Abstract
Tumor necrosis factor α (TNFα) is a cytokine implicated in the pathogenesis of numerous chronic and acute inflammatory conditions. In the present study, we have characterized the ability of TNFα in inducing eosinophil accumulation in rat skin and have shown the inhibitory effects of anti-α4 integrin and anti–vascular cell adhesion molecule-1 (VCAM-1) antibodies on this response. The intradermal injection of recombinant human TNFα induced a slowly developing, dose-dependent accumulation of 111In-eosinophils in rat skin that was maximal at the dose of 10−11 mol/site. Coadministration of TNFα with the soluble TNFα receptor (p55)-IgG fusion protein (TNFR-IgG) totally inhibited the 111In-eosinophil accumulation induced by the cytokine. The TNFα-induced 111In-eosinophil accumulation was not affected after pretreatment of rats with the platelet-activating factor (PAF) receptor antagonist UK-74,505 or the antihuman interleukin-8 monoclonal antibody (MoAb) DM/C7. In contrast, the intravenous administration of an anti-α4 integrin MoAb, HP2/1 (3.5 mg/kg), or an anti–VCAM-1 MoAb, 5F10 (2 mg/kg), greatly inhibited the 111In-eosinophil accumulation induced by TNFα (the responses detected at 10−11 mol/site were inhibited by 78% and 50%, respectively). These results show that TNFα is an effective inducer of eosinophil accumulation in vivo, with this response being dependent on an interaction between α4 integrins and VCAM-1.
TUMOR NECROSIS FACTOR α (TNFα), a multifunctional cytokine with potent immunomodulatory and proinflammatory properties, has been implicated in the pathogenesis of several disease states, including septic shock, acquired immune deficiency syndrome, rheumatoid arthritis, multiple sclerosis, and adult respiratory distress syndrome.1-3 After cellular stimulation, TNFα can be generated and released by numerous cell types, including monocytes, T cells, eosinophils, and mast cells.4-6 TNFα exerts its biologic effects via interaction with specific cell surface receptors. To date, at least two distinct receptors have been characterized and designated as the 75-kD and 55-kD receptors, the latter mediating much of the proinflammatory effects of TNFα.7,8 Among its proinflammatory properties, TNFα can induce neutrophil degranulation and generation of superoxide anions from adherent leukocytes9 and stimulate endothelial cells, resulting in the generation of inflammatory mediators such as interleukin-8 (IL-8) and platelet-activating factor (PAF)10,11 and increased surface expression of endothelial cell adhesion molecules such as E-selectin, intercellular cell adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1).12-15 The enhanced adhesion of neutrophils and eosinophils to TNFα-stimulated cultured endothelial cells can be blocked by neutralizing anti-β2 integrin, anti–ICAM-1, or anti–E-selectin monoclonal antibodies (MoAbs).16-18 In contrast, anti–VCAM-1 and anti-α4 integrin MoAbs inhibit the adhesion of eosinophils but not neutrophils to cytokine-treated endothelial cells.16,19-22 This relatively selective effect is explained by the fact that the principal leukocyte ligands for VCAM-1, namely α4β1 (VLA-4) and α4β7 , are expressed on eosinophils but not normally on neutrophils.23
In vivo, TNFα is a potent inducer of neutrophil, lymphocyte, and monocyte accumulation.24-27 More recently, TNFα has also been indirectly implicated in the process of eosinophil accumulation in vivo. A number of studies have associated elevated levels of TNFα in bronchoalveolar lavage fluid with the pathophysiology of asthma.28-30 Furthermore, in animal models of asthma, TNFα or TNFα-like bioactivity has been detected in lungs and correlated with eosinophil infiltration and airway hyperreactivity.31 In addition, a soluble TNFα-receptor-IgG fusion protein inhibited eosinophil accumulation in a murine model of airway inflammation32 and the eosinophil accumulation induced by lipopolysaccharide in guinea-pig skin.33 Despite these studies, there have been no direct investigations into the ability of TNFα to induce eosinophil accumulation in vivo. In the present study, using a rat model that we have previously used to characterize the eosinophil accumulation induced by IL-1β,34 we now report on the profile of eosinophil accumulation induced by TNFα. Furthermore, using antagonists and neutralizing MoAbs, we show the dependency of this response on α4 integrins and VCAM-1 but not PAF or an IL-8–like molecule.
MATERIALS AND METHODS
Animals. Male Sprague-Dawley cell donor rats (400 to 500 g) and male Sprague-Dawley test rats (200 to 300 g) were purchased from Harlan-Olac (Oxfordshire, UK).
Materials. Pentobarbitone sodium (Sagatal; 60 mg/mL) was purchased from May and Baker Ltd (Dagenham, UK). Hypnorm (0.315 mg/mL fentanyl citrate and 10 mg/mL fluanisone) was purchased from Janssen Pharmaceutical Ltd (Grove, UK). Hypnovel (5 mg/mL midazolam hydrochloride) was purchased from Roche Products Ltd (Welwyn Garden City, UK). 111Indium chloride (111InCl3 ; 10 mCi/mL in pyrogen-free 0.04 N hydrochloric acid) was purchased from Amersham International (Amersham, UK). Bovine serum albumin, 2-mercaptopyridine-N-oxide, oyster glycogen, control MoAb MOPC-21 (mouse myeloma IgG1), PAF, glycogen, hexadecyltrimethylammonium bromide (HTAB), o-phenylenediamine dihydrochloride, and H2O2 were purchased from Sigma Chemical Co (Dorset, UK). Horse serum, sterile Hanks' balanced salt solution (HBSS), HEPES, and Tyrode's salt solution were purchased from GIBCO Ltd (Paisley, UK). Percoll was purchased from Pharmacia Fine Chemicals (Uppsala, Sweden). Pyrogen-and preservative-free heparin sodium (5,000 U/mL) was purchased from Pabyrn Laboratories (Greenford, UK). Recombinant rat TNFα was from Autogen Bioclear UK Ltd (Wiltshire, UK; >107 U/mg). OCT compound and hematoxilin were from BDH (Essex, UK). ABC Kit and Fast red substrate were from Vector (Peterborough, UK). FACS Flow and FACSR Lysing Solution were from Becton Dickinson (Oxford, UK).
The following were generous gifts: recombinant human TNFα (TNFα) was from Biogen Inc (Cambridge, MA; >107 U/mg). TNFR-IgG (p55sf2) and control chimeric antibody (cSF25), reactive to a human pan adeno carcinoma antigen, were from Dr B.J. Scallon and Dr J. Ghrayeb (Centocor Inc, Malvern, PA). UK-74,505 {4-(2-chlorophenyl)-1,4-dihydro-3-ethoxycarbonyl-6-methyl-2-[4-(2-methylimidazo [4,5-c]pyrid-1-yl ) phenyl]-5-[N-(2-pyridylcarbamoyl)pyridine]}, initially dissolved in 0.1 mol/L HCl and diluted in saline, was from Dr M.J. Parry (Pfizer Central Research, Sandwich, UK).
MoAbs. The MoAb DM/C7 is a mouse IgG1κ that was raised against human IL-877 and isolated and purified as previously described.35 Mouse antirat β2 MoAb (TA-4, IgG2a) was from Seikagaku Corp (Tokyo, Japan). Hamster antirat L-selectin MoAb (HRL1, IgG) and goat antihamster IgG-fluorescein isothiocyanate (FITC) were purchased from Pharmingen (San Diego, CA). Goat antimouse IgG-FITC was from DAKO Ltd (Buckinghamshire, UK). The antihuman α4 integrin MoAb HP2/1 (IgG1) that recognizes rat α364 was from Biogen Inc. The antirat VCAM-1 MoAb 5F10 (mouse IgG2a) was generated as follows.
For hybridoma generation, 8- to 10-week-old female RBF/DnJ mice (Jackson Labs, Bar Harbor, ME) were immunized intraperitoneally (0.4 mL) with 2 to 5 × 106 COS cells transiently expressing rat VCAM-1,37 suspended in phosphate-buffered saline (PBS), and emulsified in an equal volume of complete Freunds's adjuvant. Seven and 15 weeks later, the mice were boosted intraperitoneally with approximately the same number of cells emulsified in incomplete Freunds adjuvant and PBS, respectively. The spleen cells were fused to the myeloma cell line HL-1 Friendly Myeloma-653 (Fisher Scientific, Pittsburgh, PA) at a ratio of 6:1 spleen cells (1 × 108) to myeloma cells (1.7 × 107). Fusions were plated into 96-well plates and hybrid cells were selected in aminopterin, adenine, and thymidine (AAT)-supplemented medium. Supernatants were screened for their ability to bind to a stable CHO cell line expressing rat VCAM-1 but not a control CHO cell line and then for inhibition of adhesion of α4 integrin-expressing human Ramos or rat RBL-1 cells to rat VCAM-1–expressing CHO cells, as described in Hession et al.37 MoAb 5F10, an IgG2a, was identified by this means and subcloned at limiting dilution. Ascites were produced and MoAb 5F10 protein A was purified using standard methods. MoAb 5F10 binds to the first two N-terminal domains of rat VCAM-1 (D. Worley, R.R. Lobb, and C. Hession, unpublished observations).
Purification and radiolabeling of rat peritoneal eosinophils and neutrophils. Rat peritoneal eosinophils were elicited, purified, and radiolabeled as previously described.34 Briefly, rats were injected intraperitoneally with 5 mL of horse serum and killed 1 to 2 days later by CO2 -induced asphyxia. Rat peritoneal neutrophils were elicited by intraperitoneal injection of 10 mL of 6% oyster glycogen and the animals were killed after 16 hours. Peritoneal cells were collected by lavage with 30 mL of heparinized saline (10 U/mL) and purified by centrifugation over a three-layer discontinuous Percoll-HBSS gradient (60%/65%/75%). With respect to eosinophils, the fractionated cell population was used only when the eosinophil purity, as determined by Kimura staining, was greater than 90%. The predominant contaminating cell type was mononuclear and a major exclusion criterion was the presence of neutrophils. The neutrophil preparation was always greater than 95% pure. The leukocytes (1 to 2 × 107) were then incubated with approximately 5 to 10 μCi of 111InCl3 chelated with 2-mercaptopyridine-N-oxide (40 μg in 0.1 mL of 50 mmol/L PBS, pH 7.4) for 15 minutes at room temperature. The labeled leukocytes were washed three times and resuspended (1 × 107 cells/mL) in HBSS solution, pH 7.4, containing cell-free citrated rat plasma to a final concentration of 10%. The final cell suspension normally carried approximately 60% of the total radioactivity used, ie, 2 to 4 μCi/1 to 2 × 107 cells, from which 5 × 106 were injected into each recipient rat (0.7 to 1.3 μCi injected into each rat).
Measurement of 111In-leukocyte accumulation in rat skin. Eosinophil or neutrophil infiltration in the rat dorsal skin was measured using the local accumulation of intravenously (IV) injected 111In-labeled leukocytes as previously described.34 Briefly, rats were anesthetized with a mixture of Hypnorm (0.1 mL/rat) and Hypnovel (0.1 mL/rat), injected intraperitoneally, and their dorsal skin was shaved. Leukocytes (5 × 106 cells in 0.5 mL HBSS) were injected IV via a tail vein. Five minutes later, the agents under investigation, freshly prepared from stock solution in Tyrode with low endotoxin bovine serum albumin (0.1%), were injected intradermally (ID; 100 μL/site), in duplicate according to a random and balanced site plan, into the back skin. At the end of a 4-hour test period, the animals were reanesthetised and a cardiac blood sample was collected. The animals were then killed by an overdose of sodium pentobarbitone, the back skin was removed, and the injection sites were punched out with a 17-mm diameter punch. Skin and plasma samples were counted in an automatic gamma counter (Canberra Packard, Pangbourne, UK) and the 111In count per cell was determined (1 to 3 counts per minute [cpm]/leukocyte) and used to express leukocyte accumulation in each skin site in terms of the number of labeled leukocytes for 5 × 106 cells injected/rat.
Preparation of skin homogenates for measurement of eosinophil peroxidase activity. TNFα-injected (10−11 mol/site) or Tyrode-injected skin sites were punched out as described above and frozen at −80°C. Skin samples were extracted for eosinophil peroxidase (EPO) using a modification of the method used by Collins et al.38 Frozen skin sites were chopped during thawing and, after the addition of 4 mL of PBS containing 0.5% HTAB and 0.6 mol/L NaCl, were homogenized (Ultra-turrax T25; Janke and KunKel GmbH and Co, Staufen, Germany; 3× for 15 seconds), sonicated (Soniprep 150; MSE Scientific Instruments, Crawley, UK; 10 seconds), and frozen at −80°C. Immediately before the assay of samples for EPO activity, the homogenates were thawed and subjected to two further cycles of rapid freeze/thawing and centrifuged (2,800g for 10 minutes at 20°C and 13,000g for 20 minutes at 20°C). The recovered supernatants were then incubated at 60°C for 2 hours before the final centrifugation step (13,000g for 10 minutes at 20°C) to produce sample supernatants suitable for measurement of EPO.
Measurement of EPO in skin homogenates. EPO was measured using the method described by Collins et al.38 Processed skin homogenates were placed in duplicate wells (100 μL/well) in a 96-well plate followed by the addition of 100 μL substrate (8.6 mmol/L o-phenylenediamine dihydrochloride and 2.9 mmol/L H2O2 in 0.1 mol/L Tris-HCl, pH 8.0). After 30 minutes at room temperature, the reaction was terminated by the addition of 50 μL of 4 mol/L H2SO4 and the absorbance was read at 492 nm.
Rat EPO standards were prepared using eosinophils recovered from the peritoneal cavity that were obtained and purified as previously described. After red blood cell lysis (resuspension in 0.2% NaCl for 20 seconds, followed by the addition of an equal volume of 1.6% NaCl), aliquots of eosinophils (106 cells/mL PBS/0.5% HTAB/0.6 mol/L NaCl) were sonicated (for 10 seconds), freeze/thawed three times, incubated at 60°C for 2 hours, and centrifuged (13,000g for 20 minutes at 20°C). Eosinophil supernatants were used to construct an EPO calibration curve (80 to 10,000 cells/well). The equivalent number of eosinophils per skin site was calculated from the standard curve. EPO standards and skin homogenates were diluted in PBS/0.5% HTAB/0.6 mol/L NaCl, pH 7.4.
Immunohistochemistry of skin sections for VCAM-1 expression. Rat skin sites, injected with TNFα or Tyrode, were embedded in OCT compound and snap frozen in isopentane cooled in liquid nitrogen and stored at −80°C. Cryostat sections (6 μm) were cut from biopsies, air-dried overnight, and then fixed in acetone. The sections were then stained using an ABC kit. Briefly, after pretreatment with normal horse serum, the sections were incubated for 60 minutes with the primary antibodies, 10 μg/mL 5F10 (mouse antirat VCAM-1) or 10 μg/mL control (mouse IgG). After washing in PBS, the sections were incubated for 30 minutes with biotinylated horse antimouse antibody, followed by 30 minutes of incubation with avidin biotin complex. The sections were then developped in fast red substrate and couterstained in hematoxylin. Positive cells stained red.
Histology. Rat skin sites were injected with TNFα (10−11 mol/site) or Tyrode. After 4 hours, the skin sites were punched out as described above, fixed in 10% phosphate-buffered formalin for 24 hours, routinely processed, and embedded in paraffin wax. Five-micron sections were stained with chromotrope 2R.
Immunofluorescence and flow cytometry. One hundred microliters of whole blood or purified peritoneal granulocytes (2.5 × 106 cells/mL) was incubated with the primary antibodies (anti-β2 , anti-α4 , or anti–L-selectin MoAbs, all at a final concentration of 10 μg/mL) or no antibody for 30 minutes on ice. After washing with PBS/0.1% NaN3 , the appropriate FITC-labeled secondary antibody was added and the cells were incubated for an additional 30 minutes. With respect to whole blood samples, to lyse erythrocytes, 2 mL of lysing solution (Becton Dickinson) was added to each tube and the cells were centrifuged after incubating for 10 minutes at room temperature, according to the manufacturer's protocol. The cells were washed twice before being analyzed on a FACScan flow cytometer (Becton Dickinson). Eosinophils comprised 1% to 2% of whole blood leukocytes and could easily be distinguished from neutrophils on the basis of forward and side scatter, with the side scatter of rat eosinophils being far greater than that of rat neutrophils. The geometric mean fluorescence intensity of eosinophils and neutrophils in each sample was obtained and the specific mean fluorescence was calculated by subtracting the mean fluorescence intensity of the appropriate negative control.
Statistical analysis. Results of eosinophil recruitment by EPO measurement and 111In-eosinophil accumulation in rat skin are expressed as the mean ± SEM for n animals, in which each datum unit is the average of responses in duplicate sites. Statistical analysis of isotopic experiments was performed by two-way analysis of variance (ANOVA) of log-transformed data and statistical significance was determined with the Newman-Keuls procedure for repeated comparisons. A P value of less than .05 was considered statistically significant. Statistical analysis of eosinophil accumulation by EPO measurement and fluorescence-activated cell sorting (FACS) analysis were performed using an unpaired two-tail Student's t-test. P < .05 was considered to be statistically significant.
RESULTS
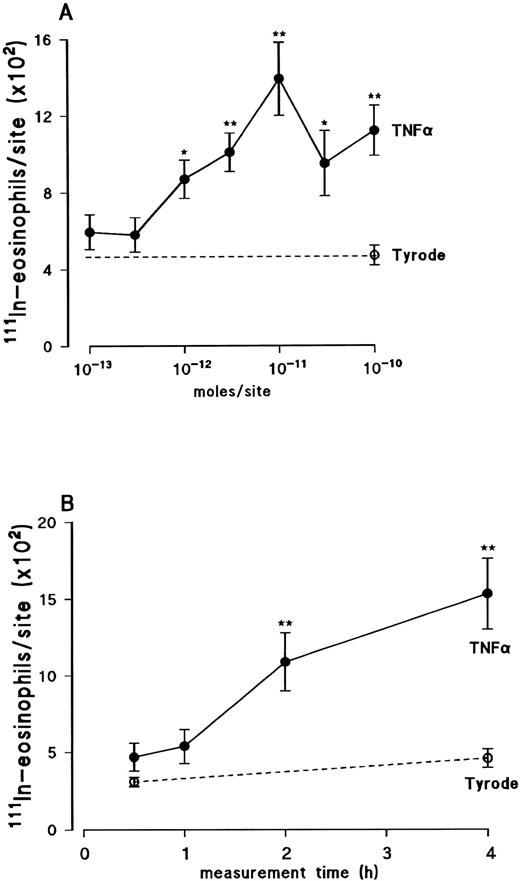
Dose-response relationship and time-course of TNFα-induced eosinophil accumulation in rat skin. Using a 4-hour in vivo test period, the intradermal injection of recombinant human TNFα (TNFα), within the dose-range of 10−12 to 10−10 mol/site, induced a significant accumulation of 111In-eosinophils above the small level of counts detected in Tyrode-injected sites (Fig 1A). The dose-response relationship of this effect was bell-shaped in that maximal response was detected at 10−11 mol/site, whereas higher doses caused a reduced level of 111In-eosinophil accumulation. Using the dose of 10−11 mol/site, the cumulative time-course profile of TNFα-induced 111In-eosinophil accumulation was investigated (Fig 1B). As previously found with IL-1β,34 the 111In-eosinophil accumulation induced by TNFα was slow in onset, progressively increasing over the 4-hour period studied. The first time-point at which the 111In-eosinophil accumulation was found to be significantly higher than the counts detected in control sites was at 2 hours. For comparison, in a number of experiments, the effect of recombinant rat TNFα was also investigated. Using a 4-hour test period, at the doses of 2 × 10−11 mol/site and 6 × 10−11 mol/site, recombinant rat TNFα caused the accumulation of 294 ± 37 and 502 ± 78 111In-eosinophils above Tyrode injected sites, respectively (n = 4 to 6 rats, P < .01).
Dose-response relation (A) and time course (B) of 111In-eosinophil accumulation induced by intradermal TNFα in rat skin. Animals were injected IV with 111In-eosinophils. (A) TNFα (•) or Tyrode solution (○) was injected ID into the previously clipped back skin of rats and responses measured over a 4-hour period. (B) TNFα (10−11 mol/site; •) or Tyrode solution (○) was injected ID at different time points within a 4-hour period. Responses are the mean ± SEM for n = 4 to 6 rats. A significant difference from the 4-hour Tyrode level is indicated by *P < .05 or **P < .01.
Dose-response relation (A) and time course (B) of 111In-eosinophil accumulation induced by intradermal TNFα in rat skin. Animals were injected IV with 111In-eosinophils. (A) TNFα (•) or Tyrode solution (○) was injected ID into the previously clipped back skin of rats and responses measured over a 4-hour period. (B) TNFα (10−11 mol/site; •) or Tyrode solution (○) was injected ID at different time points within a 4-hour period. Responses are the mean ± SEM for n = 4 to 6 rats. A significant difference from the 4-hour Tyrode level is indicated by *P < .05 or **P < .01.
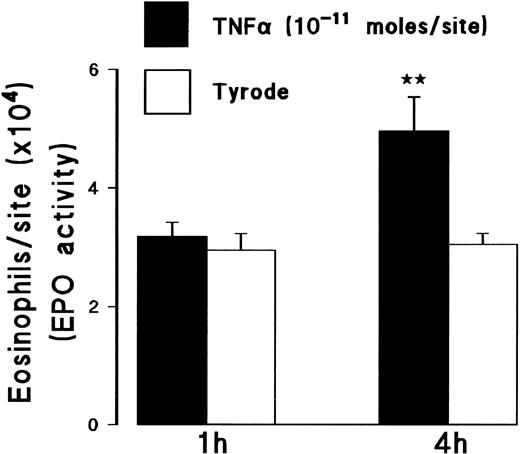
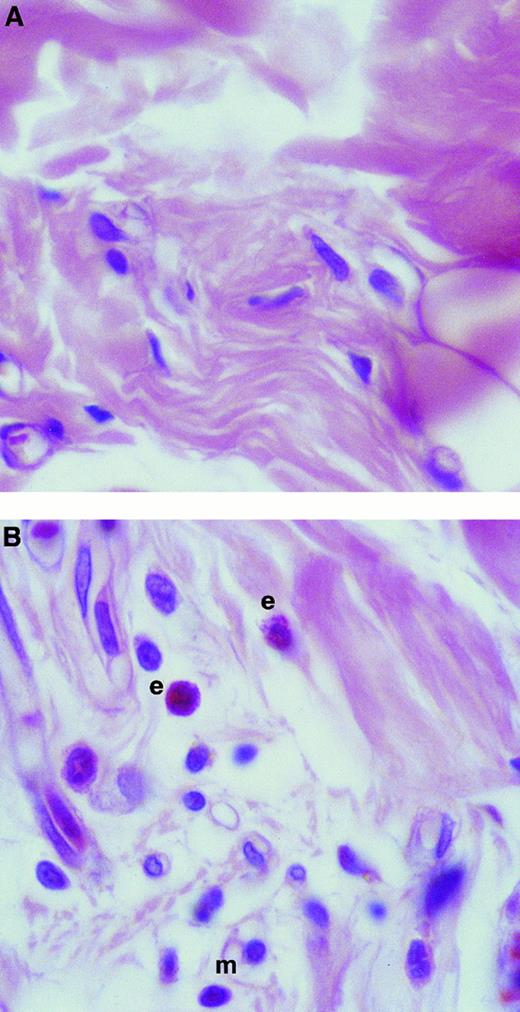
The results obtained using the assay of 111In-eosinophil accumulation agreed with measurements of eosinophil accumulation as quantified by EPO activity in homogenized skin sites (Fig 2). Intradermal TNFα (10−11 mol/site), at 4 hours but not at 1 hour, induced a significant increase in eosinophil accumulation above levels detected in Tyrode-injected sites, as measured by EPO activity. Furthermore, histologic analysis of TNFα-injected skin sites showed that, whereas the majority of the infiltrating leukocytes were mononuclear leukocytes and neutrophils, there was a marked infiltration of eosinophils as indicated in Fig 3.
Effect of TNFα on local eosinophil accumulation as measured by EPO activity in skin sites. The graph shows EPO activity in skin sites injected with TNFα (10−11 mol/site) or Tyrode, using a 4-hour or 1-hour measurement period. Results represent the number of eosinophils per skin site ± SEM (n = 3). A significant difference over Tyrode injected sites is indicated by **P < .01.
Effect of TNFα on local eosinophil accumulation as measured by EPO activity in skin sites. The graph shows EPO activity in skin sites injected with TNFα (10−11 mol/site) or Tyrode, using a 4-hour or 1-hour measurement period. Results represent the number of eosinophils per skin site ± SEM (n = 3). A significant difference over Tyrode injected sites is indicated by **P < .01.
Histologic analysis of rat skin sites injected with TNFα. Skin sites were injected with (A) Tyrode or (B) TNFα (10−11 mol/site). After a 4-hour in vivo test period, the skin sites were processed and stained as described in the Materials and Methods. In photomicrographs, examples of eosinophils and mononuclear cells are labeled e and m, respectively. Original magnification × 600.
Histologic analysis of rat skin sites injected with TNFα. Skin sites were injected with (A) Tyrode or (B) TNFα (10−11 mol/site). After a 4-hour in vivo test period, the skin sites were processed and stained as described in the Materials and Methods. In photomicrographs, examples of eosinophils and mononuclear cells are labeled e and m, respectively. Original magnification × 600.
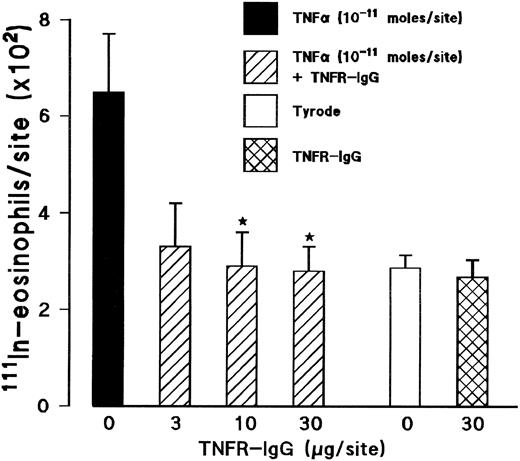
Effect of a soluble TNFα receptor (p55)-IgG fusion protein (TNFR-IgG) on TNFα-induced 111In-eosinophil accumulation. The coinjection of a TNFR-IgG (3 to 30 μg/site) with TNFα into skin sites totally inhibited the 111In-eosinophil accumulation induced by the cytokine (Fig 4). In contrast, TNFR-IgG had no significant effect on the responses elicited by other inflammatory stimuli such as zymosan-activated plasma (ZAP). The 111In-eosinophil accumulation induced by ZAP (above Tyrode injected sites) in the absence and presence of TNFR-IgG (30 μg/site) was 435 ± 68 and 658 ± 138, respectively, for n = 5 rats. The responses detected in skin sites where TNFα was coinjected with the control chimeric antibody cSF25 (791 ± 56 cells/site) were not significantly different from sites injected with TNFα alone (659 ± 120 cells/site).
Effect of a soluble TNFα receptor (p55)-IgG fusion protein (TNFR-IgG) on the TNFα-induced 111In-eosinophil accumulation in rat skin. Using a 4-hour in vivo test period, the graph shows responses to TNFα alone (10−11 mol/site; ▪), TNFα coinjected with TNFR-IgG (3 to 30 μg/site; ▨), TNFR-IgG alone (30 μg/site; ), and Tyrode (□). Results are the mean ± SEM for n = 5 animals. Asterisks indicate a significant difference from sites injected with TNFα alone (*P < .05).
Effect of a soluble TNFα receptor (p55)-IgG fusion protein (TNFR-IgG) on the TNFα-induced 111In-eosinophil accumulation in rat skin. Using a 4-hour in vivo test period, the graph shows responses to TNFα alone (10−11 mol/site; ▪), TNFα coinjected with TNFR-IgG (3 to 30 μg/site; ▨), TNFR-IgG alone (30 μg/site; ), and Tyrode (□). Results are the mean ± SEM for n = 5 animals. Asterisks indicate a significant difference from sites injected with TNFα alone (*P < .05).
Effects of the PAF receptor antagonist UK-74,505 and an anti–IL-8 MoAb DM/C7 on 111In-eosinophil accumulation induced by TNFα in rat skin. We have previously found roles for endogenously generated PAF and an IL-8–like molecule in 111In-eosinophil accumulation induced by IL-1β.34 To investigate the possible roles of these mediators in the TNFα-induced responses, we have tested the effects of the potent and long-acting PAF receptor antagonist UK-74,505 and the anti–IL-8 MoAb DM/C7. UK-74,505 (0.5 mg/kg IV), although totally inhibiting the PAF-induced 111In-eosinophil accumulation (eg, the 111In-eosinophil accumulation induced by 10−11 mol/site PAF was inhibited by 96.3%), had no significant effect on the 111In-eosinophil accumulation elicited by TNFα (20.1% ± 8.2% inhibition, n = 7).
The antihuman IL-877 MoAb, DM/C7 (3.5 mg/kg IV), which was previously shown to inhibit IL-1β–induced 111In-eosinophil accumulation in rat skin,34 had an apparent inhibitory effect on the TNFα-induced 111In-eosinophil accumulation in all the experiments performed, but this difference was not statistically significant (44.4% ± 12.5% inhibition, n = 8). UK-74,505 and MoAb DM/C7 had no affect on circulating 111In-leukocyte cell numbers.
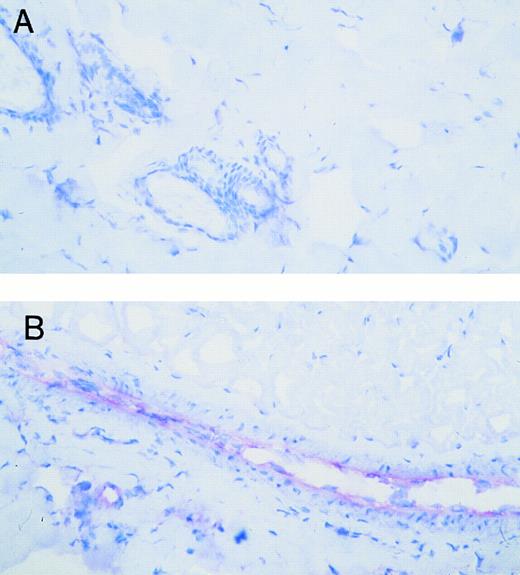
Effect of intradermal TNFα on VCAM-1 expression in rat skin microvasculature. Tyrode or TNFα (10−11 mol/site) was ID injected into the back skin of the animal. Photomicrographs show VCAM-1 expression (red staining) in Tyrode-injected (A) or TNFα-injected sites (B) after a 4-hour in vivo test period. Original magnification × 200.
Effect of intradermal TNFα on VCAM-1 expression in rat skin microvasculature. Tyrode or TNFα (10−11 mol/site) was ID injected into the back skin of the animal. Photomicrographs show VCAM-1 expression (red staining) in Tyrode-injected (A) or TNFα-injected sites (B) after a 4-hour in vivo test period. Original magnification × 200.
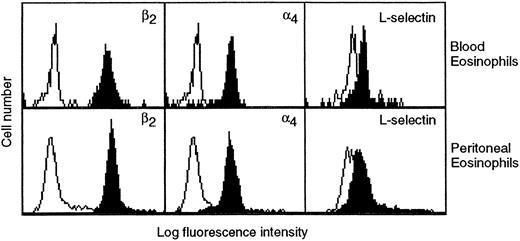
Effects of an anti-α4 integrin MoAb HP2/1 and an anti–VCAM-1 MoAb 5F10 on TNFα-induced 111In-eosinophil accumulation in rat skin. The aim of the experiments described below was to investigate the role of the α4 integrin/VCAM-1 adhesion pathways in the TNFα-induced eosinophil accumulation in vivo. In vitro studies performed before the in vivo experiments showed that rat peritoneal eosinophils expressed key leukocyte adhesion molecules β2 integrins, α4 integrins, and L-selectin on their surface, as determined by FACS analysis (Table 1 and Fig 5). Furthermore, there was no significant difference between the expression of α4 integrins or L-selectin on blood and peritoneal eosinophils (Table I and Fig 5). However, peritoneal eosinophils did express a significantly enhanced level of β2 integrins. For comparison, Table 1 also shows the expression of adhesion molecules on rat neutrophils. As compared with blood neutrophils, peritoneal neutrophils expressed a significantly greater level of β2 integrins and a significantly lower level of L-selectin. Interestingly, a very low level of α4 integrin expression was also detected on rat whole blood neutrophils. The expression of this molecule was significantly greater on peritoneal neutrophils.
Expression of Adhesion Molecules on Rat Blood and Peritoneal Eosinophils and Neutrophils
| . | β2 Integrins . | α4 Integrins . | L-Selectin . |
|---|---|---|---|
| Blood eosinophils | 170.0 ± 13.5 | 61.5 ± 3.4 | 14.9 ± 3.7 |
| Peritoneal eosinophils | 278.0 ± 32.5 | 72.2 ± 12.1 | 6.7 ± 2.0 |
| (P = .022*) | (P = .429) | (P = .099) | |
| Blood neutrophils | 167.9 ± 12.2 | 5.4 ± 1.2 | 14.8 ± 1.5 |
| Peritoneal neutrophils | 331.4 ± 45.3 | 14.8 ± 2.8 | 2.4 ± 0.7 |
| (P = .025*) | (P = .038*) | (P = .002*) |
| . | β2 Integrins . | α4 Integrins . | L-Selectin . |
|---|---|---|---|
| Blood eosinophils | 170.0 ± 13.5 | 61.5 ± 3.4 | 14.9 ± 3.7 |
| Peritoneal eosinophils | 278.0 ± 32.5 | 72.2 ± 12.1 | 6.7 ± 2.0 |
| (P = .022*) | (P = .429) | (P = .099) | |
| Blood neutrophils | 167.9 ± 12.2 | 5.4 ± 1.2 | 14.8 ± 1.5 |
| Peritoneal neutrophils | 331.4 ± 45.3 | 14.8 ± 2.8 | 2.4 ± 0.7 |
| (P = .025*) | (P = .038*) | (P = .002*) |
Expression of adhesion molecules, β2 integrins, α4 integrins, or L-selectin on blood and peritoneal eosinophils (n = 4 preparations) and neutrophils (n = 3 preparations) were quantified and analyzed by indirect immunofluorescence and FACS analysis and are expressed as specific mean fluorescence (the results are corrected for the relevant control values). For methodological details, see the Materials and Methods. Data are the mean ± SEM and the numbers in the parenthesis indicate the P value comparing the difference between blood and peritoneal leukocytes.
Significance, P < .05.
Expression of β2 and α4 integrins and L-selectin on eosinophils in whole blood (upper panels) and eosinophils purified from the peritoneal fluid (lower panels) as determined by flow cytometry. Cells were stained with anti-β2 integrin (TA-4), anti-α4 integrin (HP2/1), or anti–L-selectin (HRL1) MoAbs (all at a final concentration of 10 μg/mL; solid histograms) or the appropriate negative controls followed by FITC-conjugated secondary antibodies and analyzed by flow cytometry. The results shown are representative of four separate experiments.
Expression of β2 and α4 integrins and L-selectin on eosinophils in whole blood (upper panels) and eosinophils purified from the peritoneal fluid (lower panels) as determined by flow cytometry. Cells were stained with anti-β2 integrin (TA-4), anti-α4 integrin (HP2/1), or anti–L-selectin (HRL1) MoAbs (all at a final concentration of 10 μg/mL; solid histograms) or the appropriate negative controls followed by FITC-conjugated secondary antibodies and analyzed by flow cytometry. The results shown are representative of four separate experiments.
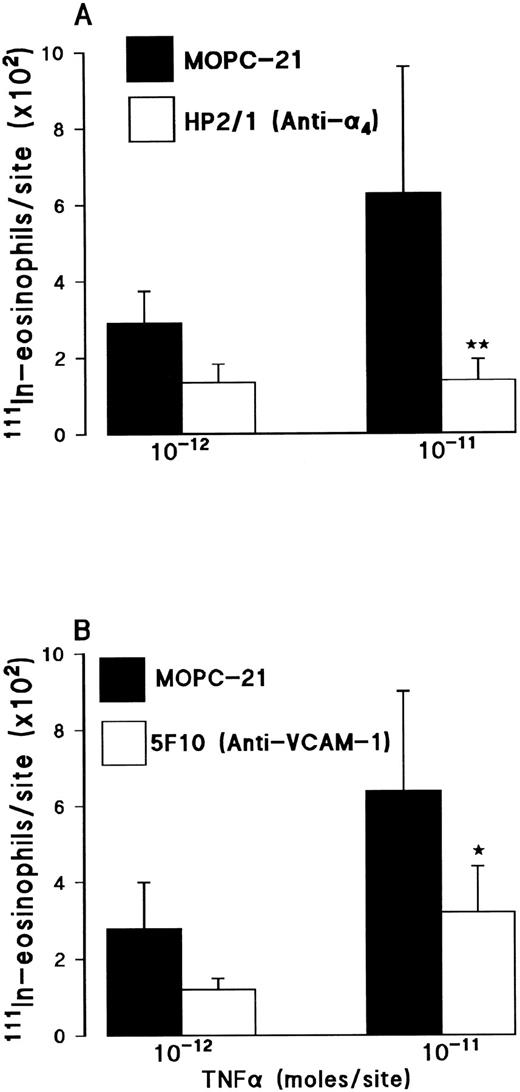
In vivo, pretreatment of rats with the anti-α4 integrin MoAb HP2/1 (3.5 mg/kg IV) significantly suppressed the 111In-eosinophil accumulation induced by TNFα (Fig 6A). The responses elicited by 10−12 mol/site and 10−11 mol/site TNFα were inhibited by 54% and 78%, respectively. A larger dose of the antibody (5 mg/kg IV) did not induce a greater level of inhibition (results not shown).
Effect of the anti-α4 integrin MoAb HP2/1 (A) or the anti–VCAM-1 MoAb 5F10 (B) on TNFα-induced 111In-eosinophil accumulation in rat skin. (A) Animals were injected with a control MoAb MOPC-21 (3.5 mg/kg, IV▪) or MoAb HP2/1 (3.5 mg/kg, IV; □) 10 minutes before the intradermal administration of TNFα (10−12 or 10−11 mol/site). (B) Animals were injected with a control MoAb MOPC-21 (2 mg/kg, IV; ▪) or MoAb 5F10 (2 mg/kg, IV; □) 10 minutes before the intradermal administration of TNFα (10−12 or 10−11 mol/site). Using a 4-hour test period, the results are presented as mean ± SEM for n = 5 to 8 pairs of rats. Asterisks indicate a significant difference from MOPC-21–treated rats (*P < .05).
Effect of the anti-α4 integrin MoAb HP2/1 (A) or the anti–VCAM-1 MoAb 5F10 (B) on TNFα-induced 111In-eosinophil accumulation in rat skin. (A) Animals were injected with a control MoAb MOPC-21 (3.5 mg/kg, IV▪) or MoAb HP2/1 (3.5 mg/kg, IV; □) 10 minutes before the intradermal administration of TNFα (10−12 or 10−11 mol/site). (B) Animals were injected with a control MoAb MOPC-21 (2 mg/kg, IV; ▪) or MoAb 5F10 (2 mg/kg, IV; □) 10 minutes before the intradermal administration of TNFα (10−12 or 10−11 mol/site). Using a 4-hour test period, the results are presented as mean ± SEM for n = 5 to 8 pairs of rats. Asterisks indicate a significant difference from MOPC-21–treated rats (*P < .05).
Similarly, administration of an anti–VCAM-1 MoAb 5F10 (2 mg/kg IV) significantly reduced the 111In-eosinophil accumulation induced by TNFα (Fig 6B). The responses elicited by 10−12 mol/site and 10−11 mol/site TNFα were inhibited by 56% and 50%, respectively. Pretreatment of rats with a larger dose of MoAb 5F10 (5 mg/kg IV) did not result in a greater level of inhibition (results not shown). These results are in agreement with the observations that increased expression of VCAM-1 was detected on venules in TNFα-injected skin sites. As can be seen in Fig 7, VCAM-1 was strongly expressed at 4 hours in TNFα-injected but not in Tyrode-injected sites. Earlier time-points did not show a marked expression of VCAM-1 (data not shown). A nonbinding control IgG (MOPC-21) and an antirat major histocompatibility complex (MHC) class I MoAb (binding to both rat leukocytes and endothelial cells) had no effect on the TNFα-induced 111In-eosinophil accumulation. The data with MOPC-21 are shown in Fig 6. The results with the anti-MHC class I (5 mg/kg IV) are as follows: the responses elicited by TNFα (10−11 mol/site) in untreated and anti-MHC class I MoAb-treated rats were 926 ± 112 versus 1,022 ± 103 111In-eosinophils/site, respectively (results are corrected for the small levels detected in Tyrode-injected sites, n = 3 pairs of rats).
Although the anti-α4 integrin MoAb had no effect on circulating 111In-eosinophil numbers, in rats treated with the anti–VCAM-1 MoAb, there was a significant increase in circulating 111In-eosinophil numbers (94.7% ± 32.2% increase as compared with rats treated with a control MoAb). Furthermore, in separate studies, in contrast to their effects on 111In-eosinophil accumulation, both antibodies were without an effect on 111In-neutrophil accumulation induced by TNFα: the responses elicited by TNFα (10−11 mol/site) in MOPC-21-treated and anti-α4 integrin MoAb-treated rats (both at 3.5 mg/kg IV) were 281 ± 73 versus 329 ± 84 111In-neutrophils/site, respectively. (Results are corrected for counts in Tyrode-injected sites, n = 4 pairs of rats. The results with the anti–VCAM-1 MoAb are not shown.)
DISCUSSION
TNFα has been indirectly implicated in the process of eosinophil accumulation in vivo. In this context, both the protein and message have been detected in lungs of asthmatic subjects and at sites of allergic inflammation in animal models.31,32,39 These measurements have been associated with the local infiltration of eosinophils. Furthermore, TNFα blockers inhibit the accumulation of eosinophils in a number of inflammatory reactions in vivo.32,33 In the present study, using an in vivo model that we have previously used to characterize the 111In-eosinophil accumulation induced by IL-1β,34 we have directly investigated the ability and profile of TNFα-induced 111In-eosinophil accumulation in rat skin. The results show that TNFα, when administered ID, is an effective inducer of 111In-eosinophil accumulation in rat skin. This response, which developed slowly, was also confirmed by histologic analysis and by measurement of eosinophil accumulation in skin sites as quantified by EPO activity.
TNFα appears to exert its effects through interaction with specific cell surface receptors. At least two distinct TNFα receptors have been characterized, namely the 75-kD (TNFRα) and the 55-kD (TNFRβ) receptors.7,8 Although the predominant role of the 75-kD receptor remains unclear, the 55-kD receptor appears to mediate much of the proinflammatory effects of TNFα. The 55-kD receptor is expressed on endothelial cells and mediates the TNFα-induced increased expression of adhesion molecules on endothelial cells and the induction of leukocyte adhesion to endothelium.7 Although both receptor types have also been detected on granulocytes, their relative numbers are contentious, and the TNFα-induced stimulation of granulocytes appears to be primarily mediated by the 55-kD receptor.8 In the present study, the TNFα-induced accumulation of radiolabeled eosinophils was totally inhibited when the cytokine was coinjected with a soluble TNFα receptor (p55)-IgG fusion protein. The slow development of this response strongly suggests that the eosinophil accumulation induced by TNFα is mediated via an interaction with TNFα receptors on venular endothelial cells as opposed to receptors on leukocytes.
The TNFα-induced eosinophil accumulation may be mediated via endothelial cell adhesion molecules and/or the local generation of inflammatory mediators. With respect to the latter, TNFα has been shown to stimulate the generation of a number of mediators, including the phospholipid PAF11 and the chemokines RANTES, macrophage inflammatory protein-1α (MIP-1α), and IL-8.10,40-42 The involvement of endogenously generated PAF and IL-8 in the TNFα-induced 111In-eosinophil accumulation was investigated by using the long-acting PAF receptor antagonist UK-74,50543-45 and the anti–IL-877 MoAb DM/C7,34,35 respectively. These inhibitors have previously been shown to suppress the eosinophil accumulation induced by IL-1β in rat skin.34 In contrast, in the present study, the PAF receptor antagonist and the anti–IL-8 MoAb did not have a significant inhibitory effect on the TNFα-induced eosinophil accumulation. These results suggest that endogenous generation of PAF or an IL-8–like molecule, on their own, do not have a major role in TNFα-stimulated eosinophil recruitment and indicate an important difference in the mechanisms by which IL-1β and TNFα elicit eosinophil accumulation in vivo. The role of other inflammatory mediators in the TNFα-induced eosinophil accumulation is currently under investigation. In this context, we have found that an antibody against eotaxin, the newly described eosinophil specific CC chemokine,46,47 although partially suppressing the eosinophil accumulation induced by IL-4, had no effect on the eosinophil accumulation induced by TNFα (Sanz et al, manuscript submitted). Alternative inflammatory mediators that may mediate this response include other members of the CC chemokine family such as RANTES 40,41 or MIP-1α.42 It is also possible that, within the 4-hour in vivo test period used in the present study, the eosinophil recruitment in response to TNFα was induced directly after stimulation of venular endothelial cells by TNFα.
The above conclusion is supported by in vitro studies demonstrating increased adhesion of eosinophils and neutrophils to TNFα-stimulated endothelial cells.16-18 This effect of TNFα is mediated via induction of endothelial cell associated adhesion molecules such as ICAM-1, VCAM-1, and E-selectin.12-15 Neutralizing antibodies to β2 integrins, ICAM-1, and E-selectin can inhibit the adhesion of both eosinophils and neutrophils to TNFα-activated cultured endothelial cells. In contrast, anti–VCAM-1 antibodies can inhibit the adhesion of eosinophils, but not neutrophils, to cytokine-activated endothelial cells.16,19-22 This selective effect is explained by the fact that the well-characterized principal leukocyte ligands for VCAM-1, namely α4β1 (VLA-4) and α4β7 , are expressed on the cell surface of eosinophils but not normally on neutrophils.23 Despite these in vitro studies, there have been no in vivo investigations into the role of the α4 integrin/VCAM-1 pathway in TNFα-induced eosinophil accumulation in vivo. Furthermore, in contrast to the increasing number of studies reporting inhibitory effects of anti-α4 reagents on eosinophil accumulation,23,48 very few studies have investigated the effect of VCAM-1 blockers on eosinophil accumulation in vivo.49 In the present study, we found that VCAM-1 was strongly expressed on venules in TNFα-injected skin sites and that both an anti-α4 integrin MoAb (HP2/1) and an antirat VCAM-1 MoAb (5F10) greatly inhibited the 111In-eosinophil accumulation induced by TNFα. The inhibitory effects of these MoAbs on TNFα-induced eosinophil accumulation were partial in that the maximum level of inhibition achieved with the anti-α4 integrin MoAb was 78% and the maximum level of inhibition achieved with the anti–VCAM-1 MoAb was 56%. These effects were the maximum attainable with either MoAb, because increasing their dose did not increase their inhibitory effects. A number of possible explanations may account for the apparent greater level of inhibition of eosinophil accumulation achieved with the anti-integrin MoAb. It is possible that a greater number of adhesion pathways are being blocked by the anti-α4 integrin antibody, ie, adhesion pathways involving α4 integrins/VCAM-1 as well as adhesion pathways involving α4 integrins/other endothelial cell adhesion ligands. The greater inhibition seen with the anti-α4 MoAb may also be because the anti-integrin MoAb probably blocks binding to domains 1 and 4 of VCAM-1, whereas the anti–VCAM-1 MoAb only blocks the domain 1/2 binding site. Furthermore, α4 integrins may have a greater role than VCAM-1 in mediating both eosinophil rolling and firm adhesion to venular endothelial cells.50 The residual level of 111In-eosinophil accumulation that was not inhibited by α4 integrin/VCAM-1 blockade may be mediated via other adhesion pathways such as β2 /ICAM-1 interactions. We have previously shown that Sephadex-induced lung eosinophilia can be totally inhibited by a combination of anti-β2 and anti-α4 integrin MoAbs51 and Issekutz27 has shown that monocyte accumulation induced by TNFα can be totally inhibited by a combination of anti-CD11a, anti-CD11b and anti-α4 MoAbs.
It is of interest that we found that, whereas rat neutrophils also expressed a small level of α4 integrins on their cell surface (the level was greater on peritoneal as compared with blood neutrophils), anti-α4 integrin or anti–VCAM-1 MoAbs were without an effect on the accumulation of 111In-neutrophils induced by TNFα. Our in vitro results are in agreement with a number of recent studies reporting low levels of expression of α4 integrins on rat neutrophils52 or on activated human neutrophils.53 However, our in vivo results do not agree with the findings of Issekutz et al,52 who have reported that an anti-α4 integrin MoAb inhibits TNFα-induced neutrophil accumulation into skin by approximately 30%. A number of methodologic explanations may account for the difference in our results and those of Issekutz et al.52 These include the fact that the two studies used different blocking MoAbs, different preparations and doses of TNFα (murine as opposed to human), and, most importantly, different procedures for obtaining and purifying rat neutrophils. Clearly, further studies are needed to clarify the functional role of α4 integrins in neutrophil accumulation in vivo.
In conclusion, we have provided direct evidence for the ability of TNFα to induce the local accumulation of eosinophils in vivo. The TNFα-induced eosinophil accumulation was slow in onset, inhibited by a soluble TNFα receptor (55 kD) fusion protein, and highly dependent on the α4 integrin/VCAM-1 adhesion pathway. Our results provide direct evidence for a role for TNFα in eosinophil recruitment in vivo and further suggest a role for this cytokine in allergic inflammatory reactions.
Supported by The Wellcome Trust, UK, and Biogen Inc, USA.
Address reprint requests to Sussan Nourshargh, PhD, National Heart and Lung Institute, ICSM, Dovehouse Street, London SW3 6LY, UK.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal