic microbeads (Miltenyi Biotec, Auburn, CA). The enriched population was stored overnight at 37°C in Iscove's modified Dulbecco's medium (IMDM; GIBCO, Grand Island, NY) with 20% fetal bovine serum (FBS; Hyclone, Logan, UT), penicillin/streptomycin, IL-3 (12.5 ng/mL), IL-6 (10 ng/mL), and KL (50 ng/mL; provided courtesy of Immunex, Seattle, WA). The next morning, cells were washed and labeled with anti-CD34-fluorescein isothiocyanate (FITC) (HPCA-2), anti-CD38-phycoerythrin (PE) (HB-7), and where specified, anti-CD33-PE (P67.6) and anti–HLA-DR peridinin chlorophyll protein (PerCP) (L243) or isotypic control antibodies (all from Becton Dickinson, Mountain View, CA). In some cases, biotinylated anti–HLA-DR/streptavidin-allophycocyanin (APC) was used (Becton Dickinson). Cell separation was performed with a Coulter ELITE ESP flow cytometer (Coulter, Miami, FL). When biotinylated anti–HLA-DR/streptavidin-APC was used in 4-color sorts, the fluorochrome was excited with a 10-mW helium neon laser (633 nm) and the fluorescent signal was processed through a gated amplifier. Propidium iodide (PI) was included as a viable stain. Viable cells (gated according to forward and right angle light scatter and PI negativity) were sorted for CD34 and CD38. The CD34+ CD38dim sort gate was positioned around CD34+ cells with the lowest 10% to 15% CD38 fluorescence. Alternatively, CD34+ CD38dim CD33dim gated events were sent to a CD34 versus HLA-DR histogram for sorting (see Fig 4). Reanalysis of sorted cells indicated ≥90% purity (or ≥95% purity if allowance is made for photo-bleaching of the fluorochromes). After suspension culture, washed cells were labeled as described above for analysis. Alternatively, early CD34+ CD45RAdim CD71dim CD64dim were identified with anti-CD34+-PerCP (HPCA-2), anti-CD45RA-FITC (L48), anti-CD71-FITC (L01.1) (all Becton Dickinson), and anti-CD64-FITC (22, Immunotec). At least 10,000 total events were collected in the light scatter gate.
Separation of early CD34+ subsets from mobilized peripheral blood. CD34+ cell-enriched suspensions were labeled with FITC-CD34, PE-CD38, PE-CD33, and PerCP-HLA-DR and PI. Viable (PIlow) cells were sorted with an ELITE ESP flow cytometer using predetermined forward and orthogonal light scatter parameters. (A) Gate for CD34+ CD38dim CD33dim cells. (B) Gated cells from (A) displayed for sorting of HLA-DRdim cells in the lower gate (25% of events shown) and HLA-DR+ cells in the upper gate (61% of events shown).
Separation of early CD34+ subsets from mobilized peripheral blood. CD34+ cell-enriched suspensions were labeled with FITC-CD34, PE-CD38, PE-CD33, and PerCP-HLA-DR and PI. Viable (PIlow) cells were sorted with an ELITE ESP flow cytometer using predetermined forward and orthogonal light scatter parameters. (A) Gate for CD34+ CD38dim CD33dim cells. (B) Gated cells from (A) displayed for sorting of HLA-DRdim cells in the lower gate (25% of events shown) and HLA-DR+ cells in the upper gate (61% of events shown).
Stroma-Free Liquid Culture
Cells were cultured in IMDM with 20% FBS, penicillin/streptomycin, and 50 ng/mL recombinant KL, 12.5 ng/mL recombinant human IL-3 (rhIL-3), 10 ng/mL rhIL-6, and (where indicated) 50 ng/mL FL (all generously supplied by Immunex). Cultures were initiated with 1 to 10 × 103 cells (dictated by sort yields) and cultured in 1 mL of medium in 24-well trays (Corning, Cambridge, MA) maintained at 37°C in a humidified atmosphere with 5% CO2 . Fresh cytokines were added 3 times a week and media were completely changed once a week with no more than 1 to 2 × 105 cells returned to the wells. Calculated yields of cells and colony-forming unit–granulocyte-macrophage (CFU-GM) were corrected for adjustments to cell numbers at the time of medium change.
Assay for Committed Myeloid Progenitor Cells
CFU-GM were assessed in agarose essentially as described35-39 using rhIL-3 (12.5 ng/mL), granulocyte-macrophage colony-stimulating factor (GM-CSF; 4 ng/mL), KL (50 ng/mL), 0.1 mmol/L hemin (Sigma, St Louis, MO), and an optimal concentration (2% vol/vol) of 5X 5637 CM. CFU-GM were also assayed in methylcellulose (Fig 1) supplemented with cytokines (Stem Cell Technologies, Vancouver, British Columbia, Canada; catalog no. 4230), as indicated. Colonies were counted after 14 days of incubation in a 5% CO2 humidified atmosphere at 37°C.
Clonogenic response of CD34+ CD38dim and CD34+ CD38+ hematopoietic precursors in different cytokine mixtures. CD34+ CD38dim or CD34+ CD38+ marrow cells (3,000/dish) were plated in methylcellulose containing 1% BSA (Stem Cell Technologies; catalogue no. 4230) supplemented with the indicated cytokines and incubated for 14 days before colonies were counted. Data were pooled from three independent trials. The number of colonies was significantly greater with FL than with the same combination lacking FL: *P < .05; **P < .008. KL stimulated the growth of CD34+ CD38dim cells (P < .02) and CD34+ CD38+ cells (P < .01) when added to [IL-3 + IL-6].
Clonogenic response of CD34+ CD38dim and CD34+ CD38+ hematopoietic precursors in different cytokine mixtures. CD34+ CD38dim or CD34+ CD38+ marrow cells (3,000/dish) were plated in methylcellulose containing 1% BSA (Stem Cell Technologies; catalogue no. 4230) supplemented with the indicated cytokines and incubated for 14 days before colonies were counted. Data were pooled from three independent trials. The number of colonies was significantly greater with FL than with the same combination lacking FL: *P < .05; **P < .008. KL stimulated the growth of CD34+ CD38dim cells (P < .02) and CD34+ CD38+ cells (P < .01) when added to [IL-3 + IL-6].
Assay for LTCIC
When the number of cells available for analysis was high, limiting dilution analysis on irradiated allogeneic stroma was used (method 1). More often, when the initial number of cells available for analysis was quite small, LTCIC were quantitated in triplicate stromal cultures at 1 or 2 concentrations (method 2, described below). For any single experiment, the same quantitative method was used throughout to ensure the validity of the analysis. Primary stromal cultures were established as previously described.38,40,41 First or second passage stromal cells were irradiated (10 Gy) and seeded at 50% to 75% confluence and were used within 3 days. Cultures were fed immediately before the addition of test cells. For method I, LTCIC were quantitated on microstromal cultures in 96-well trays. Six blocks of 24 wells were recharged with varying numbers of cells. Wells were fed by weekly demi-depopulation. After 5 weeks, nonadherent cells and trypsinized adherent cells from each well were plated for CFU-GM. In the absence of added test cells, no CFU-GM were recovered from the wells. The frequency of LTCIC was determined by plotting the logarithm of the percentage of negative wells against the number of cells added per well.42,43 A curve was fitted using logistic regression and the method of maximum likelihood, with (LTCIC frequency)−1 found at 37% negative wells.44 For method 2, test cells were plated at 1 or 2 concentrations in triplicate wells (24-well trays) containing stroma and maintained as described above. After 35 days, all cells were harvested as above and plated at two concentrations in triplicate in agarose. From these data, the total number of day-35 CFU-GM in the liquid culture was calculated and converted to LTCIC by multiplying by 0.25.39 43 For both methods, 95% confidence intervals are given.
Statistics
Tests of significance were conducting using the Student's t-test.
RESULTS
Identification of Cells Responsive to FL
Comparison of CD34+ CD38dim and CD34+ CD38+ cells. The first goal of this study was to determine how early and late hematopoietic cells from adult donors responded to stimulation by FL. Towards that end, CD34+ CD38dim and CD34+ CD38+ fractions were isolated as representative early and late cells. Before use, they were characterized to confirm their developmental status. When plated in agarose containing IL-3 and GM-CSF, the cloning efficiency of CD34+ CD38dim cells was lower than CD34+ CD38+ cells (data not shown), the results expected for the primitive CD34+ CD38dim fraction.11,32 45 Additional experiments showed that LTCIC frequencies were higher in the CD34+ CD38dim fraction than in the CD34+ CD38+ fraction (7.4- ± 3.2-fold greater with blood [n = 5] and 3.7- ± 1.1-fold greater with marrow [n = 3]). These data confirmed the early and late nature of these fractions. We therefore analyzed their responses to FL in both short- and long-term culture.
When CD34+ CD38dim marrow cells were cultured in methylcellulose, little growth was observed with single factors FL or GM-CSF (Fig 1). Cloning efficiency improved with multifactor combinations and, in each case, the addition of FL significantly enhanced the number of colonies in the CD34+ CD38dim fraction. In contrast, FL provided no significant stimulation to the CD34+ CD38+ fraction. To better understand the effect of FL on cell proliferation, marrow fractions were grown in liquid culture (Fig 2). The addition of FL to [IL-3 + IL-6] and to [IL-3 + IL-6 + KL] was nearly 10 times more stimulatory for the CD34+ CD38dim fraction than for the CD34+ CD38+ fraction (Table 1). Of the 4 cytokine combinations examined, [IL-3 + IL-6 + KL + FL] was the most supportive for CD34+ CD38dim cell expansion (P < .05 in each case, see legend to Table 1). Interestingly, cultivation in [IL-3 + IL-6] confirmed the greater stimulatory requirements of the early CD34+ CD38dim population, ie, CD34+ CD38dim cells expanded only fourfold, whereas CD34+ CD38+ cells expanded 267-fold (Table 1).
Effect of FL and KL on the proliferation of CD34+ CD38dim cells and CD34+ CD38+ in liquid culture. CD34+ CD38dim cells and CD34+ CD38+ cells from normal marrow were cultured in [IL-3 + IL-6] alone or with FL, KL, or [KL + FL]. The figure shows the maximum fold cell expansion obtained in each of 6 or 7 independent experiments as well as the mean ± SD for each condition. Note that the marked variability of CD34+ CD38dim cell expansion in [IL-3 + IL-6 + FL] was largely overcome when cells were cultured in [IL-3 + IL-6 + KL + FL].
Effect of FL and KL on the proliferation of CD34+ CD38dim cells and CD34+ CD38+ in liquid culture. CD34+ CD38dim cells and CD34+ CD38+ cells from normal marrow were cultured in [IL-3 + IL-6] alone or with FL, KL, or [KL + FL]. The figure shows the maximum fold cell expansion obtained in each of 6 or 7 independent experiments as well as the mean ± SD for each condition. Note that the marked variability of CD34+ CD38dim cell expansion in [IL-3 + IL-6 + FL] was largely overcome when cells were cultured in [IL-3 + IL-6 + KL + FL].
Expansion of Marrow CD34+ CD38dim Cells and CD34+ CD38+ Cells in Liquid Culture
| Cytokines . | Cell Yield (fold-increase ± SEM) . | |||||
|---|---|---|---|---|---|---|
| . | CD34+ CD38dim . | CD34+ CD38+ . | . | . | ||
| . | Average Fold Increase* . | FL Enhancement† . | Average Fold Increase* . | FL Enhancement† . | . | . |
| IL-3 + IL-6 | 3.8 ± 2.2 | 267 ± 141 | ||||
| IL-3 + IL-6 + FL | 280 ± 129 | 50.0 ± 22.6‡ | 1,467 ± 873 | 5.56 ± 2.08 | ||
| (P = .15) | ||||||
| IL-3 + IL-6 + KL | 1,333 ± 857 | 3,244 ± 2,320 | ||||
| IL-3 + IL-6 + KL + FL | 6,867 ± 2,059ρ | 14.6 ± 5.21-155 | 4,243 ± 1,980 | 1.89 ± 0.54 | ||
| (P = .034) | ||||||
| Cytokines . | Cell Yield (fold-increase ± SEM) . | |||||
|---|---|---|---|---|---|---|
| . | CD34+ CD38dim . | CD34+ CD38+ . | . | . | ||
| . | Average Fold Increase* . | FL Enhancement† . | Average Fold Increase* . | FL Enhancement† . | . | . |
| IL-3 + IL-6 | 3.8 ± 2.2 | 267 ± 141 | ||||
| IL-3 + IL-6 + FL | 280 ± 129 | 50.0 ± 22.6‡ | 1,467 ± 873 | 5.56 ± 2.08 | ||
| (P = .15) | ||||||
| IL-3 + IL-6 + KL | 1,333 ± 857 | 3,244 ± 2,320 | ||||
| IL-3 + IL-6 + KL + FL | 6,867 ± 2,059ρ | 14.6 ± 5.21-155 | 4,243 ± 1,980 | 1.89 ± 0.54 | ||
| (P = .034) | ||||||
Average fold increase in total cells was calculated by averaging absolute peak values from 6 or 7 trials (Fig 2).
Fold enhancement of cell growth observed when FL was added to either [IL-3 + IL-6] or [IL-3 + IL-6 + KL]. P values show significance of difference of enhancement of CD34+ CD38dim versus CD34+ CD38+ fractions.
The modest P value reflects the highly variable response obtained with CD34+ CD38dim cells from different donors when cultured in [IL-3 + IL-6 + FL] (Fig 2). In columns 3 and 5, FL enhancement was calculated by dividing (fold cell increase with FL) by (fold cell increase without FL) in each experiment and averaging the results. This calculation reduces the impact of donor variability. Resultant values for FL stimulation consequently differ somewhat from those obtained by comparing entries in column 2 or in column 4.
ρ For CD34+ CD38dim cells, growth in [IL-3 + IL-6 + KL + FL] was significantly greater than in [IL-3 + IL-6] (P = .012), [IL-3 + IL-6 + FL] (P = .009), and [IL-3 + IL-6 + KL] (P = .047).
Addition of FL to [IL-3 + IL-6 + KL] enhanced the growth of CD34+ CD38dim cells significantly more than the growth of CD34+ CD38+ cells (P = .034).
Cultures were also examined for expansion of myeloid progenitors. Unlike CFU-GM in the CD34+ CD38+ fraction, progenitors in the CD34+ CD38dim fraction did not survive cultivation in [IL-3 + IL-6] (Fig 3). CD34+ CD38dim progenitors did grow when FL was added to [IL-3 + IL-6], although the response was highly varied (see legend to Fig 3). The greatest progenitor output was achieved by adding FL to [IL-3 + IL-6 + KL], a combination that significantly enhanced production of CFU-GM from CD34+ CD38dim cells (P = .01) but not CD34+ CD38+ cells (P = .18).
Expansion of CFU-GM from CD34+ CD38dim cells and CD34+ CD38+ cells. Normal marrow MNC were sorted into CD34+ CD38dim and CD34+ CD38+ fractions and subsequently cultured in liquid medium with 4 different combinations of cytokines. In each trial (n = 5), the number of CFU-GM was assessed at weekly intervals to determine the maximum number produced for each condition. The figure shows average values for CFU-GM input and the maximum number of CFU-GM generated with each of the combinations. [IL-3 + IL-6] could not sustain CFU-GM from the CD34+ CD38dim fraction. [IL-3 + IL-6 + FL] supported a significant expansion of CFU-GM from the CD34+ CD38dim fraction in 3 trials, whereas none were recovered in 2 others. Such variability (also seen in Fig 2) decreased the mean yield to less than that of the CD34+ CD38+ fraction. The greatest expansion was obtained with [IL-3 + IL-6 + KL + FL]. *CFU-GM yield from CD34+ CD38dim cells in [IL-3 + IL-6 + KL + FL] significantly greater than same cells in [IL-3 + IL-6 + KL] (P = .01). **CFU-GM yield from CD34+ CD38dim cells in [IL-3 + IL-6 + KL + FL] significantly greater than CD34+ CD38+ yield in same cytokine mix (P = .003). #CFU-GM yield from CD34+ CD38+ cells in [IL-3 + IL-6 + KL + FL] not significantly greater than same cells in [IL-3 + IL-6 + KL] (P = .18). ##Generation of CFU-GM in the presence of [IL-3 + IL-6 + FL] was not significantly greater in the CD34+ CD38+ fraction than in the CD34+ CD38dim fraction (P = .30).
Expansion of CFU-GM from CD34+ CD38dim cells and CD34+ CD38+ cells. Normal marrow MNC were sorted into CD34+ CD38dim and CD34+ CD38+ fractions and subsequently cultured in liquid medium with 4 different combinations of cytokines. In each trial (n = 5), the number of CFU-GM was assessed at weekly intervals to determine the maximum number produced for each condition. The figure shows average values for CFU-GM input and the maximum number of CFU-GM generated with each of the combinations. [IL-3 + IL-6] could not sustain CFU-GM from the CD34+ CD38dim fraction. [IL-3 + IL-6 + FL] supported a significant expansion of CFU-GM from the CD34+ CD38dim fraction in 3 trials, whereas none were recovered in 2 others. Such variability (also seen in Fig 2) decreased the mean yield to less than that of the CD34+ CD38+ fraction. The greatest expansion was obtained with [IL-3 + IL-6 + KL + FL]. *CFU-GM yield from CD34+ CD38dim cells in [IL-3 + IL-6 + KL + FL] significantly greater than same cells in [IL-3 + IL-6 + KL] (P = .01). **CFU-GM yield from CD34+ CD38dim cells in [IL-3 + IL-6 + KL + FL] significantly greater than CD34+ CD38+ yield in same cytokine mix (P = .003). #CFU-GM yield from CD34+ CD38+ cells in [IL-3 + IL-6 + KL + FL] not significantly greater than same cells in [IL-3 + IL-6 + KL] (P = .18). ##Generation of CFU-GM in the presence of [IL-3 + IL-6 + FL] was not significantly greater in the CD34+ CD38+ fraction than in the CD34+ CD38dim fraction (P = .30).
Substantiating the FL-responsiveness of CD34+ CD38dim cells, 9 additional trials showed that inclusion of FL with [IL-3 + IL-6 + KL] increased CFU-GM output 14.5- ± 5.6-fold (total CFU-GM expansion, 426- ± 154-fold). FL enhancement was significant for 7 normal marrows (all P values <.02) and 1 mobilized peripheral blood sample (P = 3 × 10−4), but not for one autologous marrow donor sample (P = .12).
FL-responsive cells within the CD34+ CD38dim fraction. From the preceding data, it was not possible to discern whether all cells within the CD34+ CD38dim fraction were uniformly responsive to FL. To answer that question, CD34+ CD38dim cells were further subdivided according to their expression of HLA-DR antigen, an approach previously used with fetal cells.46 47 To ensure that early cells were depleted of committed progenitors, we selected for cells that were also CD33dim. Thus, CD34+ CD38dim CD33dim cells from mobilized blood were separated into the HLA-DR+ subset (denoted HLA-DR+) and the HLA-DRdim subset (HLA-DRdim; Fig 4). The fractions were cultured with [IL-3 + IL-6 + KL] ± FL.
After 6 to 9 days of incubation, HLA-DRdim cultures showed no growth with [IL-3 + IL-6 + KL] and a modest increase (10-fold) with the addition of FL (Table 2). In contrast, the HLA-DR+culture increased 16-fold without FL and 93-fold with it (P = .035). Furthermore, with FL, the HLA-DR+ subset grew more than the HLA-DRdim subset (P = .042). CFU-GM in HLA-DR+ cultures expanded 43-fold without FL and 326-fold with FL (day 14, n = 5, Table 2). Thus, the CD34+ CD38dim fraction from mobilized blood was immunophenotypically and functionally heterogeneous, with the CD34+ CD38dim CD33dim HLA-DR+ fraction showing the greatest responsiveness to FL. We next asked how FL stimulation affected the number of primitive cells in culture.
FL-Responsive Cells Are Primarily in the HLA-DR+ Fraction
| Subset Input . | FL . | Cell Yield (fold increase ± SEM) . | |
|---|---|---|---|
| . | . | Total Cells . | CFU-GM . |
| HLA-DRdim | − | 1.49 ± 0.83 | |
| (n = 6) | |||
| HLA-DRdim | + | 10.6 ± 4.9* | |
| (n = 7) | |||
| HLA-DR+ | − | 16.4 ± 7.3 | 43 ± 20 |
| (n = 9) | (range, 2.2-87) | ||
| (n = 5) | |||
| HLA-DR+ | + | 93 ± 31†‡ | 326 ± 152ρ |
| (n = 10) | (range, 40-897) | ||
| (n = 5) | |||
| Subset Input . | FL . | Cell Yield (fold increase ± SEM) . | |
|---|---|---|---|
| . | . | Total Cells . | CFU-GM . |
| HLA-DRdim | − | 1.49 ± 0.83 | |
| (n = 6) | |||
| HLA-DRdim | + | 10.6 ± 4.9* | |
| (n = 7) | |||
| HLA-DR+ | − | 16.4 ± 7.3 | 43 ± 20 |
| (n = 9) | (range, 2.2-87) | ||
| (n = 5) | |||
| HLA-DR+ | + | 93 ± 31†‡ | 326 ± 152ρ |
| (n = 10) | (range, 40-897) | ||
| (n = 5) | |||
HLA-DR+ cells and HLA-DRdim cells from mobilized peripheral blood were cultured in [IL-3 + IL-6 + KL] ± FL. Cultures were analyzed for total cells (days 6 through 9) and CFU-GM (day 14).
Not significantly different from HLA-DRdim lacking FL (P = .11).
Significantly different from HLA-DR+ lacking FL (P = .035).
Significantly different from HLA-DRdim with FL (P = .042).
ρ Not significantly different from HLA-DR+ lacking FL (P = .14). Insufficient cells were available to assess HLA-DRdim cultures for CFU-GM.
Fate of Primitive Cells in the Presence of FL
Effect of FL on early immunophenotypes. The effects of FL on the maintenance of early cells was first assessed by flow cytometric analyses. CD34+ CD38dim blood cells were cultured in [IL-3 + IL-6 + KL] ± FL for 5 to 12 days. FL-supplemented cultures contained 11.5- ± 3.1-fold more CD34+ cells than cultures lacking FL (n = 7). The number of CD34+ cells averaged 196% ± 108% of input with FL and only 30% ± 16% of input without FL, differences that did not reach significance (P = .15) due to the variability of donor responsiveness. To show the persistence of primitive cells, cultures were analyzed for early CD34+ CD33dim cells.48 49 In 3 trials, the number of CD34+ CD33dim cells in the FL-supplemented culture was greater than the number in the FL-deprived culture (Fig 5).
Effect of FL on hematopoietic expansion in liquid culture. CD34+ CD38dim cells from mobilized peripheral blood were cultured in [IL-3 + IL-6 + KL] ± FL. Cultures were terminated for analysis when sufficient cells had been generated (day 12, 5, and 6 are shown in the top, middle, and bottom panels, respectively). In the absence of FL, CD34+ cells were below detection in the top panel and CD34+ CD33dim cells were below detection in the top and bottom panels. In each case, FL enhanced the number of total cells, CD34+ cells, and early CD34+ CD33dim cells.
Effect of FL on hematopoietic expansion in liquid culture. CD34+ CD38dim cells from mobilized peripheral blood were cultured in [IL-3 + IL-6 + KL] ± FL. Cultures were terminated for analysis when sufficient cells had been generated (day 12, 5, and 6 are shown in the top, middle, and bottom panels, respectively). In the absence of FL, CD34+ cells were below detection in the top panel and CD34+ CD33dim cells were below detection in the top and bottom panels. In each case, FL enhanced the number of total cells, CD34+ cells, and early CD34+ CD33dim cells.
A similar approach was used to analyze the FL-responsiveness of HLA-DRdim and HLA-DR+ subsets. After 6 to 8 days of culture of HLA-DR+ blood cells, suspensions with FL contained 5.8- ± 1.1-fold more CD34+ cells than FL-deprived cultures (n = 4; P = .024). FL enhanced the number of early CD34+ CD33dim cells 6-, 15-, and 17-fold in 3 separate trials. The analysis was extended to quantitate cells with a primitive CD34+ HLA-DRdim phenotype.15,43,50 51 When HLA-DR+ cells were cultured with FL, the number of CD34+ HLA-DRdim cells was elevated 3.5-, 5.9-, and 45-fold compared with cultures lacking FL.
Figure 6 shows a representative flow cytometric pattern obtained from HLA-DR+ cells cultured with or without FL. In the culture supplemented with FL (Fig 6A), one observes that a higher proportion of gated CD34+ cells were CD33dim or HLA-DRdim. Data from Fig 6 are displayed quantitatively in Fig 7. Figure 7 also shows the comparatively poor yield of early cells from the HLA-DRdim fraction cultured with FL. In 3 trials, CD34+ cells in the HLA-DRdim fraction did not respond to FL (104% ± 8% of control). In a direct comparison of cell outputs from these fractions, FL stimulated 2.4, 3.2, and 7.8 times greater production of CD34+ CD33dim cells from the HLA-DR+ fraction than from the HLA-DRdim fraction. Furthermore, the enhancement of CD34+ HLA-DRdim levels by FL was 8.4, 8.4, and 1.2 times greater in the HLA-DR+ subset than in the HLA-DRdim subset.
Persistence of early CD34+ CD33dim and CD34+ HLA-DRdim subsets is enhanced by FL. HLA-DR+ cells from mobilized peripheral blood were sorted as shown in Fig 4 and cultured for 6 days in stroma-free medium containing [IL-3 + IL-6 + KL] ± FL. Harvested cells were stained for CD34, CD33, and HLA-DR antigens as well as PI. Histograms show PIlow CD34+ cells gated for analysis of CD33 and HLA-DR. Growth with (A) or without (B) FL. Inclusion of FL increased the proportion of cells with early phenotypes. Larger number of events in (A) reflects the enhanced production of CD34+ cells (and primitive subsets) in the presence of FL. Data representative of 3 separate trials. Data from this trial (including those for the HLA-DRdim culture not included in this figure) are displayed quantitatively in Fig 7.
Persistence of early CD34+ CD33dim and CD34+ HLA-DRdim subsets is enhanced by FL. HLA-DR+ cells from mobilized peripheral blood were sorted as shown in Fig 4 and cultured for 6 days in stroma-free medium containing [IL-3 + IL-6 + KL] ± FL. Harvested cells were stained for CD34, CD33, and HLA-DR antigens as well as PI. Histograms show PIlow CD34+ cells gated for analysis of CD33 and HLA-DR. Growth with (A) or without (B) FL. Inclusion of FL increased the proportion of cells with early phenotypes. Larger number of events in (A) reflects the enhanced production of CD34+ cells (and primitive subsets) in the presence of FL. Data representative of 3 separate trials. Data from this trial (including those for the HLA-DRdim culture not included in this figure) are displayed quantitatively in Fig 7.
Effect of FL on the number of primitive precursors after cultivation of HLA-DR+ cells and HLA-DRdim cells. Mobilized peripheral blood cells were sorted into HLA-DR+ and HLA-DRdim fractions and cultured in [IL-3 + IL-6 + KL] ± FL. After 6 days, cultures were analyzed for the indicated phenotypes. The figure shows the absolute yield of CD34+ cells and subsets. FL supported a greater yield of CD34+ cells, CD34+ CD33dim cells, and CD34+ HLA-DRdim cells in HLA-DR+ cultures than in the HLA-DRdim cultures. Data are representative of 3 independent trials.
Effect of FL on the number of primitive precursors after cultivation of HLA-DR+ cells and HLA-DRdim cells. Mobilized peripheral blood cells were sorted into HLA-DR+ and HLA-DRdim fractions and cultured in [IL-3 + IL-6 + KL] ± FL. After 6 days, cultures were analyzed for the indicated phenotypes. The figure shows the absolute yield of CD34+ cells and subsets. FL supported a greater yield of CD34+ cells, CD34+ CD33dim cells, and CD34+ HLA-DRdim cells in HLA-DR+ cultures than in the HLA-DRdim cultures. Data are representative of 3 independent trials.
Thus, the addition of FL enhanced the size of early populations with CD34+ CD33dim and CD34+ HLA-DRdim phenotypes. Greater enhancement of early populations was obtained in cultures of CD34+ CD38dim cells and HLA-DR+ cells than in cultures of HLA-DRdim cells. Further support for this conclusion was obtained in a single trial analyzing cultured HLA-DR+ and HLA-DRdim blood cells for their contents of early CD34+ CD45RAdim CD71dim CD64dim cells. FL enhanced this early fraction 15.4-fold in the HLA-DR+ culture, but only 1.8-fold in the HLA-DRdim culture.
Effect of FL on maintenance/expansion of LTCIC. The preceding data suggested that FL might enhance the recovery of LTCIC after stroma-free cultivation. To test that hypothesis, LTCIC levels were assessed after stroma-free cultivation in [IL-3 + IL-6 + KL] ± FL. Initially, CD34+ CD38dim cells from bone marrow were examined. After 2 weeks of incubation, the number of LTCIC in the liquid cultures was close to input levels, provided that FL was present (Fig 8, trials 1 and 2). In the absence of FL, LTCIC yields were significantly smaller (P < .05). Similar results were obtained using CD34+ CD38dim cells from blood (trial 3). To better understand how blood LTCIC respond to FL, 5 additional experiments were conducted using HLA-DR+ and HLA-DRdim fractions (Fig 8, trials 4 through 8). A comparison of HLA-DRdim and HLA-DR+ populations was of interest, because Figs 6 and 7 suggested that LTCIC in the HLA-DR+ fraction might be more responsive to FL than those in the HLA-DRdim fraction. In freshly isolated HLA-DR+ and HLA-DRdim subsets, LTCIC frequencies were not significantly different (3.6% ± 1.4% v 2.6% ± 0.9%, respectively; n = 5; P > .05). The HLA-DR+ and HLA-DRdim fractions were cultured in [IL-3 + IL-6 + KL] ± FL for 14 days. The mean yield of LTCIC from the HLA-DR+ fraction was 214% ± 87% of input with FL and only 24% ± 16% without FL. Direct comparisons of LTCIC yields with and without FL were possible in 3 trials and the differences were significant in 2. Overall, Fig 8 shows that LTCIC yields were enhanced by FL in 6 of 6 experiments and that the differences were significant in 5 of the 6 studies. In contrast to these results, HLA-DRdim LTCIC responded poorly to FL. In 3 trials, HLA-DRdim LTCIC decreased below the level of detection within 14 days of culture (data not shown). Finally, in Fig 8, CFU-GM expansion in the presence of FL averaged 481- ± 169-fold. Thus, FL-supplemented, stroma-free cultures of CD34+ CD38dim cells and HLA-DR+ cells can generate large numbers of committed progenitors without losing significant numbers of primitive LTCIC.
FL enhances the yield of LTCIC in stroma-free culture. CD34+ CD38dim cells and CD34+ CD38dim CD33dim HLA-DR+ cells were cultured with [IL-3 + IL-6 + KL] ± FL for 14 to 19 days and analyzed for CFU-GM and LTCIC. This figure shows the fold change in LTCIC number relative to input. In trials 7 and 8, low sort yields did not permit the establishment of cultures without FL. Cell sources: trials 1 and 2, normal bone marrow; trials 3 through 8, mobilized blood. Average CFU-GM expansions: 481- ± 169-fold with FL; 74- ± 34-fold without FL. Error bars show 95% confidence intervals; bars on trials 1, 3, and 8 are too short to be visible. Significance of difference for (▪) +FL versus (□) −FL in individual trials: *P < .05; **P < .02; ***P < .0004.
FL enhances the yield of LTCIC in stroma-free culture. CD34+ CD38dim cells and CD34+ CD38dim CD33dim HLA-DR+ cells were cultured with [IL-3 + IL-6 + KL] ± FL for 14 to 19 days and analyzed for CFU-GM and LTCIC. This figure shows the fold change in LTCIC number relative to input. In trials 7 and 8, low sort yields did not permit the establishment of cultures without FL. Cell sources: trials 1 and 2, normal bone marrow; trials 3 through 8, mobilized blood. Average CFU-GM expansions: 481- ± 169-fold with FL; 74- ± 34-fold without FL. Error bars show 95% confidence intervals; bars on trials 1, 3, and 8 are too short to be visible. Significance of difference for (▪) +FL versus (□) −FL in individual trials: *P < .05; **P < .02; ***P < .0004.
DISCUSSION
The ability to generate hematopoietic precursors ex vivo will profoundly affect future approaches to stem cell collection, transplantation, and gene therapy. In the present study, we determined how hematopoietic cell expansion was modulated by FL, a recently discovered cytokine that acts early in blood cell development. Our goal was to precisely identify hematopoietic populations responsive to FL and to determine the impact of FL on primitive cell numbers. Towards that end, four CD34+ subsets were isolated for growth and analysis in stroma-free culture. To ensure clinical relevance, this study focused on hematopoietic cells collected from living, adult donors.
CD34+ Subsets Responsive to FL
The stimulatory effects of FL were largely confined to primitive hematopoietic subsets. FL increased the proportion of CD34+ CD38dim cells (but not CD34+ CD38+ cells) that grew in methylcellulose (Fig 1). In suspension culture, the addition of FL to [IL-3 + IL-6] and to [IL-3 + IL-6 + KL] stimulated proliferation of CD34+ CD38dim cells far more than proliferation of CD34+ CD38+ cells (Table 1 and Fig 2). FL also enhanced the generation of CFU-GM from CD34+ CD38dim cells (Fig 3). Interestingly, the proliferative response of CD34+ CD38dim cells obtained from different donors was quite variable in [IL-3 + IL-6 + FL]. Such variability was greatly reduced when KL was added to [IL-3 + IL-6 + FL] (Fig 2). Finally, in some experiments, FL stimulated the growth of CFU-GM from late CD34+ CD38+ cells (Fig 3), but on average, it was less than that obtained with early populations.
To extend the analysis of early cells, CD34+ CD38dim precursors from blood were separated into CD34+ CD38dim CD33dim HLA-DR+ (HLA-DR+) and CD34+ CD38dim CD33dim HLA-DRdim (HLA-DRdim) populations. Although the populations were similarly enriched for LTCIC, the HLA-DR+ fraction was far more responsive to FL stimulation than the HLA-DRdim fraction (Table 2). The HLA-DRdim population also responded poorly to KL. That is, in [IL-3 + IL-6 + KL], the HLA-DRdim fraction failed to grow (Table 2), whereas CD34+ CD38dim cells (Table 1) and the HLA-DR+ fraction (Table 2) grew significantly.
Fate of Primitive Cells
FL enhanced the number of CD34+ cells in 6 of 7 cultures initiated with CD34+ CD38dim blood cells and all 4 cultures initiated with HLA-DR+ blood cells. Cultures were also examined for the presence of early immunophenotypes. After 5 to 12 days of growth of CD34+ CD38dim cells, early CD34+ CD33dim cells were present and their numbers were enhanced by the addition of FL (Fig 5). When the analysis was extended to the HLA-DR+ subset, it was found that FL stimulated the absolute number of CD34+ CD33dim cells and CD34+HLA-DRdim cells (Figs 6 and 7). To validate flow cytometric data, suspension cultures of CD34+ CD38dim cells and HLA-DR+ cells were evaluated for LTCIC after 2 weeks of incubation. Cultures supplemented with FL had significantly (P < .05) greater numbers of LTCIC in 5 of 6 trials.
In summary, both functional and immunophenotypic assays show that early cells are considerably more responsive to FL than are late cells. Instead of accelerating the loss of early cells, FL enhances the number of primitive precursors in expansion cultures. It is notable that stroma-free growth with [IL-3 + IL-6 + KL + FL] yielded a 300- to 400-fold expansion of committed progenitor cells under conditions supporting the maintenance or slight expansion of more primitive precursors. The enhanced generation of late cells (progenitors and mature cells) results from the stimulation of early HLA-DR+ cells, with weaker effects on CD34+ CD38+ cells.
Our finding that adult CD34+ CD38dim cells are highly responsive to FL agrees with recent data obtained with human fetal liver31 and cadaveric marrow.32,33 Other investigators used stromal cultures to document the FL responsiveness of CD34+ CD38dim cells.52 Our data are also consistent with a recent report that CD34+ lin− high proliferative potential colony-forming cells (HPP-CFC) and CFU-blast respond to FL.53 To our knowledge, this is the first study to show that early blood cells are heterogeneous in their response to FL, ie, CD34+ CD38dim CD33dim subfractions differing in HLA-DR antigen density vary in their capacities to respond to FL. This report also demonstrates that late cells, which express FLT3,22,34 respond weakly to FL. Low FL-responsiveness has also been observed with CD34+ CD38+ cells in a stroma-based system52 and with late FLT3+ murine cells.29 In flow analysis, the proportion of cells coexpressing FLT3 and c-kit increases with the intensity of CD34 staining.34 Our results appear to support that finding, because the most primitive cell fractions responded to the simultaneous presence of FL and KL (Figs 1-3 and Table 1). In that regard, adult CD34+ CD38dim cells and fetal liver CD34+ CD38dim cells differ, because the latter are not further stimulated by combining FL with KL.31 When FL and KL were examined separately, ie, [IL-3 + IL-6 + KL] versus [IL-3 + IL-6 + FL], no clear distinction could be made in terms of total cell expansion. However, KL differed from FL in that KL stimulated both early and late populations (Figs 1 and 2).
The effect of FL on the number of precommitted cells in liquid culture has been examined previously, although generally with different cell sources and less highly fractionated cell populations. A role for FL in the maintenance of primitive cells was proposed based on studies of long-term cultures,23,53,54 but neither the target population nor the identity of synergizing factors nor the change in LTCIC number could be defined. In stroma-free culture, a 4.5-fold increase in cord blood LTCIC was reported, which was somewhat greater with FL than without.16 In studies of cadaveric CD34+ CD38dim cells, Petzer et al32,33 reported that FL, KL, and IL-3 were key to stimulating a greater than 10-fold expansion of LTCIC. Although it is not clear why LTCIC expansion was greater in the latter studies than in the present work, methodologic differences are apparent. For example, Petzer et al32,33 used serum-free media for expansion. Furthermore, LTCIC and CFU-GM detection systems used by Petzer et al32,33 differed from those used in the present study. It is also possible that marrow harvested from cadaveric bone possesses an enhanced growth potential. For example, mechanical disruption of the bone could release cells adjacent to the endosteum, a region enriched for early precursors, including marrow repopulating cells.55-57 In other studies conducted without FL, recovery of LTCIC from nonperfused, stroma-free cultures generally decreases.12-15 Enhanced maintenance or expansion in the presence of stromal layers,14 stromal CM,15,58,59 or low-density marrow spinner cultures19 has been reported. Elegant experiments showed a fivefold expansion of LTCIC in stroma-noncontact cultures.48
In this study, the HLA-DR+ population gave rise to CD34+ HLA-DRdim cells, a fraction generally accepted as primitive in nature and enriched for LTCIC.15,43,51 The possibility that early cells may be either HLA-DR+ or HLA-DRdim was addressed previously.60 Because the CD34+ HLA-DRdim fraction and the HLA-DR+ fraction each constitute less than 10% of total CD34+ cells, the enrichment of primitive cells in both populations is not contradictory. The precise relationship between HLA-DR antigen expression and the nature and properties of primitive cells remains to be clarified. In the case of human fetal liver, HLA-DR+ and HLA-DRdim subsets of the CD34+ CD38dim linlo population contained similar numbers of HPP-CFC and had similar proliferative capacities.47 In the present study, the HLA-DR+ and HLA-DRdim subsets contained similar numbers of LTCIC when freshly isolated, yet only HLA-DR+ LTCIC survived in stroma-free culture containing [IL-3 + IL-6 + KL + FL]. The superior proliferative potential of adult HLA-DR+ cells is similar to that observed in fetal marrow with CD34+ CD38dim HLA-DR+ cells.60 Although not directly tested in this study, these results suggest that maintenance or expansion of LTCIC in the HLA-DR+ fraction should provide superior results to that obtained with CD34+ CD38dim cells, because the latter include unresponsive HLA-DRdim cells. The nature of the HLA-DRdim fraction is under investigation.
In conclusion, functional and flow cytometric studies show that FL enhances the productivity of stroma-free expansion cultures. FL had its greatest stimulatory effects on a subfraction of the precommitted CD34+ CD38dim population (HLA-DR+), which constitutes just a few percent of the total CD34+ cell pool. FL stimulated the HLA-DR+ fraction to generate increased numbers of cells, CFU-GM, and CD34+ cells as well as greater numbers of primitive CD34+ CD33dim cells, CD34+ HLA-DRdim cells, and LTCIC. The strong synergism between FL and KL suggests that these cytokines are not functionally redundant61 and that FL may make a distinct contribution to early hematopoietic development. Finally, these data show that a simple, stroma-free culture system can be used with cells from adult donors to expand progenitors several hundred fold while maintaining primitive precursors. These results may have clinical significance. Maintenance of LTCIC numbers during a time of extensive progenitor expansion raises important questions regarding the effect of FL on the dynamics of self-renewal and maturation in the primitive cell pool.
Address reprint requests to Douglas C. Dooley, PhD, Pacific Northwest Regional Blood Services, American Red Cross, PO Box 3200, Portland, OR 97208.

![Fig. 1. Clonogenic response of CD34+ CD38dim and CD34+ CD38+ hematopoietic precursors in different cytokine mixtures. CD34+ CD38dim or CD34+ CD38+ marrow cells (3,000/dish) were plated in methylcellulose containing 1% BSA (Stem Cell Technologies; catalogue no. 4230) supplemented with the indicated cytokines and incubated for 14 days before colonies were counted. Data were pooled from three independent trials. The number of colonies was significantly greater with FL than with the same combination lacking FL: *P < .05; **P < .008. KL stimulated the growth of CD34+ CD38dim cells (P < .02) and CD34+ CD38+ cells (P < .01) when added to [IL-3 + IL-6].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/10/10.1182_blood.v90.10.3903/3/m_bl_0040f1.jpeg?Expires=1767889174&Signature=L32bMvE62jYoDr-t00yk4g2oRsFMJLlPuKAbLdT0eNb52CV~hgf0r1NvKZ~UEeAfh~FX-OnsdN6GfxfzGkYYtKOFsAolHH3rZWf8FLsocEd47zeV9I7cq389msyvtZngWWMxjvaR1t0SPIST0bEsJqYBubxWKASQsbOTIDLjpwSuefWttH27sE0MMhaaV3iToY4XClCGjaZHIH6D3ALHvpnJj~hxlNG4mVe89oER3enysGYaL3POOXG7YYi1L5jqsuh-CjgZZrUbfJODe8f8wKmCbmZ0T~wv0LepOEY2vZxG707FsHGBJ079efuGUM5RF0orJe0NOg3J2CMWbRlCuA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Effect of FL and KL on the proliferation of CD34+ CD38dim cells and CD34+ CD38+ in liquid culture. CD34+ CD38dim cells and CD34+ CD38+ cells from normal marrow were cultured in [IL-3 + IL-6] alone or with FL, KL, or [KL + FL]. The figure shows the maximum fold cell expansion obtained in each of 6 or 7 independent experiments as well as the mean ± SD for each condition. Note that the marked variability of CD34+ CD38dim cell expansion in [IL-3 + IL-6 + FL] was largely overcome when cells were cultured in [IL-3 + IL-6 + KL + FL].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/10/10.1182_blood.v90.10.3903/3/m_bl_0040f2.jpeg?Expires=1767889174&Signature=a0GzqGYnQ7qF2sePxMlDZOJCJinCQPI0YZj702iBep-tRahNS2WUGD5llxYygWu8tNBMX~nOzgvKN59G9F19rvMQQ98~WlSvwy~aqUB5RjWB-p500jKUhyK4eHtPa4qxfD7hRlCSuZISRLCmIZqL-NFiKavtcoSki4UCNDkEk8iDeNeXpStF68ovvQI3e4XheXgVQYShSXIG3DqNUOauC9Egt1ngkjOjsNmEPs8S5qsZ7Kjzy3lbOCmNM3BC2fRf6~e7oj87lySm0QGIcG~dhklp1UDABcsXvkHWvmii-DpDUsWbLf0Xm2zaGF3Cdo6O~d0fuCsLRsw81g~xWrE22g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Expansion of CFU-GM from CD34+ CD38dim cells and CD34+ CD38+ cells. Normal marrow MNC were sorted into CD34+ CD38dim and CD34+ CD38+ fractions and subsequently cultured in liquid medium with 4 different combinations of cytokines. In each trial (n = 5), the number of CFU-GM was assessed at weekly intervals to determine the maximum number produced for each condition. The figure shows average values for CFU-GM input and the maximum number of CFU-GM generated with each of the combinations. [IL-3 + IL-6] could not sustain CFU-GM from the CD34+ CD38dim fraction. [IL-3 + IL-6 + FL] supported a significant expansion of CFU-GM from the CD34+ CD38dim fraction in 3 trials, whereas none were recovered in 2 others. Such variability (also seen in Fig 2) decreased the mean yield to less than that of the CD34+ CD38+ fraction. The greatest expansion was obtained with [IL-3 + IL-6 + KL + FL]. *CFU-GM yield from CD34+ CD38dim cells in [IL-3 + IL-6 + KL + FL] significantly greater than same cells in [IL-3 + IL-6 + KL] (P = .01). **CFU-GM yield from CD34+ CD38dim cells in [IL-3 + IL-6 + KL + FL] significantly greater than CD34+ CD38+ yield in same cytokine mix (P = .003). #CFU-GM yield from CD34+ CD38+ cells in [IL-3 + IL-6 + KL + FL] not significantly greater than same cells in [IL-3 + IL-6 + KL] (P = .18). ##Generation of CFU-GM in the presence of [IL-3 + IL-6 + FL] was not significantly greater in the CD34+ CD38+ fraction than in the CD34+ CD38dim fraction (P = .30).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/10/10.1182_blood.v90.10.3903/3/m_bl_0040f3.jpeg?Expires=1767889174&Signature=UsmQ3D8bd3ZGOtr9FFaLmFyIA2sv7bjhp8fzRLj28smhF3lCmS5YjuqnLPHVxjJIEJxO1YMKJ8E4e1akSp4e6k3g3x6~6J7dMfTLBJQJe7r-nLRexWBuJR3HpNzcJ0UGcnv0I9qkDhWdoRdngcFxD4u2O0y-QQ~rLazWUREwtvU9wJc1djHK4aOVhICmxCQw~29YF0~tEDXJVm2~CpMU-wf3KV4HowxOxKBv4RwfXVzozvl9XiSDACm-1ogCTnajXUyxkxtkvBXEoJFFQasYpFcVVQS2XnK54sFMgTSAtw8W7IGb4pEFfYfMLnq0hWz8F-dS4PQ8JPLumjJvKulPeA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Effect of FL on hematopoietic expansion in liquid culture. CD34+ CD38dim cells from mobilized peripheral blood were cultured in [IL-3 + IL-6 + KL] ± FL. Cultures were terminated for analysis when sufficient cells had been generated (day 12, 5, and 6 are shown in the top, middle, and bottom panels, respectively). In the absence of FL, CD34+ cells were below detection in the top panel and CD34+ CD33dim cells were below detection in the top and bottom panels. In each case, FL enhanced the number of total cells, CD34+ cells, and early CD34+ CD33dim cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/10/10.1182_blood.v90.10.3903/3/m_bl_0040f5.jpeg?Expires=1767889174&Signature=m5NP8bNKgAlBmy4KFGTppzbSay9OeIjAbzlXbi8MGG9ECqLzgsbbe7ri~XrQJR7qWQUdeCLzG4CPM7KagxFWjFt8260le4m25OKUitD8NnJQo4Y1EV8zaB1WXNtNRurd9x8cVYz7cJQzztrDMgpMwqjqjf6sYLn8R35reEbceF5I~GWIX-AdP4vYJymrRK9bymdyZA-b62L7K0tHma8sXs8qjA3WGWhlJhgxCGz9~V9xy88pzKZs2IZIPayUa~Uu3BbFrwR5sK81Vk5gHozT7p6iHYVkwS6QB0olI-D8t0bo020Q8dizCs8DuNZNUEWZbToRUiF5iL3a2QUcYOcctg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Persistence of early CD34+ CD33dim and CD34+ HLA-DRdim subsets is enhanced by FL. HLA-DR+ cells from mobilized peripheral blood were sorted as shown in Fig 4 and cultured for 6 days in stroma-free medium containing [IL-3 + IL-6 + KL] ± FL. Harvested cells were stained for CD34, CD33, and HLA-DR antigens as well as PI. Histograms show PIlow CD34+ cells gated for analysis of CD33 and HLA-DR. Growth with (A) or without (B) FL. Inclusion of FL increased the proportion of cells with early phenotypes. Larger number of events in (A) reflects the enhanced production of CD34+ cells (and primitive subsets) in the presence of FL. Data representative of 3 separate trials. Data from this trial (including those for the HLA-DRdim culture not included in this figure) are displayed quantitatively in Fig 7.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/10/10.1182_blood.v90.10.3903/3/m_bl_0040f6.jpeg?Expires=1767889174&Signature=VGcTE1Pt7grcFAYYVl2H5gMaHMejarepvNQ9xe8Vo5Ahb-LcgP0JvRJsDUFglDzBNOMQYcHic1K4S6wn7KtdroCqQgvy9ePzEY7TevXNTiS55D045iLz3cgQYsWYfBAbX32jzHDbH9HCSqtneNACe0ksACei1WXtUSdqst-dmBr3xr97VFCL-JqIa9VFnru-FsrfiZM3OP1ZjQnOFkXQExZDgvVHbqw1VG2VSwZysPapLYD-4V3YgjJMcjygoa4A34VhkK9YxD2Dvp-Sffh9aSd3F-Fnxp5fAOrgAAKCBnAZ6LhXhA2JcV~-J-wU53xpalWjHQXCIoMIZpeS112ZKw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Effect of FL on the number of primitive precursors after cultivation of HLA-DR+ cells and HLA-DRdim cells. Mobilized peripheral blood cells were sorted into HLA-DR+ and HLA-DRdim fractions and cultured in [IL-3 + IL-6 + KL] ± FL. After 6 days, cultures were analyzed for the indicated phenotypes. The figure shows the absolute yield of CD34+ cells and subsets. FL supported a greater yield of CD34+ cells, CD34+ CD33dim cells, and CD34+ HLA-DRdim cells in HLA-DR+ cultures than in the HLA-DRdim cultures. Data are representative of 3 independent trials.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/10/10.1182_blood.v90.10.3903/3/m_bl_0040f7.jpeg?Expires=1767889174&Signature=J-q2obktB8rcyj~LGiqaGyudtPGRT-tbHvHVTASTUyHJFgh9y5EbgpVwzVaI~7~79Ex0GzXNmheCUyDFCvebuAszXTruWnxgjVCSaXpV855000gFLWYI95p7fFg3~j7lmXaKlTwktn24v0DJuOT2W~EDdjdmKP6hQDSt~S0BizbugAC7-iDou6xO7i-Wi0dPLaSJlGzs2cIf9iD7m6wptniK1iky9EgCZQOWrxPimAOtAjNDE4xeCu9iT18GYppCV6zOXK95fn-GI-KwSySmoUs8O9HC17~brUJYXIvFtflFxMgyPJETeTUUZSvGpWcraRZVl5ciabfRgIdJi5HPRw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. FL enhances the yield of LTCIC in stroma-free culture. CD34+ CD38dim cells and CD34+ CD38dim CD33dim HLA-DR+ cells were cultured with [IL-3 + IL-6 + KL] ± FL for 14 to 19 days and analyzed for CFU-GM and LTCIC. This figure shows the fold change in LTCIC number relative to input. In trials 7 and 8, low sort yields did not permit the establishment of cultures without FL. Cell sources: trials 1 and 2, normal bone marrow; trials 3 through 8, mobilized blood. Average CFU-GM expansions: 481- ± 169-fold with FL; 74- ± 34-fold without FL. Error bars show 95% confidence intervals; bars on trials 1, 3, and 8 are too short to be visible. Significance of difference for (▪) +FL versus (□) −FL in individual trials: *P < .05; **P < .02; ***P < .0004.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/10/10.1182_blood.v90.10.3903/3/m_bl_0040f8.jpeg?Expires=1767889174&Signature=dJfX1432vcuY0vCzWbGEzhWYLZCjqGbz4Tpz3WIy1dsX4TKTn1RvziCPITpyTPvTdaVpti34dUCyUFJJtqa2IA~dE-RiZ9C9puSjy9zeykbjFu0BZiB~OMKOLXG5UyVmJ~E1kDKxlYP6UG6G8Y8ZzdObZ1ivEM00AIpRwCGqTqJfqlSMuc1ytEZWk5X1AoLbIoK-FYST6po24hxa2hQBMVNqENaX-HNk3wDBGK1XYZNlLt5c~4my~B39m1YppVmO1mp8r4gKNHU-POoEi1vrx6fHV8KY-IL~vEFFKsZhjn44F7DD3FCZ7VlCCspX-HvEfM4Ez0Q3PePmWgrrr5msDA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal